-2548G>A LEP Polymorphism Is Positively Associated with Increased Leptin and Glucose Levels in Obese Saudi Patients Irrespective of Blood Pressure Status
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Anthropometric and Blood Pressure Measurements
2.3. Blood Sampling and Biochemical Analyses
2.4. Genetic Analyses of LEP G-2548A Polymorphism
2.5. Statistical Analysis
3. Results
3.1. Anthropometric and Biochemical Characteristics of Participants
3.2. Distribution of Alleles and Genotype Frequency for -2548G>A LEP Polymorphism
3.3. Anthropometric and Clinical Characteristics of the Study Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Mokdad, A.H.; Ford, E.; Narayan, K.V.; Giles, W.H.; Vinicor, F.; Deedwania, P.C. Obesity, metabolic syndrome, and type 2 diabetes: Emerging epidemics and their cardiovascular implications. Cardiol. Clin. 2004, 22, 485–504. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Lu, W.; Zong, X.F.; Ruan, H.Y.; Liu, Y. Obesity and hypertension. Exp. Ther. Med. 2016, 12, 2395–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Lawes, C.M.; Vander Hoorn, S.; Rodgers, A. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Hall, J.E. The kidney, hypertension, and obesity. Hypertension 2003, 41, 625–633. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Barba, G.; Russo, O.; Siani, A.; Iacone, R.; Farinaro, E.; Gerardi, M.C.; Russo, P.; Della Valle, E.; Strazzullo, P. Plasma leptin and blood pressure in men: Graded association independent of body mass and fat pattern. Obes. Res. 2003, 11, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Mammes, O.; Betoulle, D.; Aubert, R.; Herbeth, B.; Siest, G.; Fumeron, F. Association of the G-2548A polymorphism in the 5′ region of the LEP gene with overweight. Ann. Hum. Genet. 2000, 64, 391–394. [Google Scholar] [CrossRef]
- Carlyle, M.; Jones, O.B.; Kuo, J.J.; Hall, J.E. Chronic cardiovascular and renal actions of leptin: Role of adrenergic activity. Hypertension 2002, 39, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Cir. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, M.L.; Haynes, W.G.; Rahmouni, K.; Morgan, D.A.; Sivitz, W.I.; Mark, A.L. The concept of selective leptin resistance. Evidence from Agouti yellow obese mice. Diabetes 2002, 51, 439–442. [Google Scholar] [PubMed] [Green Version]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mammes, O.; Betoulle, D.; Aubert, R.; Giraud, V.; Tuzet, S.; Petiet, A.; Colas-Linhart, N.; Fumeron, F. Novel polymorphisms in the 5’ region of the LEP gene: Association with leptin levels and response to low-calorie diet in human obesity. Diabetes 1998, 47, 487–489. [Google Scholar] [CrossRef]
