Clinical Characteristics and In-Hospital Outcomes in Dialysis Patients with Septic Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Selection and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Microbiology Results
3.3. Treatment and Outcomes
3.4. Univariate and Multivariate Analyses of Predictors of Longer LOS, Positive Blood Culture, and In-Hospital Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirtliff, M.E.; Mader, J.T. Acute septic arthritis. Clin. Microbiol. Rev. 2002, 15, 527–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, B.; Koyfman, A.; Gottlieb, M. Evaluation and Management of Septic Arthritis and its Mimics in the Emergency Department. West. J. Emerg. Med. 2019, 20, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Hammel, J.M.; Kwon, N. Septic arthritis of the acromioclavicular joint. J. Emerg. Med. 2005, 29, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Arieli, M.M.; Fowler, M.L.; Lieber, S.B.; Shmerling, R.H.; Paz, Z. The Profile of the Causative Organisms which Lead to Septic Arthritis of Native Joints Over the Last Two Decades in a Single Tertiary Medical Center in the East Coast of the United States. Int. J. Clin. Pract. 2021, 75, e15003. [Google Scholar] [CrossRef]
- Hassan, A.S.; Rao, A.; Manadan, A.M.; Block, J.A. Peripheral Bacterial Septic Arthritis: Review of Diagnosis and Management. J. Clin. Rheumatol. 2017, 23, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Margaretten, M.E.; Kohlwes, J.; Moore, D.; Bent, S. Does this adult patient have septic arthritis? JAMA 2007, 297, 1478–1488. [Google Scholar] [CrossRef]
- Winn, M.; Waller, J.; Nahman, N.S.; Huber, L.; Baer, S.; Kheda, M.; Colombo, R. Septic Arthritis in End-Stage Renal Disease. Open Forum Infect. Dis. 2016, 3 (Suppl. 1), 1135. [Google Scholar] [CrossRef]
- Aitkens, L.; Winn, M.; Waller, J.L.; Huber, L.; Baer, S.L.; Mohammed, A.; Kheda, M.; Tran, S.; Siddiqui, B.; Padala, S.; et al. Septic arthritis in the end-stage renal disease population. J. Investig. Med. 2021, 70, 383–390. [Google Scholar] [CrossRef]
- Al-Nammari, S.; Gulati, V.; Patel, R.; Bejjanki, N.; Wright, M. Septic Arthritis in Haemodialysis Patients: A Seven-Year Multi-Centre Review. J. Orthop. Surg. 2008, 16, 54–57. [Google Scholar] [CrossRef]
- Sasaki, S.; Raita, Y.; Murakami, M.; Yamamoto, S.; Tochitani, K.; Hasegawa, T.; Fujisaki, K.; Fukuhara, S. Added value of clinical prediction rules for bacteremia in hemodialysis patients: An external validation study. PLoS ONE 2021, 16, e0247624. [Google Scholar] [CrossRef]
