Microendoscopic Lumbar Posterior Decompression Surgery for Lumbar Spinal Stenosis: Literature Review
Abstract
:1. Introduction
2. Surgical Indication
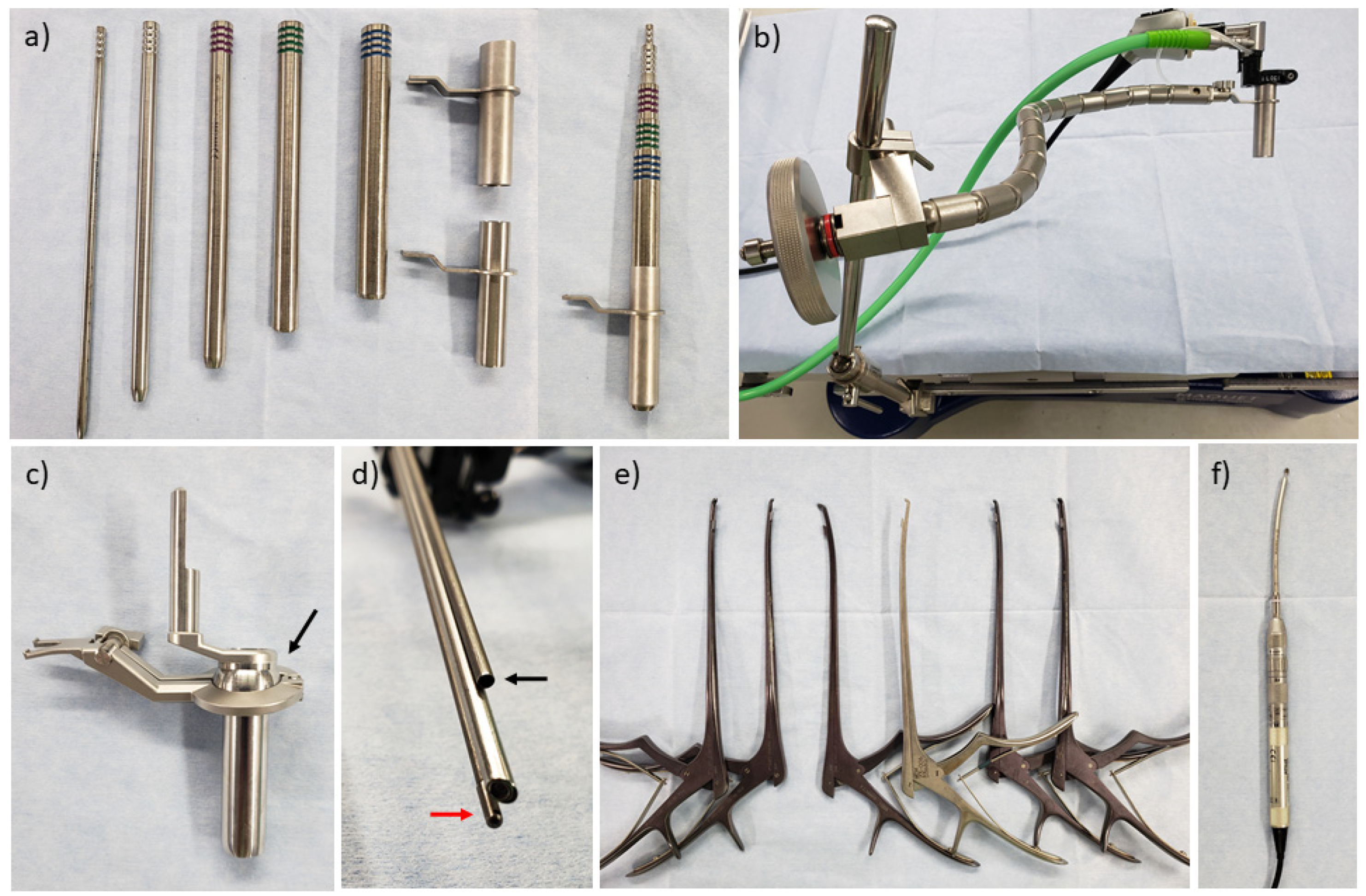
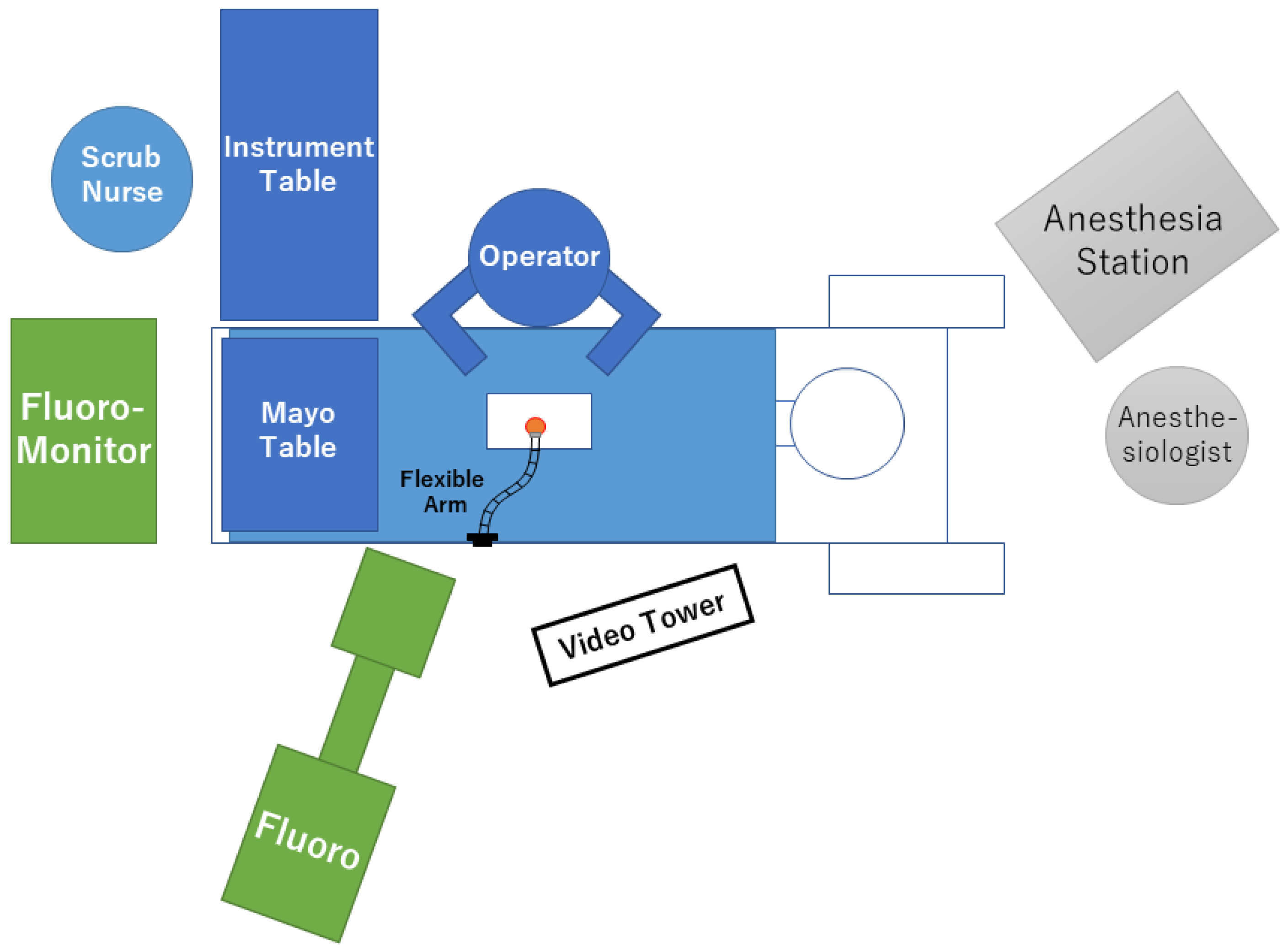
3. Surgical Equipment, Patient Positioning, and Room Setup
4. Surgical Technique for Spinal Canal Stenosis
4.1. Paramedian Approach
4.2. Midline Approach
5. Surgical Technique for Foraminal Stenosis
6. Learning Curve
7. Clinical Outcomes of Microendoscopic Lumbar Decompression
7.1. Spinal Canal Stenosis
7.2. Spinal Canal Stenosis with Spondylolisthesis
7.3. Complications
7.4. Comparison with Other Surgical Techniques
7.5. Foraminal/Extraforaminal Stenosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, R.K.; Jensen, T.S.; Koes, B.; Hartvigsen, J. Prevalence of lumbar spinal stenosis in general and clinical populations: A systematic review and meta-analysis. Eur. Spine J. 2020, 29, 2143–2163. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.A.; Hanscom, B.; Skinner, J.S.; Abdu, W.A.; Hilibrand, A.S.; Boden, S.D.; Deyo, R.A. Surgical vs nonoperative treatment for lumbar disk herniation: The spine patient outcomes research trial (SPORT): A randomized trial. JAMA 2006, 296, 2441–2450. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.; Blood, E.; Hanscom, B.; Herkowitz, H.; Cammisa, F.; Albert, T.; Boden, S.D.; et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N. Engl. J. Med. 2008, 358, 794–810. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Hanscom, B.; Tosteson, A.N.; Blood, E.A.; Birkmeyer, N.J.; Hilibrand, A.S.; Herkowitz, H.; Cammisa, F.P.; et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N. Engl. J. Med. 2007, 356, 2257–2270. [Google Scholar] [CrossRef] [Green Version]
- Grotle, M.; Småstuen, M.C.; Fjeld, O.; Grøvle, L.; Helgeland, J.; Storheim, K.; Solberg, T.K.; Zwart, J.-A. Lumbar spine surgery across 15 years: Trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open 2019, 9, e028743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, J.N.; Lurie, J.D.; Olson, P.R.; Bronner, K.K.; Fisher, E.S. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine 2006, 31, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Steiger, H.J.; Krämer, M.; Reulen, H.J. Development of neurosurgery in germany: Comparison of data collected by polls for 1997, 2003, and 2008 among providers of neurosurgical care. World Neurosurg. 2012, 77, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramaniam, V.; Patel, H.C.; Ozdemir, B.A.; Papadopoulos, M.C. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: A 15-year time-series study. BMJ Open 2015, 5, e009011. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, H.; Krause, F. Ueber einklemmung bzw. Strangulation der Cauda equina. DMW-Dtsch. Med. Wochenschr. 1909, 35, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Foley, K.T.; Smith, M.M. Microendoscopic discectomy. Tech. Neurosurg. 1997, 3, 301–307. [Google Scholar]