- Shintani, M.; Ikegami, H.; Fujisawa, T.; Kawaguchi, Y.; Ohishi, M.; Katsuya, T.; Higaki, J.; Shimamoto, K.; Ogihara, T. Leptin Gene Polymorphism Is Associated with Hypertension Independent of Obesity. J. Clin. Endocrinol. Metab. 2002, 87, 2909–2912. [Google Scholar] [CrossRef]
- Hoffstedt, J.; Eriksson, P.; Mottagui-Tabar, S.; Arner, P. A polymorphism in the leptin promoter region (-2548G/A) influences gene expression and adipose tissue secretion of leptin. Horm. Metab. Res. 2002, 34, 355–359. [Google Scholar] [CrossRef]
- Ali, S.B.; Kallel, A.; Ftouhi, B.; Sediri, Y.; Feki, M.; Slimane, H.; Jemaa, R.; Kaabachi, N. Association of G-2548A LEP polymorphism with plasma leptin levels in Tunisian obese patients. Clin. Biochem. 2009, 42, 584–588. [Google Scholar]
- Hinuy, H.M.; Hirata, M.H.; Forti, N.; Diament, J.; Sampaio, M.F.; Armaganijan, D.; Salazar, L.A.; Hirata, R.D. Leptin G-2548A promoter polymorphism is associated with increased plasma leptin and BMI in Brazilian women. Arq. Bras. Endocrinol. Metabol. 2008, 52, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.N.; Huang, M.C.; Chang, W.T.; Ko, A.M.S.; Tsai, E.M.; Liu, C.S.; Lee, C.H.; Ko, Y.C. G-2548A polymorphism of the leptin gene is correlated with extreme obesity in Taiwanese aborigines. Obesity 2006, 14, 183–187. [Google Scholar] [CrossRef]
- Ortega-Azorín, C.; Coltell, O.; Asensio, E.M.; Sorlí, J.V.; González, J.I.; Portolés, O.; Saiz, C.; Estruch, R.; Ramírez-Sabio, J.B.; Pérez-Fidalgo, A.; et al. Candidate gene and genome-wide association studies for circulating leptin levels reveal population and sex-specific associations in high cardiovascular risk Mediterranean subjects. Nutrients 2019, 11, 2751. [Google Scholar] [CrossRef] [Green Version]
- Kargasheh, F.B.; Ansaripour, S.; Borumandnia, N.; Moradi, N.; Zandieh, Z.; Maleki, M.; Mokhtar, S.; Karimi, A.; Fatemi, F.; Kheirollahi, A.; et al. Association of leptin G2548A and leptin receptor Q223R polymorphisms and their serum levels with infertility and recurrent pregnancy loss in Iranian women with polycystic ovary syndrome. PLoS ONE 2021, 16, e0255920. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry (1995) Report of WHO Expert Committee; WHO Technical Report Series, no. 854; WHO: Geneva, Switzerland, 1995; pp. 321–344. [Google Scholar]