- Zhang, J.; You, X. Clinical features, risk factors, and outcomes of septic arthritis in patients on maintenance hemodialysis. Clin. Rheumatol. 2020, 39, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, L.; Yee, J. Current Concepts in Hemodialysis Vascular Access Infections. Adv. Chronic Kidney Dis. 2019, 26, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Samuel, V.; Le, C.; Khan, M.; Alexandraki, I.; Cuhaci, B.; Nahman, N.S. Hemodialysis Vascular Catheter-Related Bacteremia. Am. J. Med Sci. 2007, 334, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, M.H.; Zhang, M.; Cohen, H.; Golestaneh, L.; Laut, J.M.; Rosenberg, S.O. Tunnelled haemodialysis catheter bacteraemia: Risk factors for bacteraemia recurrence, infectious complications and mortality. Nephrol. Dial. Transplant. 2006, 21, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.M.; Clark, E.; Dipchand, C.; Hiremath, S.; Kappel, J.; Kiaii, M.; Lok, C.; Luscombe, R.; Moist, L.; Oliver, M.; et al. Hemodialysis Tunneled Catheter-Related Infections. Can. J. Kidney Heal. Dis. 2016, 3, 2054358116669129. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.E.; Mokrzycki, M.H. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011, 79, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Ruangpin, C.; Rodchuae, M.; Katchamart, W. Factors Related to Surgical Treatment and Outcomes of Thai Patients with Septic Arthritis. JCR: J. Clin. Rheumatol. 2019, 25, 176–180. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S. The burden of septic arthritis on the U.S. inpatient care: A national study. PLoS ONE 2017, 12, e0182577. [Google Scholar] [CrossRef] [Green Version]
- Elsissy, J.G.; Liu, J.N.; Wilton, P.J.; Nwachuku, I.; Gowd, A.K.; Amin, N.H. Bacterial Septic Arthritis of the Adult Native Knee Joint: A Review. JBJS Rev. 2020, 8, e0059. [Google Scholar] [CrossRef]
- Mabille, C.; El-Samad, Y.; Joseph, C.; Brunschweiler, B.; Goeb, V.; Grados, F.; Lanoix, J.P. Medical versus surgical treatment in native hip and knee septic arthritis. Infect. Dis. Now. 2021, 51, 164–169. [Google Scholar] [CrossRef]
- Harada, K.; McConnell, I.; DeRycke, E.C.; Holleck, J.L.; Gupta, S. Native Joint Septic Arthritis: Comparison of Outcomes with Medical and Surgical Management. South. Med. J. 2019, 112, 238–243. [Google Scholar] [CrossRef]
