Inflammaging and Vascular Function in Metabolic Syndrome: The Role of Hyperuricemia
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Assessment of Study Population
2.3. Non-Invasive Assessment of Arterial Stiffness
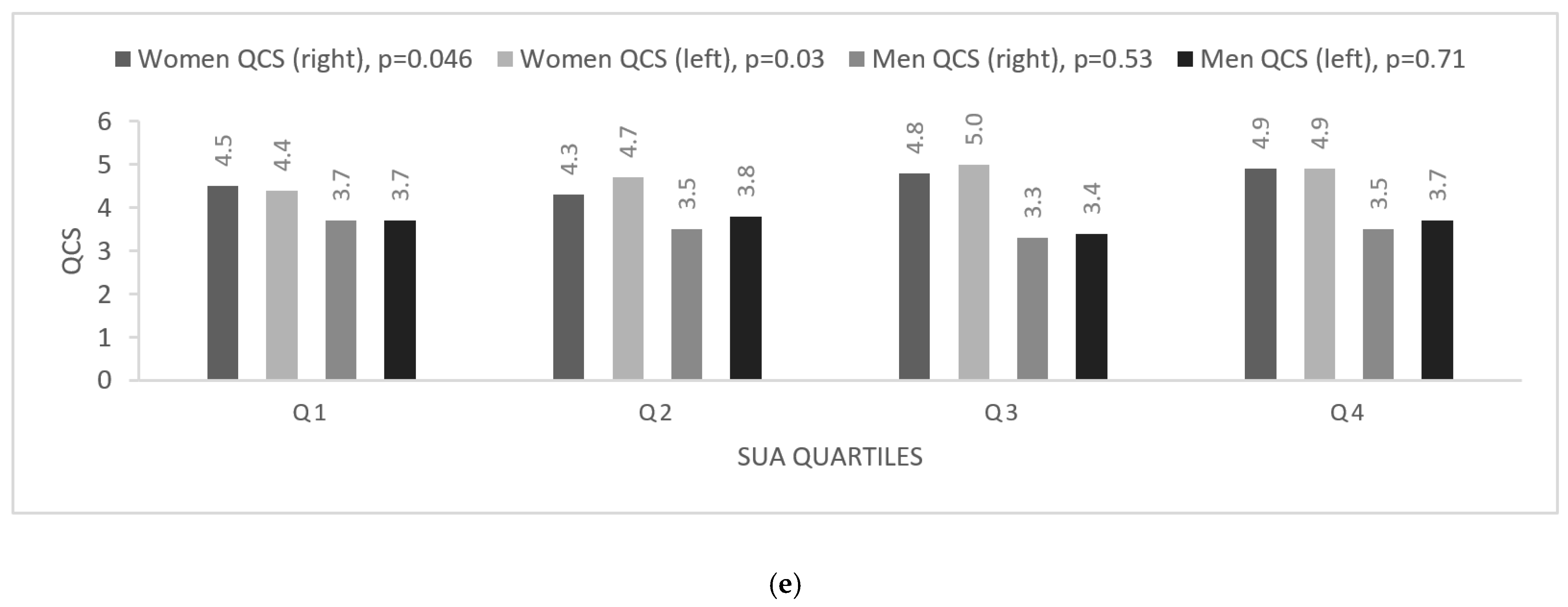
2.3.1. Assessment of Carotid Artery Intima-Media Thickness (CIMT) and Quality Carotid Stiffness (QCS)
2.3.2. Assessment of Pulse Wave Velocity (PWV)
2.3.3. Assessment of Flow-Mediated Dilatation (FMD)
2.4. Statistical Analysis
3. Results
3.1. Baseline and Vascular Characteristics
3.2. Association between Objective Data, Vascular Parameters, and SUA
3.3. Linear Regression Analysis of cfPWV and SUA
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, P.M. Early Vascular Aging (EVA): Consequences and Prevention. Vasc. Health Risk Manag. 2008, 4, 547–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Moreno, P.; Burgos-Angulo, G.; Martinez-Ceballos, M.A.; Pizano, A.; Echeverri, D.; Bautista-Niño, P.K.; Roks, A.J.M.; Rojas-Villarraga, A. Inflammaging as a Link between Autoimmunity and Cardiovascular Disease: The Case of Rheumatoid Arthritis. RMD Open 2021, 7, e001470. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef] [Green Version]
- Zanoli, L.; Rastelli, S.; Granata, A.; Inserra, G.; Empana, J.-P.; Boutouyrie, P.; Laurent, S.; Castellino, P. Arterial Stiffness in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Hypertens. 2016, 34, 822–829. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2022, 11, 79. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The Metabolic Syndrome—What Is It and How Should It Be Managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef]
- Kanikowska, D.; Kanikowska, A.; Swora-Cwynar, E.; Grzymisławski, M.; Sato, M.; Bręborowicz, A.; Witowski, J.; Korybalska, K. Moderate Caloric Restriction Partially Improved Oxidative Stress Markers in Obese Humans. Antioxidants 2021, 10, 1018. [Google Scholar] [CrossRef]
- Ali, N.; Miah, R.; Hasan, M.; Barman, Z.; Mou, A.D.; Hafsa, J.M.; Trisha, A.D.; Hasan, A.; Islam, F. Association between Serum Uric Acid and Metabolic Syndrome: A Cross-Sectional Study in Bangladeshi Adults. Sci. Rep. 2020, 10, 7841. [Google Scholar] [CrossRef] [PubMed]
- Spiga, R.; Marini, M.A.; Mancuso, E.; Di Fatta, C.; Fuoco, A.; Perticone, F.; Andreozzi, F.; Mannino, G.C.; Sesti, G. Uric Acid Is Associated With Inflammatory Biomarkers and Induces Inflammation Via Activating the NF-ΚB Signaling Pathway in HepG2 Cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Albu, A.; Para, I.; Porojan, M. Uric Acid and Arterial Stiffness. Ther. Clin. Risk Manag. 2020, 16, 39–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Monticone, R.E.; McGraw, K.R. Proinflammatory Arterial Stiffness Syndrome: A Signature of Large Arterial Aging. J. Vasc. Res. 2018, 55, 210–223. [Google Scholar] [CrossRef]
