Does the SDMQ-9 Predict Changes in HbA1c Levels? An Ecuadorian Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Share Decision Making
2.3. Procedure
2.4. Statistical Analysis
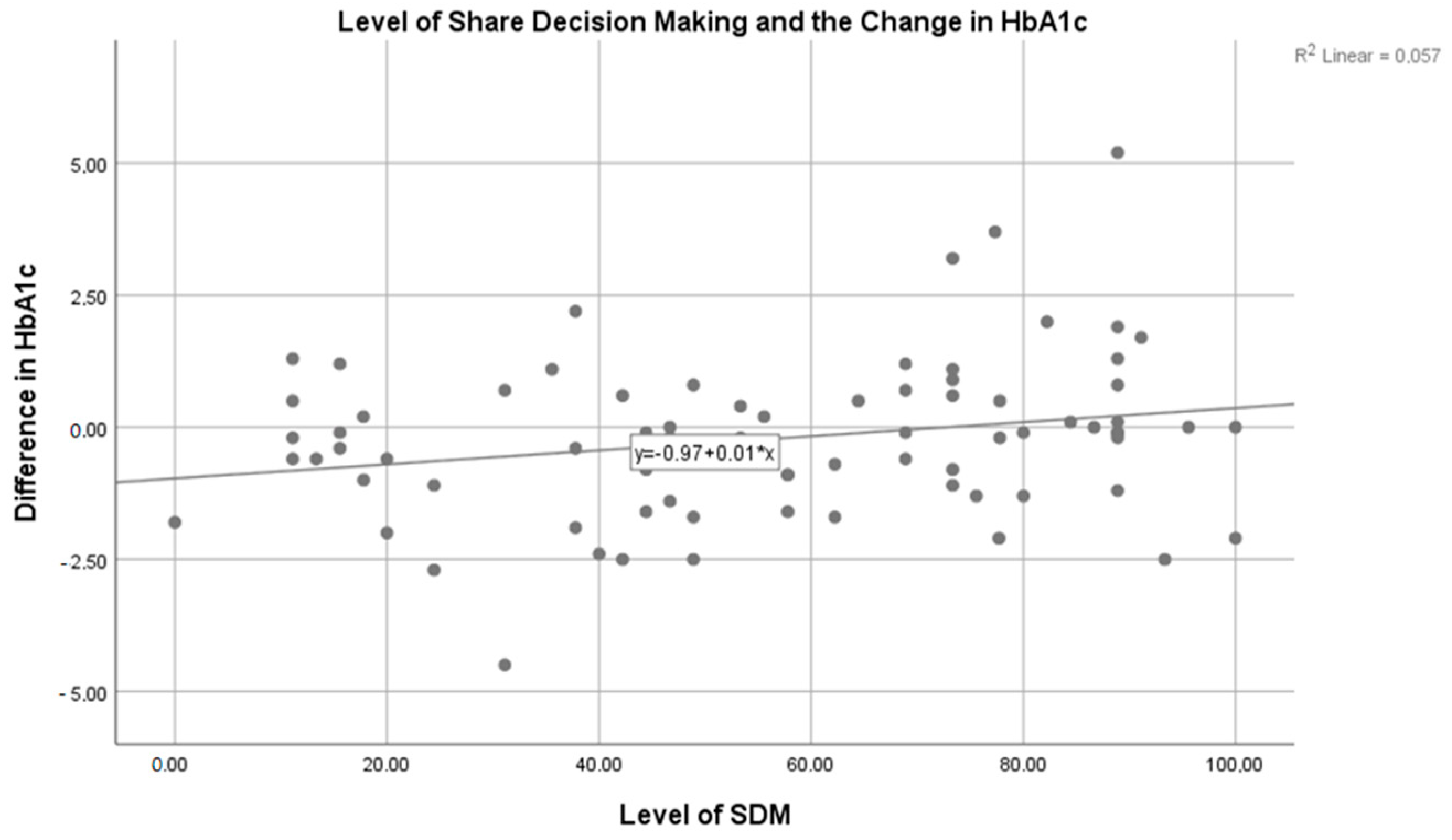
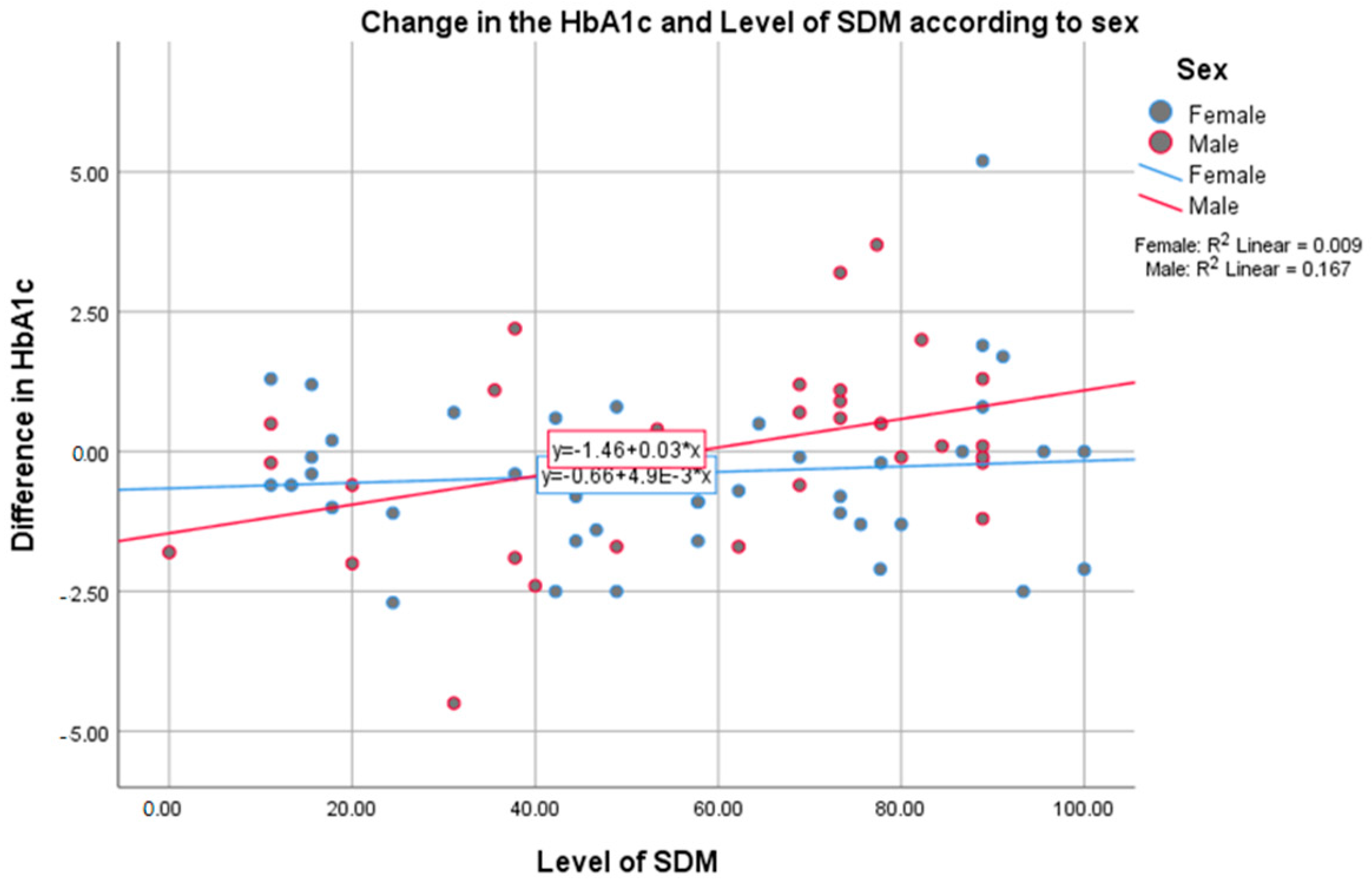
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/westernpacific/health-topics/diabetes (accessed on 15 January 2022).
