Six Revision Surgeries for Massive Epidural Fibrosis with Recurrent Pain and Weakness in the Left Lower Extremity
Abstract
:1. Introduction
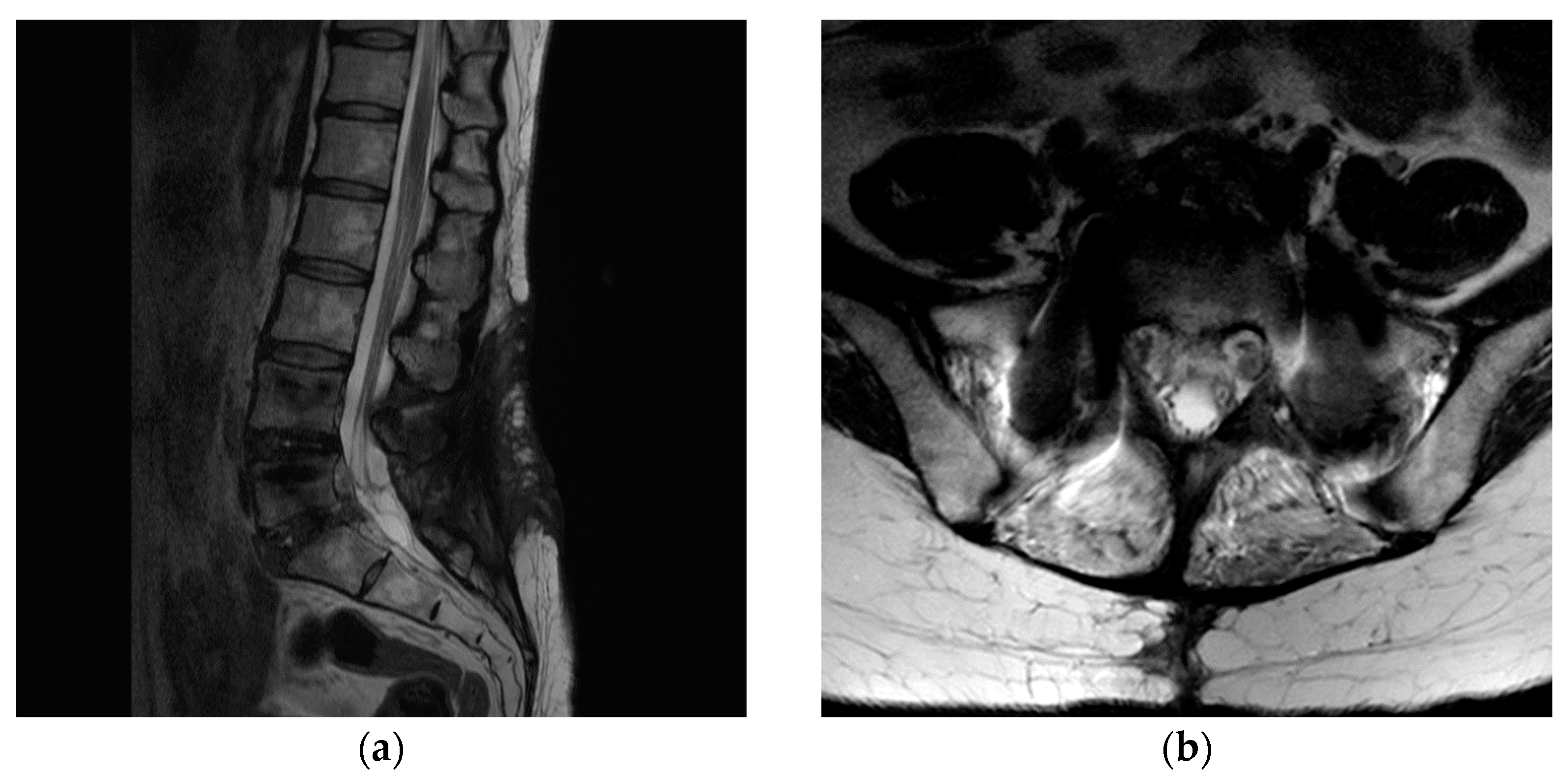
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carragee, E.J.; Han, M.Y.; Suen, P.W.; Kim, D. Clinical outcomes after lumbar discectomy for sciatica: The effects of fragment type and anular competence. J. Bone Jt. Surg. Am. 2003, 85, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Andrews, D.W.; Lavyne, M.H. Retrospective analysis of microsurgical and standard lumbar discectomy. Spine 1990, 15, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Ahmed, M.; Arrotegui, J.; Bjornsson, A.; Gillstrom, P. Microsurgery versus standard removal of the herniated lumbar disc. A 3-year comparison in 150 cases. Acta Orthop. Scand. 1990, 61, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Gauchat, M.H.; Valach, L. The outcome of surgery for lumbar disc herniation. I. A 4–17 years’ follow-up with emphasis on somatic aspects. Spine 1988, 13, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Eismont, F.J.; Currier, B. Surgical management of lumbar intervertebral-disc disease. J. Bone Jt. Surg Am. 1989, 71, 1266–1271. [Google Scholar] [CrossRef]
- Kahanovitz, N.; Viola, K.; Muculloch, J. Limited surgical discectomy and microdiscectomy. A clinical comparison. Spine 1989, 14, 79–81. [Google Scholar] [CrossRef]
- Kotilainen, E.; Valtonen, S. Clinical instability of the lumbar spine after microdiscectomy. Acta Neurochir. 1993, 125, 120–126. [Google Scholar] [CrossRef]
- Spengler, D.M.; Ouellette, E.A.; Battie, M.; Zeh, J. Elective discectomy for herniation of a lumbar disc. Additional experience with an objective method. J. Bone Jt. Surg. Am. 1990, 72, 230–237. [Google Scholar] [CrossRef]
- Weber, H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 1983, 8, 131–140. [Google Scholar] [CrossRef]
- Fritsch, E.W.; Heisel, J.; Rupp, S. The failed back surgery syndrome: Reasons, intraoperative findings, and long-term results: A report of 182 operative treatments. Spine 1996, 21, 626–633. [Google Scholar] [CrossRef]
- Ross, D.J.; Marchevsky, A.; Kass, R.M. “Nonspecific chronic inflammation” in lung allografts: Association with respiratory pathogens. Transplant. Proc. 1996, 28, 2983–2986. [Google Scholar]
- Anderson, S.R. A rationale for the treatment algorithm of failed back surgery syndrome. Curr. Rev. Pain 2000, 4, 395–406. [Google Scholar] [CrossRef]
- Burton, C.V. Causes of failure of surgery on the lumbar spine: Ten-year follow-up. Mt. Sinai J. Med. 1991, 58, 183–187. [Google Scholar]
- Long, D.M.; Filtzer, D.L.; BenDebba, M.; Hendler, N.H. Clinical features of the failed-back syndrome. J. Neurosurg. 1988, 69, 61–71. [Google Scholar] [CrossRef]
- Annertz, M.; Jonsson, B.; Stromqvist, B.; Holtas, S. No relationship between epidural fibrosis and sciatica in the lumbar postdiscectomy syndrome. A study with contrast-enhanced magnetic resonance imaging in symptomatic and asymptomatic patients. Spine 1995, 20, 449–453. [Google Scholar] [CrossRef]