- Al-Daghri, N.; Alokail, M.; Al-Attas, O.; Sabico, S.; Kumar, S. Establishing abdominal height cut-offs and their association with conventional indices of obesity among Arab children and adolescents. Ann. Saudi Med. 2010, 30, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Standards of Medical Care in Diabetes, 2016. Diabetes Care 2016, 39, S4–S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Boumaiza, I.; Omezzine, A.; Rejeb, J.; Rebhi, L.; Ouedrani, A.; Ben Rejeb, N.; Nabli, N.; Ben Abdelaziz, A.; Bouslama, A. Relationship between leptin G2548A and leptin receptor Q223R gene polymorphisms and obesity and metabolic syndrome risk in Tunisian volunteers. Genet. Test. Mol. Biomark. 2012, 16, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Huuskonen, A.; Lappalainen, J.; Tanskanen, M.; Oksala, N.; Kyröläinen, H.; Atalay, M. Genetic variations of leptin and leptin receptor are associated with body composition changes in response to physical training. Cell Biochem. Funct. 2010, 28, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wilk, J.B.; Borecki, I.; Williamson, S.; DeStefano, A.L.; Xu, G.; Liu, J.; Ellison, R.C.; Province, M.; Myers, R.H. Common variants in the 5′ region of the leptin gene are associated with body mass index in men from the National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Hum. Genet. 2004, 75, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Şahın, S.; Rüstemoğlu, A.; Tekcan, A.; Taşliyurt, T.; Güven, H.; Yığıt, S. Investigation of associations between obesity and LEP G2548A and LEPR 668A/G polymorphisms in a Turkish population. Dis. Markers 2013, 35, 673–677. [Google Scholar] [CrossRef] [Green Version]
- Constantin, A.; Costache, G.; Sima, A.V.; Glavce, C.S.; Vladica, M.; Popov, D.L. Leptin G-2548A and leptin receptor Q223R gene polymorphisms are not associated with obesity in Romanian subjects. Biochem. Biophys. Res. Commun. 2010, 391, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Soskic, S.; Stokic, E.; Obradovic, M.; Sudar, E.; Tanic, N.; Kupusinac, A.; Djordjevic, J.; Isenovic, E.R. Association of leptin gene polymorphism G-2548A with metabolic and anthropometric parameters in obese patients in a Serbian population: Pilot study. Clin. Lipidol. 2014, 9, 505–513. [Google Scholar] [CrossRef]
- Portolés, O.; Sorlí, J.V.; Francés, F.; Coltell, O.; González, J.I.; Sáiz, C.; Corella, D. Effect of genetic variation in the leptin gene promoter and the leptin receptor gene on obesity risk in a population-based case-control study in Spain. Eur. J. Epidemiol. 2006, 21, 605–612. [Google Scholar] [CrossRef]
- Duarte, S.F.; Francischetti, E.A.; Genelhu, V.A.; Cabello, P.H.; Pimentel, M.M. LEPR p.Q223R, beta3-AR p.W64R and LEP c.-2548G>A gene variants in obese Brazilian subjects. Genet. Mol. Res. 2007, 6, 1035–1043. [Google Scholar]