- Minamide, A.; Yoshida, M.; Yamada, H.; Nakagawa, Y.; Hashizume, H.; Iwasaki, H.; Tsutsui, S. Clinical outcomes after microendoscopic laminotomy for lumbar spinal stenosis: A 5-year follow-up study. Eur. Spine J. 2014, 24, 396–403. [Google Scholar] [CrossRef]
- Minamide, A.; Yoshida, M.; Simpson, A.K.; Nakagawa, Y.; Iwasaki, H.; Tsutsui, S.; Takami, M.; Hashizume, H.; Yukawa, Y.; Yamada, H. Minimally invasive spinal decompression for degenerative lumbar spondylolisthesis and stenosis maintains stability and may avoid the need for fusion. Bone Jt. J. 2018, 100-B, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Toyone, T.; Murata, Y.; Inage, K.; Urushibara, M.; Ouchi, J. Degenerative lumbar spondylolisthesis with spinal stenosis: A comparative study of 5-year outcomes following decompression with fusion and microendoscopic decompression. Asian Spine J. 2018, 12, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamide, A.; Simpson, A.K.; Okada, M.; Enyo, Y.; Nakagawa, Y.; Iwasaki, H.; Tsutsui, S.; Takami, M.; Nagata, K.; Hashizume, H.; et al. Microendoscopic decompression for lumbar spinal stenosis with degenerative spondylolisthesis: The influence of spondylolisthesis stage (disc height and static and dynamic translation) on clinical outcomes. Clin. Spine Surg. A Spine Publ. 2019, 32, E20–E26. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tamai, K.; Toyoda, H.; Terai, H.; Hoshino, M.; Suzuki, A.; Takahashi, S.; Hori, Y.; Yabu, A.; Nakamura, H. Clinical outcomes of minimally invasive posterior decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine 2021, 46, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Minamide, A.; Takami, M.; Iwasaki, H.; Okada, S.; Nonaka, K.; Taneichi, H.; Schoenfeld, A.J.; Simpson, A.K.; Yamada, H. Microendoscopic decompression for lumbar spinal stenosis caused by facet-joint cysts: A novel technique with a cyst-dyeing protocol and cohort comparison study. J. Neurosurg. Spine 2021, 34, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Minamide, A.; Yoshida, M.; Yamada, H.; Nakagawa, Y.; Kawai, M.; Maio, K.; Hashizume, H.; Iwasaki, H.; Tsutsui, S. Endoscope-assisted spinal decompression surgery for lumbar spinal stenosis. J. Neurosurg. Spine 2013, 19, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Nomura, K.; Yoshida, M. Microendoscopic decompression surgery for lumbar spinal canal stenosis via the paramedian approach: Preliminary results. Glob. Spine J. 2012, 2, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Ikuta, K.; Arima, J.; Tanaka, T.; Oga, M.; Nakano, S.; Sasaki, K.; Goshi, K.; Yo, M.; Fukagawa, S. Short-term results of microendoscopic posterior decompression for lumbar spinal stenosis. J. Neurosurg. Spine 2005, 2, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Asgarzadie, F.; Khoo, L.T. Minimally