- Kuwabara, M.; Kanbay, M.; Hisatome, I. Uric Acid and Hypertension Because of Arterial Stiffness. Hypertension 2018, 72, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Shiina, K.; Vlachopoulos, C.; Iwasaki, Y.; Matsumoto, C.; Kimura, K.; Fujii, M.; Chikamori, T.; Yamashina, A. Involvement of Arterial Stiffness and Inflammation in Hyperuricemia-Related Development of Hypertension. Hypertension 2018, 72, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Laucevičius, A.; Kasiulevičius, V.; Jatužis, D.; Petrulionienė, Ž.; Ryliškytė, L.; Rinkūnienė, E.; Badarienė, J.; Ypienė, A.; Gustienė, O.; Šlapikas, R. Lithuanian High Cardiovascular Risk (LitHiR) Primary Prevention Programme—Rationale and Design. Semin. Cardiovasc. Med. 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice|European Heart Journal|Oxford Academic. Available online: https://academic.oup.com/eurheartj/article/42/34/3227/6358713?login=true (accessed on 18 February 2022).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Sesso, H.D.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Gaziano, J.M.; Manson, J.E.; Glynn, R.J. Systolic and Diastolic Blood Pressure, Pulse Pressure, and Mean Arterial Pressure as Predictors of Cardiovascular Disease Risk in Men. Hypertension 2000, 36, 801–807. [Google Scholar] [CrossRef]
- Rácz, O.; Lepej, J.; Fodor, B.; Lepejová, K.; Jarčuška, P.; Kováčová, A. Pitfalls in the Measurements and Assesment of Glomerular Filtration Rate and How to Escape Them. EJIFCC 2012, 23, 33–40. [Google Scholar] [PubMed]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert Consensus and Evidence-Based Recommendations for the Assessment of Flow-Mediated Dilation in Humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Rebora, P.; Andreano, A.; Triglione, N.; Piccinelli, E.; Palazzini, M.; Occhi, L.; Grassi, G.; Valsecchi, M.G.; Giannattasio, C.; Maloberti, A. Association between Uric Acid and Pulse Wave Velocity in Hypertensive Patients and in the General Population: A Systematic Review and Meta-Analysis. Blood Press. 2020, 29, 220–231. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Zhou, N.; Teng, F.; Zhao, J.; Zou, C.; Qi, L. Synergistic Effects of Serum Uric Acid and Cardiometabolic Risk Factors on Early Stage Atherosclerosis: The Cardiometabolic Risk in Chinese Study. PLoS ONE 2012, 7, e51101. [Google Scholar] [CrossRef] [Green Version]
- Bian, S.; Guo, H.; Ye, P.; Luo, L.; Wu, H.; Xiao, W. Serum Uric Acid Level and Diverse Impacts on Regional Arterial Stiffness and Wave Reflection. Iran. J. Public Health 2012, 41, 33–41. [Google Scholar] [PubMed]