- Becer, E.; Kizilkanat, M.; Tinazli, M.; Serakinci, N. Association between leptin G-2548A gene polymorphism, plasma leptin levels and lipid profiles in Turkish Cypriot obese subjects. Turk. J. Biochem. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Gong, D.W.; Bi, S.; Pratley, R.E.; Weintraub, B.D. Genomic structure and promoter analysis of the human obese gene. J. Biol. Chem. 1996, 271, 3971–3974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, M.M.; He, Y.; Chen, H.; Quon, M.J.; Reitman, M. Regulation of leptin promoter function by Sp1, C/EBP, and a novel factor. Endocrinology 1998, 139, 1013–1022. [Google Scholar] [CrossRef]
- Moreno-Aliaga, M.J.; Swarbrick, M.M.; Lorente-Cebrian, S.; Stanhope, K.L.; Havel, P.J.; Martinez, J.A. Sp1-mediated transcription is involved in the induction of leptin by insulin-stimulated glucose metabolism. J. Mol. Endocrinol. 2007, 38, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Yiannakouris, N.; Melistas, L.; Yannakoulia, M. The -2548G/A polymorphism in the human leptin gene promoter region is associated with plasma free leptin levels; interaction with adiposity and gender in healthy subjects. Hormones 2003, 2, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Han, H.R.; Ryu, H.J.; Cha, H.S.; Go, M.J.; Ahn, Y.; Koo, B.K.; Cho, Y.M.; Lee, H.K.; Cho, N.H.; Shin, C.; et al. Genetic variations in the leptin and leptin receptor genes are associated with type 2 diabetes mellitus and metabolic traits in the Korean female population. Clin. Genet. 2008, 74, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Vaškù, J.A.B.; Vaškù, A.; Dostálová, Z.; Bienert, P. Association of leptin genetic polymorphism -2548G/A with gestational diabetes mellitus. Genes Nutr. 2006, 1, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bains, V.; Kaur, H.; Badaruddoza, B. Association analysis of polymorphisms in LEP (rs7799039 and rs2167270) and LEPR (rs1137101) gene towards the development of type 2 diabetes in North Indian Punjabi population. Gene 2020, 754, 144846. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Lin, Y.G.; Wu, J.; Sun, H.; Gong, Z.C.; Hu, P.C.; Yin, J.Y.; Zhang, W.; Wang, D.; Zhou, H.H.; et al. Impact of genetic polymorphisms of leptin and TNF-α on rosiglitazone response in Chinese patients with type 2 diabetes. Eur. J. Clin. Pharmacol. 