invasive operative management for lumbar spinal stenosis: Overview of early and long-term outcomes. Orthop. Clin. N. Am. 2007, 38, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Okada, E.; Ninomiya, K.; Kihara, M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J. Neurosurg. Spine 2009, 10, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Nagae, M.; Ikeda, T.; Tonomura, H.; Fujiwara, H.; Kubo, T. Tubular surgery with the assistance of endoscopic surgery via midline approach for lumbar spinal canal stenosis: A technical note. Eur. Spine J. 2013, 22, 2105–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Yoshida, M.; Hashizume, H.; Minamide, A.; Nakagawa, Y.; Kawai, M.; Iwasaki, H.; Tsutsui, S. Efficacy of novel minimally invasive surgery using spinal microendoscope for treating extraforaminal stenosis at the lumbosacral junction. J. Spinal Disord. Tech. 2012, 25, 268–276. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Iesato, N.; Terashima, Y.; Tanimoto, K.; Oshigiri, T.; Emori, M.; Teramoto, A.; Yamashita, T. Mid-term clinical results of microendoscopic decompression for lumbar foraminal stenosis. Spine Surg. Relat. Res. 2019, 3, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Enyo, Y.; Yamada, H.; Kim, J.H.; Yoshida, M.; Hutton, W.C. Microendoscopic lateral decompression for lumbar foraminal stenosis. J. Spinal Disord. Tech. 2014, 27, 257–262. [Google Scholar] [CrossRef]
- Murata, S.; Minamide, A.; Iwasaki, H.; Nakagawa, Y.; Hashizume, H.; Yukawa, Y.; Tsutsui, S.; Takami, M.; Okada, M.; Nagata, K.; et al. Microendoscopic decompression for lumbosacral foraminal stenosis: A novel surgical strategy based on anatomical considerations using 3D image fusion with MRI/CT. J. Neurosurg. Spine 2020, 33, 789–795. [Google Scholar] [CrossRef]
- Matsumoto, M.; Chiba, K.; Ishii, K.; Watanabe, K.; Nakamura, M.; Toyama, Y. Microendoscopic partial resection of the sacral ala to relieve extraforaminal entrapment of the L-5 spinal nerve at the lumbosacral tunnel. J. Neurosurg. Spine 2006, 4, 342–346. [Google Scholar] [CrossRef]
- Nowitzke, A.M. Assessment of the learning curve for lumbar microendoscopic discectomy. Neurosurgery 2005, 56, 755–762. [Google Scholar] [CrossRef]
- Nomura, K.; Yoshida, M. Assessment of the learning curve for microendoscopic decompression surgery for lumbar spinal canal stenosis through an analysis of 480 cases involving a single surgeon. Glob. Spine J. 2017, 7, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.; Turner, R.; Palmer, R. Bilateral decompressive surgery in lumbar spinal stenosis associated with spondylolisthesis: Unilateral approach and use of a microscope and tubular retractor system. Neurosurg. Focus 2002, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Turner, R.; Palmer, R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J. Neurosurg. 2002, 97, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Khoo, L.T.; Fessler, R.G. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery 2002, 51, S2–S146. [Google Scholar] [CrossRef]