- Trott, D.W.; Fadel, P.J. Inflammation as a Mediator of Arterial Ageing. Exp. Physiol. 2019, 104, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhang, K.; Wang, Y.; Chen, Y.; Zhang, W.; Xia, F.; Zhang, Y.; Wang, N.; Lu, Y. The Associations between Gonadal Hormones and Serum Uric Acid Levels in Men and Postmenopausal Women With Diabetes. Front. Endocrinol. 2020, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochlani, Y.; Pothineni, N.V.; Mehta, J.L. Metabolic Syndrome: Does It Differ Between Women and Men? Cardiovasc. Drugs Ther. 2015, 29, 329–338. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Crawford, S.; Lasley, B.; Sutton-Tyrrell, K. Menopause and the Metabolic Syndrome: The Study of Women’s Health Across the Nation. Arch. Intern. Med. 2008, 168, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Tarhouni, K.; Guihot, A.L.; Freidja, M.L.; Toutain, B.; Henrion, B.; Baufreton, C.; Pinaud, F.; Procaccio, V.; Grimaud, L.; Ayer, A.; et al. Key Role of Estrogens and Endothelial Estrogen Receptor α in Blood Flow-Mediated Remodeling of Resistance Arteries. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Fappi, A.; Mittendorfer, B. Different Physiological Mechanisms Underlie an Adverse Cardiovascular Disease Risk Profile in Men and Women. Proc. Nutr. Soc. 2020, 79, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, T.; Nuccio, E.; McFann, K.; Madero, M.; Sarnak, M.J.; Jalal, D. Association of Uric Acid With Vascular Stiffness in the Framingham Heart Study. Am. J. Hypertens. 2015, 28, 877–883. [Google Scholar] [CrossRef]
- Ma, M.; Wang, L.; Zhong, X.; Zhong, L.; Chen, R.; Li, L.; Mao, M. Correlation between Carotid Intima Media Thickness and Serum Uric Acid, Results from 15843 Subjects in 2016–2020. 2020. Available online: https://assets.researchsquare.com/files/rs-729479/v1/83b82aff-968d-4b24-868d-238ac7bb5c63.pdf?c=1631886819 (accessed on 27 February 2022).
- Buzas, R.; Tautu, O.-F.; Dorobantu, M.; Ivan, V.; Lighezan, D. Serum Uric Acid and Arterial Hypertension—Data from Sephar III Survey. PLoS ONE 2018, 13, e0199865. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-H.; He, Y.; Xu, W.-D.; Zhao, Z.-X.; Liu, M.; Luo, X.; He, C.-S.; Chen, J. Carotid Intima-Media Thickness in Patients with Hyperuricemia: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2021, 33, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Jain, S.; Dhawan, V.; Kalra, N.; Kumari, S. Uric Acid as a Predictor of Endothelial Dysfunction in Patients with Metabolic Syndrome. Arch. Endocrinol. Metab. 2021, 64, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xian, Z.; Zou, Y.; Liao, Z.; Yang, R.; Zou, C.; Wang, X.; Sun, Y. Flow-Mediated Dilation Can Be Used to Predict Incident Hypertension in Patients with Hyperuricemia. Arch. Med. Sci 2019, 15, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Relationship between Uric Acid and Endothelial Function in Hypertensive Patients with Metabolic Syndrome|Abstract. Available online: https://www.longdom.org/abstract/relationship-between-uric-acid-and-endothelial-function-in-hypertensive-patients-with-metabolic-syndrome-50657.html (accessed on 6 November 2021).