2008, 64, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Antic, V.; Dulloo, A.; Montani, J.P. Multiple mechanisms involved in obesity-induced hypertension. Heart Lung Circ. 2003, 12, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.C.; Chuang, T.Y.; Lien, C.C.; Lin, Y.J.; Huang, S.W.; Kwok, C.F.; Ho, L.T. Leptin increases endothelin type A receptor levels in vascular smooth muscle cells. Am. J. Physiol. Endocrinol. Metab. 2008, 294, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Ali, S.; Kallel, A.; Ftouhi, B.; Sediri, Y.; Feki, M.; Slimane, H.; Jemaa, R.; Kaabachi, N. The-2548G/A LEP polymorphism is associated with blood pressure in Tunisian obese patients. Blood Press. 2008, 17, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Genelhu, V.A.; Celoria, B.M.; Pimentel, M.M.; Duarte, S.F.; Cabello, P.H.; Francischetti, E.A. Association of a common variant of the leptin gene with blood pressure in an obese Brazilian population. Am. J. Hypertens. 2009, 22, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Koh, K.K. Effects of estrogen on the vascular wall: Vasomotor function and inflammation. Cardiovasc. Res. 2002, 55, 714–726. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B.; Chen, C.; Wang, B.; Stampfer, M.J.; Xu, X. Leptin concentrations in relation to overall adiposity, fat distribution, and blood pressure in rural Chinese population. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 121–125. [Google Scholar] [CrossRef] [Green Version]
- El-Gharbawy, A.H.; Kotchen, J.M.; Grim, C.E.; Kaldunski, M.; Hoffmann, R.G.; Pausova, Z.; Hamet, P.; Kotchen, T.A. Gender specific correlates of leptin with hypertension-related phenotypes African Americans. Am. J. Hypertens. 2002, 15, 989–993. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Normotensive ND Control | Obese Normotensive Nondiabetics (ND) | Obese Hypertensives with T2D | p-Value |
|---|---|---|---|---|
| N | 50 | 80 | 76 | |
| Age | 47.9 ± 5.4 | 47.7 ± 6.0 | 49.4 ± 5.9 | 0.14 |
| Gender (M\F) | (32/18) | (37/43) | (25/51) | 0.003 |
| BMI (kg/m2) | 22.9 ± 2.1 | 34.1 ± 4.2 * | 35.1 ± 4.7 * | <0.001 |
| Waist (cm) | 84.7 ± 14.6 | 99.1 ± 16.6 * | 99.1 ± 20.3 * | <0.001 |
| Hips (cm) | 96.5 ± 17.1 | 110.4 ± 19.6 * | 111.3 ± 24.0 * | <0.001 |
| SAD (cm) | 19.6 ± 4.0 | 24.9 ± 5.5 * | 25.5 ± 4.1* | <0.001 |
| Systolic BP (mmHg) | 116.7 ± 8.6 | 121.9 ± 10.4 * | 144.1 ± 11.2 *,† | <0.001 |
| Diastolic BP (mmHg) | 75.3 ± 5.6 | 78.8 ± 6.2 * | 91.2 ± 4.0 *,† | <0.001 |
| Leptin (ng/mL) | 15.6 ± 5.2 | 38.8 ± 6.2 * | 38.6 ± 5.1 * | <0.001 |
| HDL (mmol/L) | 0.83 ± 0.30 | 0.74 ± 0.31 | 0.86 ± 0.32 † | 0.04 |
| TC (mmol/L) | 5.3 ± 1.1 | 5.4 ± 1.2 | 5.5 ± 1.1 | 0.59 |