- Castro-Menéndez, M.; Bravo-Ricoy, J.A.; Casal-Moro, R.; Hernández-Blanco, M.; Jorge-Barreiro, F.J. Midterm outcome after microendoscopic decompressive laminotomy for lumbar spinal stenosis. Neurosurgery 2009, 65, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Endo, K.; Suzuki, H.; Kojima, A.; Sawaji, Y.; Urushibara, M.; Matsuoka, Y.; Takamatsu, T.; Murata, K.; Konishi, T.; et al. Long-term outcomes following lumbar microendoscopic decompression for lumbar spinal stenosis with and without degenerative spondylolisthesis: Minimum 10-year follow-up. World Neurosurg. 2020, 146, e1219–e1225. [Google Scholar] [CrossRef]
- Gupta, S.; Marathe, N.; Chhabra, H.S.; Destandau, J. Long-term functional outcomes of endoscopic decompression with destandau technique for lumbar canal stenosis. Asian Spine J. 2021, 15, 431–440. [Google Scholar] [CrossRef]
- Ikuta, K.; Tono, O.; Oga, M. Clinical outcome of microendoscopic posterior decompression for spinal stenosis associated with degenerative spondylolisthesis–minimum 2-year outcome of 37 patients. Min-Minim. Invasive Neurosurg. 2008, 51, 267–271. [Google Scholar] [CrossRef]
- Tsutsumimoto, T.; Yui, M.; Uehara, M.; Ohta, H.; Kosaku, H.; Misawa, H. A prospective study of the incidence and outcomes of incidental dural tears in microendoscopic lumbar decompressive surgery. Bone Jt. J. 2014, 96-B, 641–645. [Google Scholar] [CrossRef]
- Pao, J.-L.; Chen, W.-C.; Chen, P.-Q. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur. Spine J. 2009, 18, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Soma, K.; Kato, S.; Oka, H.; Matsudaira, K.; Fukushima, M.; Oshina, M.; Koga, H.; Takano, Y.; Iwai, H.; Ganau, M.; et al. Influence of incidental dural tears and their primary microendoscopic repairs on surgical outcomes in patients undergoing microendoscopic lumbar surgery. Spine J. 2019, 19, 1559–1565. [Google Scholar] [CrossRef]
- Kothe, R.; Quante, M.; Engler, N.; Heider, F.; Kneißl, J.; Pirchner, S.; Siepe, C. The effect of incidental dural lesions on outcome after decompression surgery for lumbar spinal stenosis: Results of a multi-center study with 800 patients. Eur. Spine J. 2016, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.H.; On Behalf of the LSOS Study Group; Burgstaller, J.M.; Brunner, F.; Porchet, F.; Farshad, M.; Pichierri, G.; Steurer, J.; Held, U. The impact of incidental durotomy on the outcome of decompression surgery in degenerative lumbar spinal canal stenosis: Analysis of the Lumbar Spinal Outcome Study (LSOS) data—A swiss prospective multi-center cohort study. BMC Musculoskelet. Disord. 2016, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.; Ball, P.A.; Bekelis, K.; Lurie, J.; Mirza, S.K.; Tosteson, T.D.; Weinstein, J.N. SPORT: Does incidental durotomy affect longterm outcomes in cases of spinal stenosis? Neurosurgery 2011, 76, S57–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pao, J.-L.; Wang, J.-L. Intraoperative myelography in minimally invasive decompression for degenerative lumbar spinal stenosis. J. Spinal Disord. Tech. 2012, 25, E117–E124. [Google Scholar] [CrossRef]