- Ravindran, V.; Logan, I.; Bourke, B.E. Medical vs surgical treatment for the native joint in septic arthritis: A 6-year, single UK academic centre experience. Rheumatology 2009, 48, 1320–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suenaga, M.; Yokoyama, Y.; Fujii, T.; Yamada, S.; Yamaguchi, J.; Hayashi, M.; Asahara, T.; Nagino, M.; Kodera, Y. Impact of Preoperative Occult-Bacterial Translocation on Surgical Site Infection in Patients Undergoing Pancreatoduodenectomy. J. Am. Coll. Surg. 2020, 232, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.L.; Musher, D.M.; Bloom, K.; Gathe, J.; Rice, L.; Sugarman, B.; Young, E.J. Manifestations of sepsis. Arch. Intern. Med. 1987, 147, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, M.; Jalali, A.; Abdollahi, A.; Anzali, B.C. A Devastating Complication of Central Venous Catheter Insertion. J. Acute Med. 2019, 9, 69–72. [Google Scholar] [PubMed]
- Weatherall, S.L.; Chambers, A.B.; Mermel, L.A. Do Bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg. Med. 2020, 20, 2. [Google Scholar] [CrossRef] [Green Version]
- Pergola, P.E.; Habiba, N.M.; Johnson, J.M. Body temperature regulation during hemodialysis in long-term patients: Is it time to change dialysate temperature prescription? Am. J. Kidney Dis. 2004, 44, 155–165. [Google Scholar] [CrossRef]
- Dubost, J.-J.; Couderc, M.; Tatar, Z.; Tournadre, A.; Lopez, J.; Mathieu, S.; Soubrier, M. Three-decade trends in the distribution of organisms causing septic arthritis in native joints: Single-center study of 374 cases. Jt. Bone Spine 2014, 81, 438–440. [Google Scholar] [CrossRef]
- Cipriano, A.; Santos, F.V.; Dias, R.; Carvalho, A.; Reis, E.; Pereira, C.; Santos, A.C.; Sousa, R.; Abreu, M.A. Adult Native Joint Septic Arthritis: A Nine-Year Retrospective Analysis in a Portuguese University Hospital. Acta Med. Port. 2021, 34, 826. [Google Scholar] [CrossRef]
- Mathews, M.; Shen, F.-H.; Lindner, A.; Sherrard, D.J. Septic Arthritis in Hemodialyzed Patients. Nephron Exp. Nephrol. 1980, 25, 87–91. [Google Scholar] [CrossRef]
- Suzuki, M.; Satoh, N.; Nakamura, M.; Horita, S.; Seki, G.; Moriya, K. Bacteremia in hemodialysis patients. World J. Nephrol. 2016, 5, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.R.; Souli, M.; Ruffin, F.; Park, L.P.; Dagher, M.; Eichenberger, E.M.; Maskarinec, S.A.; Thaden, J.T.; Mohnasky, M.; Wyatt, C.M.; et al. Staphylococcus aureus Bacteremia Among Patients Receiving Maintenance Hemodialysis: Trends in Clinical Characteristics and Outcomes. Am. J. Kidney Dis. 2022, 79, 393–403.e1. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Genet. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.; Sader, H.S.; Ribeiro, J.; Zoccoli, C.; Barth, A.; Pignatari, A.C.C. Antimicrobial susceptibility of gram-positive bacteria isolated in brazilian hospitals participating in the SENTRY Program (2005–2008). Braz. J. Infect. Dis. 2009, 13, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udo, E.E. Community-acquired methicillin-resistant Staphylococcus aureus: The new face of an old foe? Med. Princ. Pract. 