| LDL (mmol/L) | 3.8 ± 1.0 | 3.8 ± 1.1 | 3.8 ± 1.0 | 0.99 |
| TG (mmol/L) | 1.5 ± 0.24 | 1.8 ± 0.39 | 1.8 ± 0.35 | 0.08 |
| FBG (mmol/L) | 5.8 ± 2.0 | 6.4 ± 2.3 | 7.2 ± 3.1 * | 0.01 |
| Insulin (IU/mL) | 9.4 ± 2.5 | 11.4 ± 1.2 | 12.3 ± 1.2 | 0.15 |
| HOMA-IR | 2.4 ± 0.85 | 3.1 ± 0.64 * | 3.8 ± 0.72 * | 0.01 |
| Normotensive | Obese Normotensive | Obese Hypertensive | |||||
|---|---|---|---|---|---|---|---|
| ND Control | ND | T2D | |||||
| N (in %) | Odds ratio (95%CI) | p-value | N (in %) | Odds ratio (95%CI) | p-value | ||
| Genotype frequency | |||||||
| GG | 23 (46.0) | 32 (40.0) | 1 | 25 (33.0) | 1 | ||
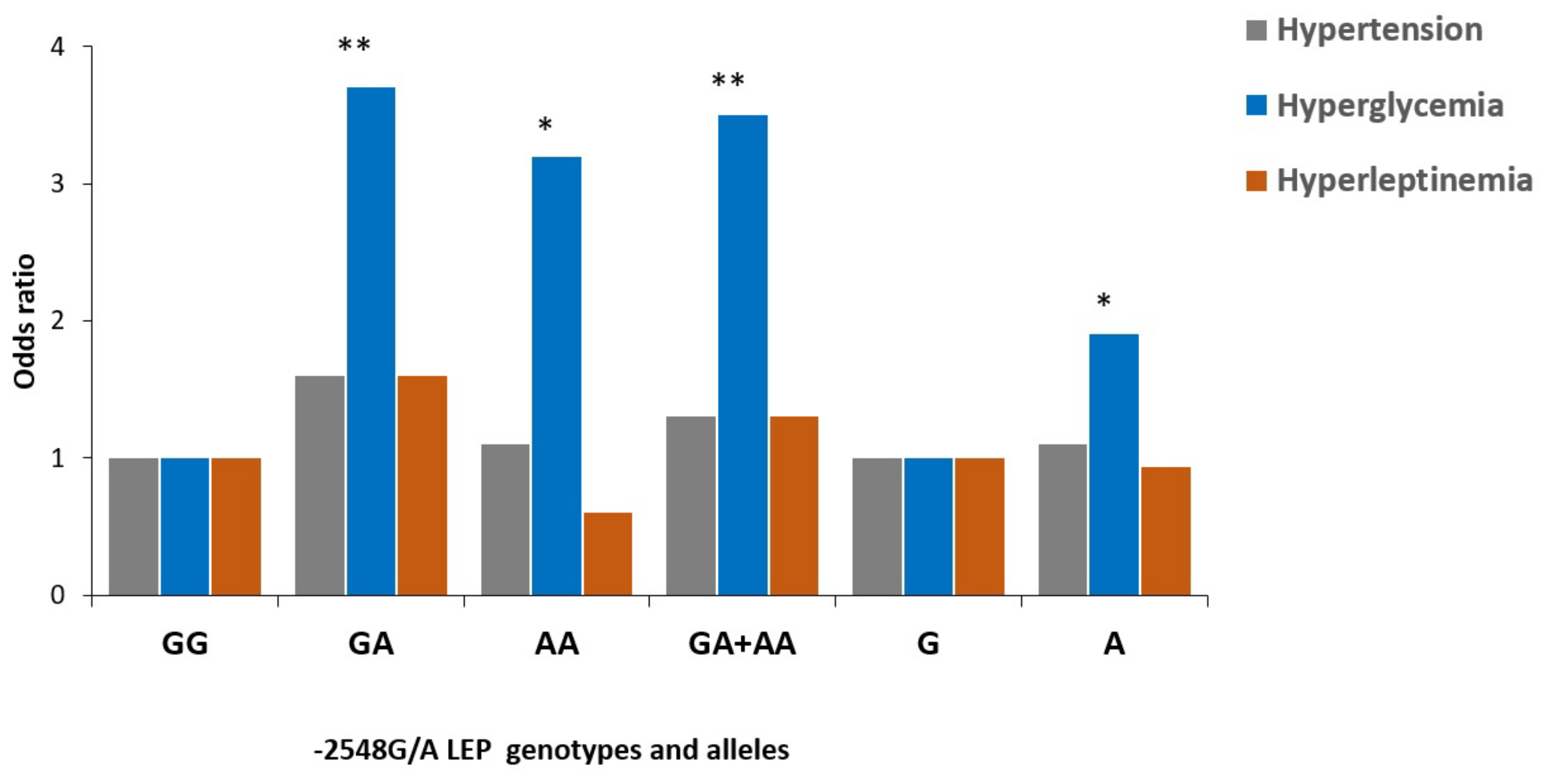
| GA | 20 (40.0) | 31 (38.8) | 1.1 (0.51–2.4) | 0.84 | 37 (48.7) | 1.7 (0.77–3.7) | 0.23 |
| AA | 7 (14.0) | 17 (21.2) | 1.7 (0.62–4.8) | 0.32 | 14 (18.3) | 1.8 (0.63–5.3) | 0.30 |
| GA + AA | 27 (64.0) | 48 (60.0) | 1.3 (0.62–2.6) | 0.58 | 51 (67.0) | 1.0 (0.49–2.1) | 0.90 |
| Allele frequency | |||||||
| G allele | 66 (66.0) | 95 (59.4) | 1 | 87 (57.2) | 1 | ||
| A allele | 34 (34.0) | 65 (40.6) | 1.3 (0.78–2.2) | 0.29 | 65 (42.8) | 1.4 (0.85–2.4) | 0.18 |
| Overall | Normotensive ND Healthy Control Group | Obese Normotensive ND Group | Obese Hypertensive T2D Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | GG | GA | AA | GG | GA | AA | GG | GA | AA | |
| N | 80 | 88 | 38 | 23 | 20 | 7 | 32 | 31 | 17 | 25 | 37 | 14 |
| Age | 48.4 ± 6.0 | 48.6 ± 5.8 | 47.9 ± 5.6 | 48.2 ± 5.7 | 46.9 ± 5.2 | 49.8 ± 5.3 | 48.5 ± 5.7 | 47.4 ± 6.1 | 46.7 ± 6.4 | 48.5 ± 7.0 | 50.4 ± 5.6 | 48.4 ± 4.8 |
| Gender (M/F) | (39/41) | (34/54) | (21/17) | (15/8) | (12/8) | (5/2) | (17/15) | (10/21) | (10/7) | (7/18) | (12/25) | (6/8) |
| BMI (kg/m2) | 31.6 ± 6.7 | 32.2 ± 6.4 | 31.5 ± 6.1 | 23.2 ± 2.6 | 23.0 ± 1.2 | 21.7 ± 1.4 | 33.9 ± 5.1 | 34.9 ± 4.0 | 32.6 ± 2.5 | 35.6 ± 4.1 | 34.8 ± 4.8 | 35.1 ± 5.3 |
| Waist (cm) | 97.1 ± 17.7 | 94.9 ± 18.8 | 93.3 ± 20.4 | 87.5 ± 9.6 | 83.8 ± 10.8 | 78.1 ± 11.6 | 100.1 ± 16.3 | 97.5 ± 19.8 | 100.4 ± 10.2 | 102.2 ± 21.9 | 98.9 ± 19.4 | 94.1 ± 20.2 |
| Hips (cm) | 109.2 ± 20.3 | 106.6 ± 22.5 | 104.7 ± 23.1 | 97.8 ± 9.3 | 100.1 ± 16.2 | 80.1 ± 11.0 | 110.4 ± 19.6 | 109.1 ± 22.8 | 100.1 ± 10.2 | 118.1 ± 24.0 | 107.9 ± 24.6 | 107.5 ± 21.8 |
| SAD (cm) | 23.3 ± 4.7 | 24.6 ± 5.4 | 22.6 ± 2.7 | 19.9 ± 3.9 | 19.8 ± 4.2 | 18.4 ± 4.4 | 23.8 ± 5.0 | 26.3 ± 5.3 | 23.4 ± 7.1 | 26.1 ± 2.8 | 25.6 ± 4.6 | 24.2 ± 4.5 |
| Systolic BP (mmHg) | 127.5 ± 16.1 | 130.2 ± 16.8 | 128.4 ± 12.1 | 114.8 ± 8.4 | 117.5 ± 9.0 | 120.7 ± 7.3 | 121.8 ± 9.8 | 122.3 ± 12.5 | 121.5 ± 7.4 | 146.5 ± 8.9 | 143.8 ± 13.3 | 140.7 ± 8.0 |