- Ikuta, K.; Tono, O.; Tanaka, T.; Arima, J.; Nakano, S.; Sasaki, K.; Oga, M. Evaluation of postoperative spinal epidural hematoma after microendoscopic posterior decompression for lumbar spinal stenosis: A clinical and magnetic resonance imaging study. J. Neurosurg. Spine 2006, 5, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Merter, A.; Shibayama, M. Does the drain placement technique affect the amount of postoperative spinal epidural hematoma after microendoscopic decompressive laminotomy for lumbar spinal stenosis? J. Orthop. Surg. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikuta, K.; Tono, O.; Tanaka, T.; Arima, J.; Nakano, S.; Sasaki, K.; Oga, M. Surgical complications of microendoscopic procedures for lumbar spinal stenosis. Min-Minim. Invasive Neurosurg. 2007, 50, 145–149. [Google Scholar] [CrossRef]
- Fourney, D.R.; Dettori, J.R.; Norvell, D.C.; Dekutoski, M.B. Does minimal access tubular assisted spine surgery increase or decrease complications in spinal decompression or fusion? Spine 2010, 35, S57–S65. [Google Scholar] [CrossRef]
- Rahman, M.; Summers, L.E.; Richter, B.; Mimran, R.I.; Jacob, R.P. Comparison of techniques for decompressive lumbar laminectomy: The minimally invasive versus the “classic” open approach. Min-Minim. Invasive Neurosurg. 2008, 51, 100–105. [Google Scholar] [CrossRef]
- Fujimoto, T.; Taniwaki, T.; Tahata, S.; Nakamura, T.; Mizuta, H. Patient outcomes for a minimally invasive approach to treat lumbar spinal canal stenosis: Is microendoscopic or microscopic decompressive laminotomy the less invasive surgery? Clin. Neurol. Neurosurg. 2015, 131, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, R.; Yoshimoto, M.; Iesato, N.; Terashima, Y.; Takebayashi, T.; Yamashita, T.; Fukushi, R. Short-term results of microendoscopic muscle-preserving interlaminar decompression versus spinal process splitting laminectomy. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2018, 79, 511–517. [Google Scholar] [CrossRef]
- Wu, B.; Xiong, C.; Tan, L.; Zhao, D.; Xu, F.; Kang, H. Clinical outcomes of MED and iLESSYS® delta for the treatment of lumbar central spinal stenosis and lateral recess stenosis: A comparison study. Exp. Ther. Med. 2020, 20, 252. [Google Scholar] [CrossRef]
- Iwai, H.; Inanami, H.; Koga, H. Comparative study between full-endoscopic laminectomy and microendoscopic laminectomy for the treatment of lumbar spinal canal stenosis. J. Spine Surg. 2020, 6, E3–E11. [Google Scholar] [CrossRef]