- Fiume, D.; Sherkat, S.; Callovini, G.M.; Parziale, G.; Gazzeri, G. Treatment of the failed back surgery syndrome due to lumbo-sacral epidural fibrosis. Acta Neurochir. Suppl. 1995, 64, 116–118. [Google Scholar] [CrossRef]
- Samy Abdou, M.; Hardy, R.W., Jr. Epidural fibrosis and the failed back surgery syndrome: History and physical findings. Neurol. Res. 1999, 21 (Suppl. 1), S5–S8. [Google Scholar] [CrossRef]
- Pereira, P.; Severo, M.; Monteiro, P.; Silva, P.A.; Rebelo, V.; Castro-Lopes, J.M.; Vaz, R. Results of Lumbar Endoscopic Adhesiolysis Using a Radiofrequency Catheter in Patients with Postoperative Fibrosis and Persistent or Recurrent Symptoms After Discectomy. Pain Pract. 2016, 16, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, H.; Dumas, G.A.; Wyss, U.P.; Ryd, L. Three-dimensional analysis of the movement of lumbar spinal nerve roots in nonsimulated and simulated adhesive conditions. Spine 2003, 28, 2373–2380. [Google Scholar] [CrossRef]
- Rydevik, B.; Holm, S.; Brown, M.D.; Lundborg, G. Diffusion from the cerebrospinal fluid as a nutritional pathway for spinal nerve roots. Acta Physiol. Scand. 1990, 138, 247–248. [Google Scholar] [CrossRef]
- Kobayashi, S.; Shizu, N.; Suzuki, Y.; Asai, T.; Yoshizawa, H. Changes in nerve root motion and intraradicular blood flow during an intraoperative straight-leg-raising test. Spine 2003, 28, 1427–1434. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takeno, K.; Yayama, T.; Awara, K.; Miyazaki, T.; Guerrero, A.; Baba, H. Pathomechanisms of sciatica in lumbar disc herniation: Effect of periradicular adhesive tissue on electrophysiological values by an intraoperative straight leg raising test. Spine 2010, 35, 2004–2014. [Google Scholar] [CrossRef]
- Takamori, Y.; Arimizu, J.; Izaki, T.; Naito, M.; Kobayashi, T. Combined measurement of nerve root blood flow and electrophysiological values: Intraoperative straight-leg-raising test for lumbar disc herniation. Spine 2011, 36, 57–62. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.Y.; Jo, D.J. Six Revision Surgeries for Massive Epidural Fibrosis with Recurrent Pain and Weakness in the Left Lower Extremity. Medicina 2022, 58, 371. https://doi.org/10.3390/medicina58030371
Choi HY, Jo DJ. Six Revision Surgeries for Massive Epidural Fibrosis with Recurrent Pain and Weakness in the Left Lower Extremity. Medicina. 2022; 58(3):371. https://doi.org/10.3390/medicina58030371
Chicago/Turabian StyleChoi, Ho Yong, and Dae Jean Jo. 2022. "Six Revision Surgeries for Massive Epidural Fibrosis with Recurrent Pain and Weakness in the Left Lower Extremity" Medicina 58, no. 3: 371. https://doi.org/10.3390/medicina58030371
APA StyleChoi, H. Y., & Jo, D. J. (2022). Six Revision Surgeries for Massive Epidural Fibrosis with Recurrent Pain and Weakness in the Left Lower Extremity. Medicina, 58(3), 371. https://doi.org/10.3390/medicina58030371