- Wong, C.-K.; Chen, Y.; Ho, L.-M.; Zhen, Z.; Siu, C.-W.; Tse, H.-F.; Yiu, K.-H. The Effects of Hyperuricaemia on Flow-Mediated and Nitroglycerin-Mediated Dilatation in High-Risk Patients. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Huang, Y.-Y.; Lin, C.-C.; Li, W.-T.; Lee, C.-H.; Chen, J.-Y.; Chen, J.-H. Uric Acid Is an Independent Predictor of Arterial Stiffness in Hypertensive Patients. Heart Vessel. 2009, 24, 371–375. [Google Scholar] [CrossRef]
- Saijo, Y.; Utsugi, M.; Yoshioka, E.; Horikawa, N.; Sato, T.; Gong, Y.Y.; Kishi, R. Relationships of C-Reactive Protein, Uric Acid, and Glomerular Filtration Rate to Arterial Stiffness in Japanese Subjects. J. Hum. Hypertens. 2005, 19, 907–913. [Google Scholar] [CrossRef]

| Characteristics | Total (n = 696) | Women (n = 439) | Men (n = 257) | p-Value |
|---|---|---|---|---|
| Age, year | 54 ± 7 | 58 ± 4 | 47 ± 4 | <0.001 |
| BMI, kg/m2 | 31.6 ± 4.5 | 31.9 ± 4.9 | 31.2 ± 3.7 | 0.03 |
| FPG, mmol/L | 6.1 (3.4; 23.5) | 6.5 (3.4; 23.5) | 6.4 (4.8; 18.1) | 0.255 |
| TC, mmol/L | 6.04 ± 1.36 | 6.11 ± 1.35 | 5.91 ± 1.34 | 0.05 |
| LDL cholesterol, mmol/L | 3.81 ± 1.16 | 3.88 ± 1.15 | 3.69 ± 1.16 | 0.04 |
| HDL cholesterol, mmol/L | 1.25 ± 0.32 | 1.36 ± 0.31 | 1.08 ± 0.24 | <0.001 |
| TG, mmol/L | 1.75 (0.48; 32.05) | 1.66 (0.48; 18.4) | 2.0 (0.49; 32.05) | <0.001 |
| MAP, mmHg | 100 ± 10 | 99 ± 10 | 102 ± 10 | <0.001 |
| SUA, µmol/L | 358.5 ± 86.38 | 333.51 ± 81.66 | 401.27 ± 78.24 | <0.001 |
| eGFR, mL/min/1.73 m2 | 91 ± 12 | 88 ± 11 | 97 ± 11 | <0.001 |
| hs-CRP, mg/L | 1.6 (0.13; 47.1) | 1.67 (0.18; 47.1) | 1.53 (0.13; 42.9) | 0.189 |
| Hyperuricemia, % (n) | 34 (234) | 35 (154) | 32 (85) | 0.464 |
| Gout, % (n) | 2 (17) | 1 (6) | 4 (11) | 0.016 |
| Smoking, % (n) | 26 (179) | 17 (75) | 41 (104) | <0.001 |
| Characteristics | Total (n = 696) | Women (n = 439) | Men (n = 257) | p-Value |
|---|---|---|---|---|
| QCS (right) | 4.1 (1.3; 17.7) | 4.6 (1.3; 17.7) | 3.6 (1.5; 16.7) | <0.001 |
| QCS (left) | 4.3 (1.1; 15.4) | 4.8 (1.4; 15.4) | 3.6 (1.1; 14) | <0.001 |
| cfPWV, m/sec | 8.5 ± 1.45 | 8.76 ± 1.49 | 8.06 ± 1.26 | <0.001 |
| crPWV, m/sec | 9.4 ± 1.2 | 9.23 ± 1.15 | 9.6 ± 1.25 | <0.001 |
| CIMT (mean of right and left), μm | 653 ± 103 | 663 ± 99 | 637 ± 108 | 0.002 |
| FMD, % | 2.39 (0.17; 15.44) | 2.4 (0.2; 15.44) | 2.35 (0.17; 9.82) | 0.15 |
| Characteristics | Women (n = 439) | p-Value | |||
|---|---|---|---|---|---|
| Quartiles | Q1 | Q2 | Q3 | Q4 | |
| Number | 112 | 111 | 108 | 108 | |
| SUA, µmol/L | ≤277 | 278–326 | 327–381 | ≥382 | |
| Age, year | 58 ± 4 | 57 ± 4 | 58 ± 4 | 57± 4 | 0.946 |