- Ito, Z.; Shibayama, M.; Nakamura, S.; Yamada, M.; Kawai, M.; Takeuchi, M.; Yoshimatsu, H.; Kuraishi, K.; Hoshi, N.; Miura, Y.; et al. Clinical comparison of unilateral biportal endoscopic laminectomy versus microendoscopic laminectomy for single-level laminectomy: A single-center, retrospective analysis. World Neurosurg. 2021, 148, e581–e588. [Google Scholar] [CrossRef]
- Aygun, H.; Abdulshafi, K. Unilateral biportal endoscopy versus tubular microendoscopy in management of single level degenerative lumbar canal stenosis. Clin. Spine Surg. A Spine Publ. 2021, 34, E323–E328. [Google Scholar] [CrossRef]
- Hayashi, K.; Toyoda, H.; Terai, H.; Hoshino, M.; Suzuki, A.; Takahashi, S.; Tamai, K.; Ohyama, S.; Hori, Y.; Yabu, A.; et al. Comparison of minimally invasive decompression and combined minimally invasive decompression and fusion in patients with degenerative spondylolisthesis with instability. J. Clin. Neurosci. 2018, 57, 79–85. [Google Scholar] [CrossRef]
- Kimura, R.; Yoshimoto, M.; Miyakoshi, N.; Hongo, M.; Kasukawa, Y.; Kobayashi, T.; Kikuchi, K.; Okuyama, K.; Kido, T.; Hirota, R.; et al. Comparison of posterior lumbar interbody fusion and microendoscopic muscle-preserving interlaminar decompression for degenerative lumbar spondylolisthesis with >5-year follow-up. Clin. Spine Surg. A Spine Publ. 2019, 32, E380–E385. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Liang, Y.; Zhu, Z.; Wang, K.; Qian, Y.; Liu, H. Decompression with fusion is not in superiority to decompression alone in lumbar stenosis based on randomized controlled trials. Medicine 2019, 98, e17849. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, P.; Feng, F.; Chhantyal, K.; Yang, Y.; Rong, L. Decompression alone versus decompression and fusion for lumbar degenerative spondylolisthesis: A meta-analysis. World Neurosurg. 2018, 111, e165–e177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, W.-J.; Wang, J.; Chu, T.-W.; Li, C.-Q.; Zhang, Z.-F.; Wang, W.-D. The clinical features of, and microendoscopic decompression for, extraforaminal entrapment of the L5 spinal nerve. Orthop. Surg. 2009, 1, 74–77. [Google Scholar] [CrossRef]
- Matsumoto, M.; Watanabe, K.; Ishii, K.; Tsuji, T.; Takaishi, H.; Nakamura, M.; Toyama, Y.; Chiba, K. Posterior decompression surgery for extraforaminal entrapment of the fifth lumbar spinal nerve at the lumbosacral junction. J. Neurosurg. Spine 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]



| First Author | Year | Comparison | Approach | No. of Patients | Follow-Up (Months) | Advantages of Microendoscopic Decompression | Disadvantages of Microendoscopic Decompression | Clinical Outcome in Microendoscopic Decompression Compared with the Opposite Arm | Complication in Microendoscopic Decompression Compared with the Opposite Arm | Ref. No. |
|---|---|---|---|---|---|---|---|---|---|---|