- Ecuador M de Salud Pública. Encuesta Nacional de Salud y Nutrición: ENSANUT-ECU 2012. Quito: INEC. 2014. Available online: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_Sociales/ENSANUT/MSP_ENSANUT-ECU_06-10-2014.pdf (accessed on 18 October 2016).
- Droumaguet, C.; Balkau, B.; Simon, D.; Caces, E.; Tichet, J.; Charles, M.A.; Eschwege, E.; DESIR Study Group. Use of HbA1c in predicting progression to diabetes in French men and women: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2006, 29, 1619–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- About Diabetes. Facts & Figures. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 15 January 2022).
- Viswanathan, M.; Golin, C.E.; Jones, C.D.; Ashok, M.; Blalock, S.J.; Wines, R.C.M.; Coker-Schwimmer, E.J.; Rosen, D.L.; Sista, P.; Lohr, K.N. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: A systematic review. Ann. Intern. Med. 2012, 157, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Marinho, F.S.; Moram, C.B.M.; Rodrigues, P.C.; Leite, N.C.; Salles, G.F.; Cardoso, C.R.L. Treatment Adherence and Its Associated Factors in Patients with Type 2 Diabetes: Results from the Rio de Janeiro Type 2 Diabetes Cohort Study. J. Diabetes Res. 2018, 2018, 8970196. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.T.; Bussell, J.K. Medication Adherence: WHO Cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaté, E.; World Health Organization (Eds.) Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; 198p. [Google Scholar]
- Castillo-Tandazo, W.; González, J.; Cabrera, D.; Ávila, V.; Salazar, H.; Alvarado, G. Attitudes, And Practice of Shared Decision Making Among Physicians from Guayaquil, Ecuador. Internet J. Intern. Med. 2016, 11, 1–5. Available online: http://ispub.com/IJIM/11/1/39191 (accessed on 16 January 2020).
- Charles, C.; Gafni, A.; Whelan, T. Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc. Sci. Med. 1997, 44, 681–692. [Google Scholar] [CrossRef]
- Ciapponi, A. Shared Decision Making. Evid. Actual. En La Práctica Ambulatorial 2012, 15, 2–4. [Google Scholar]
- Elwyn, G.; Laitner, S.; Coulter, A.; Walker, E.; Watson, P.; Thomson, R. Implementing shared decision making in the NHS. BMJ 2010, 341, c5146. [Google Scholar] [CrossRef] [Green Version]
- Makoul, G.; Clayman, M.L. An integrative model of shared decision making in medical encounters. Patient Educ. Couns. 2006, 60, 301–312. [Google Scholar] [CrossRef]
- Asche, C.; LaFleur, J.; Conner, C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin. Ther. 2011, 33, 74–109. [Google Scholar] [CrossRef]
- Kriston, L.; Scholl, I.; Hölzel, L.; Simon, D.; Loh, A.; Härter, M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ. Couns. 2010, 80, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Scholl, I.; Kriston, L.; Dirmaier, J.; Buchholz, A.; Härter, M. Development and psychometric properties of the Shared Decision Making Questionnaire—Physician version (SDM-Q-Doc). Patient Educ. Couns. 2012, 88, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.; Moral, E.; Brieva, L.; Ruiz-Beato, E.; Prefasi, D.; Maurino, J. Psychometric properties of the SDM-Q-9 questionnaire for shared decision-making in multiple sclerosis: Item response theory modelling and confirmatory factor analysis. Health Qual. Life Outcomes 2017, 15, 79. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5401467/ (accessed on 16 January 2020). [CrossRef] [Green Version]
- Alvarado-Villa, G.E.; Moncayo-Rizzo, J.D.; Gallardo-Rumbea, J.A. Spanish validation endorsement of SDM-Q-9, a new approach. BMC Public Health 2019, 19, 106. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6343252/ (accessed on 26 December 2020). [CrossRef] [PubMed] [Green Version]
- Alzubaidi, H.; Hussein, A.; Mc Namara, K.; Scholl, I. Psychometric properties of the Arabic version of the 9-item Shared Decision-Making Questionnaire: The entire process from translation to validation. BMJ Open 2019, 9, e026672. [Google Scholar] [CrossRef] [PubMed]
- Rencz, F.; Tamási, B.; Brodszky, V.; Gulácsi, L.; Weszl, M.; Péntek, M. Validity and reliability of the 9-item Shared Decision Making Questionnaire (SDM-Q-9) in a national survey in Hungary. Eur. J. Health Econ. 2019, 20 (Suppl. 1), 43–55. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Miura, H.; Son, D.; Arai, H.; Kriston, L.; Scholl, I.; Härter, M.; Sato, K.; Kusaba, T. Psychometric Evaluation of the Japanese 9-Item Shared Decision-Making Questionnaire and Its Association with Decision Conflict and Patient Factors in Japanese Primary Care. JMA J. 2020, 3, 208–215. [Google Scholar]
- National Institute for Health and Care Excellence (UK). Blood Glucose Management. Type 2 Diabetes in Adults: Management. 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553510/ (accessed on 16 February 2022).