| BMI, kg/m2 | 30.2 ± 4.6 | 30.7 ± 4.4 | 32.9 ± 4.7 | 33.9 ± 5 | <0.001 |
| FPG, mmol/L | 5.9 (3.4; 16.8) | 6.1 (5; 23.5) | 6 (4.6; 12.1) | 6.3 (5; 16.1) | 0.014 |
| TC, mmol/L | 6.16 ± 1.43 | 5.95 ± 1.32 | 6.15 ± 1.23 | 6.18 ± 1.44 | 0.532 |
| LDL cholesterol, mmol/L | 3.92 ± 1.22 | 3.79 ± 1.16 | 3.99 ± 1.0 | 3.82 ± 1.21 | 0.585 |
| HDL cholesterol, mmol/L | 1.46 ± 0.34 | 1.35 ± 0.28 | 1.34 ± 0.25 | 1.29 ± 0.33 | <0.001 |
| TG, mmol/L | 1.51 (0.48; 8.58) | 1.50 (0.52; 18.40) | 1.68 (0.64; 5.06) | 1.93 (0.58; 7.3) | <0.001 |
| MAP, mmHg | 98 ± 10 | 98 ± 10 | 101 ± 11 | 101 ± 10 | 0.032 |
| eGFR, mL/min/1.73 m2 | 92.1 ± 8.9 | 88.8 ± 8.6 | 86.3 ± 10.4 | 84.2 ± 13 | <0.001 |
| hs-CRP, mg/L | 1.37 (0.18; 28.4) | 1.34 (0.19; 47.1) | 1.98 (0.33; 18.1) | 2.75 (0.33; 25.3) | <0.001 |
| Smoking, % (n) | 21 (24) | 14 (15) | 17 (18) | 17 (18) | 0.472 |
| Characteristics | Men (n = 257) | p-Value | |||
|---|---|---|---|---|---|
| Quartiles | Q1 | Q2 | Q3 | Q4 | |
| Number | 65 | 67 | 61 | 64 | |
| SUA, µmol/L | ≤352 | 353–393 | 394–452 | ≥453 | |
| Age, year | 49 ± 4 | 48 ± 4 | 46 ± 4 | 46 ± 4 | <0.001 |
| BMI, kg/m2 | 30.2 ± 3.1 | 30.2 ± 3.5 | 31.7 ± 3.4 | 32.7 ± 4.1 | <0.001 |
| FPG, mmol/L | 6.05 (5.03; 17.54) | 6.04 (4.83; 18.07) | 5.94 (4.93; 7.78) | 6.08 (4.9; 11.49) | 0.151 |
| TC, mmol/L | 5.57 ± 1.36 | 6.08 ± 1.38 | 6 ± 1.17 | 5.98 ± 1.39 | 0.132 |
| LDL cholesterol, mmol/L | 3.46 ± 1.2 | 3.83 ± 1.13 | 3.74 ± 1.06 | 3.74 ±1.23 | 0.312 |
| HDL cholesterol, mmol/L | 1.06 ± 0.25 | 1.15 ± 0.27 | 1.05 ±0.2 | 1.05 ± 0.24 | 0.039 |
| TG, mmol/L | 1.65 (0.49; 32.05) | 1.78 (0.53; 6.93) | 2.23 (0.96; 6.61) | 2.51 (0.79; 7.8) | <0.001 |
| MAP, mmHg | 101 ± 9 | 100 ± 10 | 101 ± 10 | 105 ± 11 | 0.057 |
| eGFR, mL/min/1.73 m2 | 99.8 ± 10.6 | 97.3 ± 9.7 | 98.7 ± 11.3 | 94.3 ± 12.7 | 0.03 |
| hs-CRP, mg/L | 1.26 (0.2; 27.3) | 1.37 (0.13; 10.3) | 1.94 (0.24; 42.9) | 1.89 (0.47; 31.8) | <0.001 |
| Smoking, % (n) | 38 (25) | 46 (31) | 38 (23) | 39 (25) | 0.752 |
| Characteristics | Women (n = 439) | Men (n = 257) | ||
|---|---|---|---|---|
| Spearman’s Correlation Coefficient r | p-Value | Spearman’s Correlation Coefficient r | p-Value | |
| Age, year | −0.02 | 0.68 | −0.26 | <0.001 |
| BMI, kg/m2 | 0.32 | <0.001 | 0.3 | <0.001 |
| FPG, mmol/L | −0.06 | 0.35 | 0.12 | 0.012 |
| TC, mmol/L | 0.01 | 0.853 | 0.1 | 0.102 |
| LDL cholesterol, mmol/L | −0.02 | 0.623 | 0.06 | 0.34 |
| HDL cholesterol, mmol/L | −0.23 | <0.001 | −0.07 | <0.27 |
| TG, mmol/L | 0.22 | <0.001 | 0.27 | <0.001 |
| MAP, mmHg | 0.14 | 0.003 | 0.14 | 0.02 |