| Khoo | 2002 | vs. open | Paramedian | 25 vs. open 25 | 12 | Less blood loss Shorter hospital stay Less use of narcotics | - | Similar changes in symptom | Dural tear 16% vs. 8% Additional fusion surgery 0% vs. 12% Transfusion 0% vs. 8% | [33] |
| Ikuta | 2005 | vs. microsurgery | Paramedian | 47 (DS 14) vs. micro 29 (DS 9) | 22 | Less blood loss Shorter hospital stay Less use of analgesics | Higher complication rate | Similar improvement in JOA score and VAS for low back pain and leg pain | Dural tear 8.5% vs. 6.8% Facet fracture 6.4% vs. 3.4% Transient neuralgia 14.9% vs. 3.4% | [20] |
| Rahman | 2008 | vs. open | Paramedian | 38 vs. open 88 | 1 | Shorter operating time Less blood loss Shorter hospital stay Lower complication rate | - | N/A | Dead 0% vs. 1.1% Wound exploration 0% vs. 2.3% Dural tear 2.6% vs. 2.3% Synovial cyst 2.6% vs. 1.1% Infection 2.6% vs. 3.4% | [49] |
| Fujimoto | 2015 | vs. microsurgery | Paramedian | 21 vs. micro 20 (Including Myerding Grade1 DS) | 24 | Shorter operating time Less blood loss Shorter hospital stay Lower CRP Less dose of NSAIDs | - | Similar improvement in JOA score and VAS for leg pain | Transient neuralgia 15% vs. 4.8% Disturbance of wound healing 10% vs. 0% | [50] |
| Yagi | 2009 | vs. open | Midline | 20 vs. open 21 | 12 | Less blood loss Shorter hospital stay Lower CPK Less atrophy of PVM | - | Less VAS for low back pain Similar improvement in JOA score | [22] | |
| Fukushi | 2015 | vs. spinous process splitting laminectomy | Midline | 58 (DS 13) vs. open 39 (DS 8) | 42 (>6) | Lower CRP | - | Similar improvement in JOABPEQ, SF-36, VAS Similar patient satisfaction scores | Superficial infection 3.4% vs. 0% | [51] |
| Hayashi | 2018 | vs. fusion (CBT-PLIF) | Paramedian | 30 vs. fusion 20 (All patients had DS) | 42 (>24) | Less blood loss Lower CRP Less dose of NSAIDs | - | Similar improvement in JOA score, and VAS for low back pain, leg pain, and leg numbness | Re-op 16% vs. 15% Neurological deficit 3.3% vs. 5.0% Epidural hematoma 0% vs. 5.0% Dural tear 6.7% vs. 0% | [56] |
| Aihara | 2018 | vs. fusion | Paramedian | 25 vs. fusion 16 (All patients had DS) | 60 | Shorter operating time Less blood loss Shorter hospital stay | - | Greater improvement in the social function domain in JOABPEQ | Re-op 12% vs. 12.5% | [14] |
| Kimura | 2019 | vs. fusion (conventional PLIF) | Midline | 37 vs. fusion 79 (Including Myerding Grade1 DS) | 60 | Shorter operating time Less blood loss | - | Similar improvement in JOA score, JOABPEQ, ZCQ, and VAS for low back pain, leg pain, leg numbness | Dural tear 2.7% vs. 1.3% Superficial infection 0% vs. 2.5% Pulmonary embolism 2.7% vs. 0% Re-op 7.1% vs. 8.0% | [57] |
| Wu | 2020 | vs. full endoscopic decompression | Paramedian | 82 vs. 52 | 20 | Shorter operating time | Longer skin incision | Similar improvement in VAS for leg pain Higher VAS for low back pain and ODI only at 1 week, and those are similar at 6 months and final follow-up | Total 3.85% vs. 3.66% Dural tear 2.4% vs. 1.9% Urinary retention 1.2% vs. 0% Dysesthesia 0% vs. 1.9% | [52] |