- WHO. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Diabetes Research and Clinical Practice; WHO: Geneva, Switzerland, 2011; Volume 93, pp. 299–309. [Google Scholar]
- Martono, D.P.; Hak, E.; Lambers Heerspink, H.; Wilffert, B.; Denig, P. Predictors of HbA1c levels in patients initiating metformin. Curr. Med. Res. Opin. 2016, 32, 2021–2028. [Google Scholar] [CrossRef] [Green Version]
- Candler, T.P.; Mahmoud, O.; Lynn, R.M.; Majbar, A.A.; Barrett, T.G.; Shield, J.P.H. Treatment adherence and BMI reduction are key predictors of HbA1c 1 year after diagnosis of childhood type 2 diabetes in the United Kingdom. Pediatric Diabetes 2018, 19, 1393–1399. [Google Scholar] [CrossRef]
- Simpson, S.H.; Lin, M.; Eurich, D.T. Medication Adherence Affects Risk of New Diabetes Complications: A Cohort Study. Ann. Pharmacother. 2016, 50, 741–746. [Google Scholar] [CrossRef]
- Bondar, A.; Popa, A.R.; Papanas, N.; Popoviciu, M.; Vesa, C.M.; Sabau, M.; Daina, C.; Stoica, R.A.; Katsiki, N.; Stoian, A.P. Diabetic neuropathy: A narrative review of risk factors, classification, screening and current pathogenic treatment options (Review). Exp. Ther. Med. 2021, 22, 690. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.R.; Pusta, C.T.J.; Vesa, C.M.; Bungau, S.; Buhas, C.L.; Sava, C.; Dimulescu, I.A.; Zaha, D.C.; Bustea, C. Prediction Models of Albumin Renal Excretion in Type 2 Diabetes Mellitus Patients. Revista de Chimie 2019, 70, 3802–3807. [Google Scholar] [CrossRef]
- Assari, S.; Lankarani, M.M.; Piette, J.D.; Aikens, J.E. Self-rated health and glycemic control in type 2 diabetes: Race by gender differences. J. Racial Ethn. Health Disparities 2018, 5, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Leandro, M.E.; Pereira, M.G. Adherence and Glycemic Control in Adolescents with Type 1 Diabetes: The Moderating Role of Age, Gender, and Family Support. J. Clin. Psychol. Med. Settings 2020, 27, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Wollny, A.; Altiner, A.; Daubmann, A.; Drewelow, E.; Helbig, C.; Löscher, S.; Pentzek, M.; Santos, S.; Wegscheider, K.; Wilm, S.; et al. Patient-centered communication and shared decision making to reduce HbA1c levels of patients with poorly controlled type 2 diabetes mellitus—Results of the cluster-randomized controlled DEBATE trial. BMC Fam. Pract. 2019, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; McFarland, M. How Are Income and Education Related to the Prevention and Management of Diabetes? J. Aging Health 2020, 32, 1063–1074. [Google Scholar] [CrossRef]
- Younes, N.; Atallah, M.; Alam, R.; Chehade, N.; Gannagé-Yared, M.-H. HbA1c and Blood Pressure Measurements: Relation with Gender, Body Mass Index, Study Field, and Lifestyle in Lebanese Students. Endocr. Pract. 2019, 25, 1101–1108. [Google Scholar] [CrossRef]