| Diastolic BP (mmHg) | 81.7 ± 8.6 | 83.1 ± 9.4 | 82.7 ± 6.6 | 74.9 ± 8.5 | 75.0 ± 6.1 | 77.1 ± 3.9 | 78.9 ± 6.1 | 78.4 ± 6.9 | 79.4 ± 5.2 | 91.6 ± 3.4 | 91.6 ± 5.0 | 89.6 ± 2.0 |
| TC (mmol/L) | 5.4 ± 1.3 | 5.5 ± 1.0 | 5.3 ± 1.1 | 5.3 ± 1.2 | 5.2 ± 0.90 | 5.9 ± 1.2 | 5.6 ± 1.4 | 5.4 ± 1.0 | 5.1 ± 1.0 | 5.3 ± 1.2 | 5.8 ± 1.1 | 5.4 ± 1.0 |
| TG (mmol/L) | 1.82 ± 0.10 | 1.85 ± 0.11 | 1.86 ± 0.21 | 1.6 ± 0.17 | 1.4 ± 0.10 | 1.5 ± 0.26 | 2.0 ± 1.0 | 1.8 ± 1.1 | 2.1 ± 1.8 | 1.8 ± 0.20 | 2.1 ± 0.20 | 1.7 ± 0.15 |
| HDL (mmol/L) | 0.75 ± 0.17 | 0.81 ± 0.17 | 0.74 ± 0.22 | 0.75 ± 0.18 | 0.86 ± 0.13 | 0.80 ± 0.24 | 0.73 ± 0.17 | 0.75 ± 0.18 | 0.57 ± 0.17 | 0.76 ± 0.16 | 0.83 ± 0.17 | 0.96 ± 0.20 |
| LDL (mmol/L) | 3.8 ± 1.0 | 3.8 ± 0.99 | 3.7 ± 0.99 | 3.7 ± 1.1 | 3.6 ± 0.75 | 4.3 ± 0.81 | 3.9 ± 1.2 | 3.7 ± 1.0 | 3.5 ± 1.1 | 3.6 ± 0.20 | 4.0 ± 1.0 | 3.5 ± 1.0 |
| Leptin (ng/mL) | 29.6 ± 2.6 | 40.0 ± 2.6 * | 26.8 ± 2.3 | 12.3 ± 1.8 | 26.1 ± 4.2 * | 23.2 ± 3.5 | 38.8 ± 2.7 | 47.7 ± 2.2 | 24.4 ± 2.1 | 38.3 ± 2.4 | 41.6 ± 1.8 | 31.5 ± 2.0 |
| FBG (mmol/L) | 5.8 ± 0.30 | 6.6 ± 0.50 | 6.8 ± 0.55 * | 5.8 ± 0.16 | 5.1 ± 0.20 | 7.1 ± 0.76 | 5.6 ± 0.29 | 6.8 ± 0.48 | 6.3 ± 0.38 | 6.1 ± 0.37 | 7.3 ± 0.55 | 7.6 ± 0.61 |
| Insulin (IU/mL) | 10.2 ± 1.2 | 11.2 ± 1.3 | 13.0 ± 1.2 | 8.2 ± 1.8 | 9.4 ± 1.6 | 13.2 ± 1.7 | 11.0 ± 1.1 | 10.6 ± 1.1 | 13.0 ± 1.2 | 11.1 ± 1.0 | 12.8 ± 1.1 | 12.8 ± 1.0 |
| HOMA-IR | 2.6 ± 0.67 | 3.3 ± 0.73 | 4.1 ± 0.84 * | 2.2 ± 0.88 | 2.2 ± 0.80 | 3.8 ± 0.88 | 2.7 ± 0.54 | 3.2 ± 0.68 | 3.7 ± 0.75 | 3.0 ± 0.62 | 4.1 ± 0.68 | 4.5 ± 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabi, E.M.; Bin Dahman, L.S.; Mohammed, A.K.; Sumaily, K.M.; Al-Daghri, N.M. -2548G>A LEP Polymorphism Is Positively Associated with Increased Leptin and Glucose Levels in Obese Saudi Patients Irrespective of Blood Pressure Status. Medicina 2022, 58, 346. https://doi.org/10.3390/medicina58030346
Sabi EM, Bin Dahman LS, Mohammed AK, Sumaily KM, Al-Daghri NM. -2548G>A LEP Polymorphism Is Positively Associated with Increased Leptin and Glucose Levels in Obese Saudi Patients Irrespective of Blood Pressure Status. Medicina. 2022; 58(3):346. https://doi.org/10.3390/medicina58030346
Chicago/Turabian StyleSabi, Essa M., Lotfi S. Bin Dahman, Abdul Khader Mohammed, Khalid M. Sumaily, and Nasser M. Al-Daghri. 2022. "-2548G>A LEP Polymorphism Is Positively Associated with Increased Leptin and Glucose Levels in Obese Saudi Patients Irrespective of Blood Pressure Status" Medicina 58, no. 3: 346. https://doi.org/10.3390/medicina58030346