| Iwai | 2020 | vs. full endoscopic decompression | Paramedian | 60 vs. 54 (Including Myerding Grade1 DS) | 3 | Shorter operating time | Longer hospital stay | Similar improvement in NRS | Dural tear 5.6% vs. 1.8% Hematoma 3.3% vs. 13.0% | [53] |
| Ito | 2021 | vs. full endoscopic decompression (biportal) | Paramedian | 139 vs. 42 | 6 | - | - | Similar improvement in VAS for low back pain and leg pain, ODI, and EQ5D | Dural tear 5.8% vs. 4.7% Hematoma 3.6% vs. 0% Re-op 1.4% vs. 0% | [54] |
| Aygun | 2021 | vs. full endoscopic decompression (biportal) | Paramedian | 77 vs. 77 (Randomized controlled trial) | 24 | - | Longer hospital stay Longer operating time More blood loss | Less improvement in ODI, ZCQ Lower results in Modified MacNab criteria | N/A | [55] |
| Author | Year | No. of Patients | Level | Follow-Up (Months) | Clinical Outcome | Complication/Revision Surgery | Ref. No. |
|---|---|---|---|---|---|---|---|
| Matsumoto | 2006 | 3 | L5/S | 31 | JOA RR 42.7% | None | [28] |
| Zhou | 2009 | 5 | L5/S | 19.7 | Improvement of VAS (10 cm); 5.9 | None | [60] |
| Matsumoto | 2010 | 2 (microendoscopic 19, surgical loupe or microscopic 9) | L5/S | 32.5 | JOA RR 68.5% (No significant difference between surgical approaches) | Intraoperative blood loss exceeded 100 mL in 4 cases Revision surgery in 4 cases | [61] |
| Yamada | 2012 | 32 | L5/S | 37.4 | JOA RR 60.1% Improvement of VAS (100 mm); 68.2 in leg pain, 31.8 in low back pain, 39.7 in leg numbness | Painful dysesthesia in 1 case Recurrence of symptom in 4 cases Revision surgery in 2 cases | [24] |
| Yoshimoto | 2019 | 20 | L5/S in 16 cases L5/6 in 2 cases L4/5 in 2 cases | 66.3 | JOA RR 63.9% | Revision surgery in 5 cases | [25] |
| Murata | 2020 | 78 | L5/s | 24 | JOA RR 56.0% Improvement of VAS (100 mm); 49.0 in leg pain, 29.8 in low back pain, | Painful dysesthesia in 5 cases | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, A.; Nakamura, H. Microendoscopic Lumbar Posterior Decompression Surgery for Lumbar Spinal Stenosis: Literature Review. Medicina 2022, 58, 384. https://doi.org/10.3390/medicina58030384
Suzuki A, Nakamura H. Microendoscopic Lumbar Posterior Decompression Surgery for Lumbar Spinal Stenosis: Literature Review. Medicina. 2022; 58(3):384. https://doi.org/10.3390/medicina58030384
Chicago/Turabian StyleSuzuki, Akinobu, and Hiroaki Nakamura. 2022. "Microendoscopic Lumbar Posterior Decompression Surgery for Lumbar Spinal Stenosis: Literature Review" Medicina 58, no. 3: 384. https://doi.org/10.3390/medicina58030384
APA StyleSuzuki, A., & Nakamura, H. (2022). Microendoscopic Lumbar Posterior Decompression Surgery for Lumbar Spinal Stenosis: Literature Review. Medicina, 58(3), 384. https://doi.org/10.3390/medicina58030384