- Claussen, A.; Møller, J.B.; Kristensen, N.R.; Klim, S.; Kjellsson, M.C.; Ingwersen, S.H.; Karlsson, M.O. Impact of demographics and disease progression on the relationship between glucose and HbA1c. Eur. J. Pharm. Sci. 2017, 104, 417–423. [Google Scholar] [CrossRef]
- Alswat, A.K. Impact of Gender on Type II Diabetes Glycemic and Cardiovascular Markers Control and Treatment. Pak. J. Biol. Sci. 2020, 23, 1643–1649. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [Green Version]
| Count (%) | SDM Level | Change in the HbA1c | p Value * | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Minimum | Maximum | Mean (SD) | ||||
| Sex | Female | 44 (57.9) | 55.66 (28.02) | −2.70 | 5.20 | −0.38 (1.41) | 0.101 |
| Male | 32 (42.1) | 57.97 (26.6) | −4.50 | 3.70 | 0.02 (1.66) | ||
| Age Group | 35–49 years old | 10 (13.2) | 58.89 (24.1) | −1.30 | 2.00 | 0.26 (1.15) | 0.193 |
| 50–64 years old | 46 (60.5) | 57.72 (27.79) | −4.50 | 5.20 | −0.41 (1.65) | ||
| ≥65 years old | 20 (26.3) | 53 (28.5) | −2.50 | 3.20 | 0.01 (1.36) | ||
| Education Level | Without Studies | 1 (1.3) | 46.67 (.) | 0.00 | 0.00 | 0.00 (.) | 0.498 |
| Primary Education | 19 (25) | 53.33 (27.1) | −4.50 | 1.90 | −0.25 (1.35) | ||
| Secondary Education | 32 (42.1) | 55.68 (27.1) | −2.70 | 3.70 | −0.49 (1.45) | ||
| University Degree | 24 (31.6) | 60.93 (27.51) | −2.50 | 5.20 | 0.18 (1.75) | ||
| Body Mass Index | Normal | 12 (15.8) | 42.22 (24.93) | −4.50 | 1.70 | −0.75 (1.71) | 0.383 |
| Overweight | 31 (40.8) | 60.27 (24.68) | −2.50 | 3.70 | 0.05 (1.43) | ||
| Obese | 33 (43.4) | 58.45 (29.4) | −2.50 | 5.20 | −0.27 (1.53) | ||
| Private Physician | Yes | 14 (18.4) | 53.17 (32.2) | −2.70 | 1.30 | −0.58 (1.27) | 0.503 |
| No | 62 (81.6) | 57.41 (26.27) | −4.50 | 5.20 | −0.13 (1.58) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farfán Bajaña, M.J.; Moncayo-Rizzo, J.; Alvarado-Villa, G.; Avila-Quintero, V.J. Does the SDMQ-9 Predict Changes in HbA1c Levels? An Ecuadorian Cohort. Medicina 2022, 58, 380. https://doi.org/10.3390/medicina58030380
Farfán Bajaña MJ, Moncayo-Rizzo J, Alvarado-Villa G, Avila-Quintero VJ. Does the SDMQ-9 Predict Changes in HbA1c Levels? An Ecuadorian Cohort. Medicina. 2022; 58(3):380. https://doi.org/10.3390/medicina58030380
Chicago/Turabian StyleFarfán Bajaña, María José, Jorge Moncayo-Rizzo, Geovanny Alvarado-Villa, and Victor J. Avila-Quintero. 2022. "Does the SDMQ-9 Predict Changes in HbA1c Levels? An Ecuadorian Cohort" Medicina 58, no. 3: 380. https://doi.org/10.3390/medicina58030380
APA StyleFarfán Bajaña, M. J., Moncayo-Rizzo, J., Alvarado-Villa, G., & Avila-Quintero, V. J. (2022). Does the SDMQ-9 Predict Changes in HbA1c Levels? An Ecuadorian Cohort. Medicina, 58(3), 380. https://doi.org/10.3390/medicina58030380