| eGFR, mL/min/1.73 m2 | −0.23 | <0.001 | −0.14 | 0.03 |
| hs-CRP, mg/L | 0.29 | <0.001 | 0.27 | <0.001 |
| QCS (right) | 0.13 | 0.008 | −0.05 | 0.39 |
| QCS (left) | 0.11 | 0.02 | −0.02 | 0.8 |
| cfPWV, m/sec | 0.19 | <0.001 | 0.13 | 0.03 |
| crPWV, m/sec | 0.02 | 0.78 | −0.04 | 0.56 |
| CIMT (mean of right and left), μm | 0.05 | 0.34 | 0.02 | 0.81 |
| FMD, % | 0.05 | 0.33 | 0.08 | 0.121 |
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Estimate | B | SE | p-Value | B | SE | p-Value |
| Model 1 | ||||||
| Q2 uric acid | 0.213 | 0.187 | 0.257 | 0.259 | 0.219 | 0.238 |
| Q3 uric acid | 0.448 | 0.195 | 0.022 | 0.17 | 0.231 | 0.462 |
| Q4 uric acid | 0.468 | 0.203 | 0.021 | 0.478 | 0.242 | 0.049 |
| Model 2 | ||||||
| Q2 uric acid | 0.214 | 0.182 | 0.238 | 0.211 | 0.209 | 0.312 |
| Q3 uric acid | 0.38 | 0.19 | 0.047 | 0.138 | 0.221 | 0.535 |
| Q4 uric acid | 0.459 | 0.198 | 0.021 | 0.34 | 0.234 | 0.147 |
| Model 3 | ||||||
| Q2 uric acid | 0.257 | 0.181 | 0.156 | 0.209 | 0.209 | 0.317 |
| Q3 uric acid | 0.384 | 0.189 | 0.042 | 0.144 | 0.222 | 0.518 |
| Q4 uric acid | 0.491 | 0.197 | 0.013 | 0.328 | 0.234 | 0.162 |
| Model 4 | ||||||
| Q2 uric acid | 0.216 | 0.18 | 0.23 | 0.288 | 0.213 | 0.179 |
| Q3 uric acid | 0.397 | 0.186 | 0.033 | 0.263 | 0.227 | 0.249 |
| Q4 uric acid | 0.533 | 0.194 | 0.006 | 0.415 | 0.24 | 0.085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laučytė-Cibulskienė, A.; Smaliukaitė, M.; Dadonienė, J.; Čypienė, A.; Mikolaitytė, J.; Ryliškytė, L.; Laucevičius, A.; Badarienė, J. Inflammaging and Vascular Function in Metabolic Syndrome: The Role of Hyperuricemia. Medicina 2022, 58, 373. https://doi.org/10.3390/medicina58030373
Laučytė-Cibulskienė A, Smaliukaitė M, Dadonienė J, Čypienė A, Mikolaitytė J, Ryliškytė L, Laucevičius A, Badarienė J. Inflammaging and Vascular Function in Metabolic Syndrome: The Role of Hyperuricemia. Medicina. 2022; 58(3):373. https://doi.org/10.3390/medicina58030373
Chicago/Turabian StyleLaučytė-Cibulskienė, Agnė, Monika Smaliukaitė, Jolanta Dadonienė, Alma Čypienė, Jurgita Mikolaitytė, Ligita Ryliškytė, Aleksandras Laucevičius, and Jolita Badarienė. 2022. "Inflammaging and Vascular Function in Metabolic Syndrome: The Role of Hyperuricemia" Medicina 58, no. 3: 373. https://doi.org/10.3390/medicina58030373
APA StyleLaučytė-Cibulskienė, A., Smaliukaitė, M., Dadonienė, J., Čypienė, A., Mikolaitytė, J., Ryliškytė, L., Laucevičius, A., & Badarienė, J. (2022). Inflammaging and Vascular Function in Metabolic Syndrome: The Role of Hyperuricemia. Medicina, 58(3), 373. https://doi.org/10.3390/medicina58030373