2013, 22 (Suppl. 1), 20–29. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Zhu, X.; Petersen, M.; Patel, E.U.; Cosgrove, S.E.; Tobian, A.A.R. Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Hospitalizations: National Inpatient Sample, 2016–2019. Open Forum Infect. Dis. 2021, 9, ofab585. [Google Scholar] [CrossRef]
- Elzorkany, K.; Elbrolosy, A.M.; Salem, E.H. Methicillin-Resistant Staphylococcus aureus Carriage in Hemodialysis Vicinity: Prevalence and Decolonization Approach. Indian J. Nephrol. 2019, 29, 282–287. [Google Scholar] [CrossRef]
- Fangtham, M.; Baer, A.N. Methicillin-Resistant Staphylococcus aureus Arthritis in Adults: Case Report and Review of the Literature. Semin. Arthritis Rheum. 2012, 41, 604–610. [Google Scholar] [CrossRef]
- Ferrand, J.; El Samad, Y.; Brunschweiler, B.; Grados, F.; Dehamchia-Rehailia, N.; Séjourne, A.; Schmit, J.-L.; Gabrion, A.; Fardellone, P.; Paccou, J. Morbimortality in adult patients with septic arthritis: A three-year hospital-based study. BMC Infect. Dis. 2016, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Egea, M.-C.; Blanco, A.; Fernández-Roblas, R.; Gadea, I.; García-Cañete, J.; Sandoval, E.; Valdazo, M.; Esteban, J. Clinical and microbiological characteristics of patients with septic arthritis: A hospital-based study. J. Orthop. 2014, 11, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.Y.; Pannikath, D.; Nugent, K. A retrospective study of septic arthritis in a tertiary hospital in West Texas with high rates of methicillin-resistant Staphylococcus aureus infection. Rheumatol. Int. 2015, 35, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
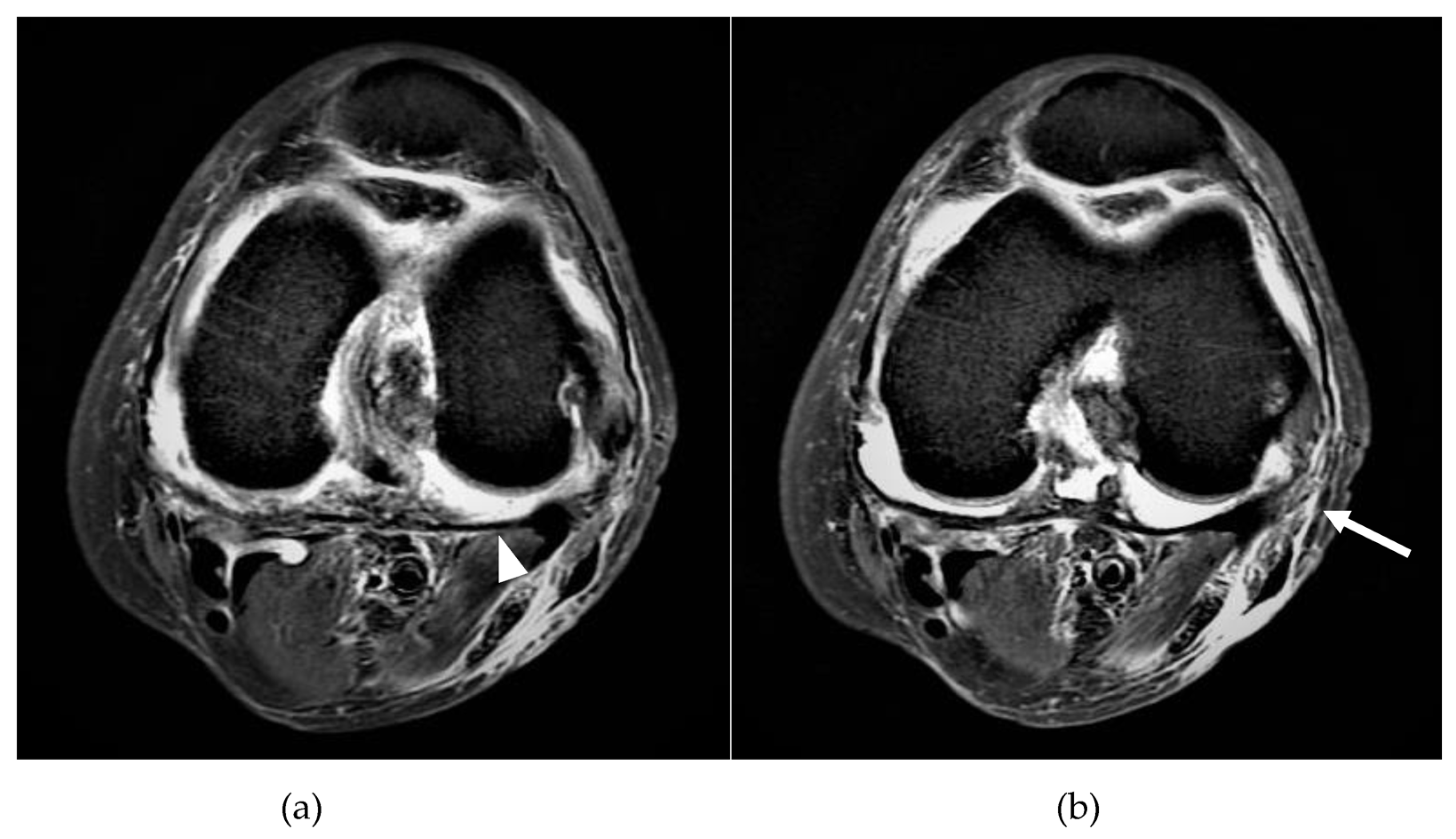
| Variable | Total (n = 52) | Tunneled Cuffed Catheter (n = 8) | Non-Tunneled Cuffed Catheter (n = 44) | p Value |
|---|---|---|---|---|
| Age (year) | 60.6 ± 12.9 | 61.9 ± 13.6 | 60.4 ± 13.0 | 0.768 |
| Male | 24 (46.2) | 3 (37.5) | 21 (47.7) | 0.711 |
| Systolic blood pressure (mmHg) | 137.4 ± 40.8 | 117 ± 38.6 | 141.5 ± 40.4 | 0.122 |
| Diastolic blood pressure (mmHg) | 74.2 ± 22.6 | 69.3 ± 15.2 | 75.2 ± 23.9 | 0.501 |
| Heart rate (beats/min) | 98.5 ± 15.3 | 102.5 ± 18.7 | 97.7 ± 14.7 | 0.422 |
| Fever † | 15 (28.8) | 4 (50.0) | 11 (25.0) | 0.207 |
| Initial presentations | ||||
| Joint pain | 52 (100) | 8 (100) | 44 (100) | |
| Joint swelling | 22 (42.3) | 1 (12.5) | 21 (47.7) | 0.118 |
| Joint redness | 16 (30.8) | 1 (12.5) | 15 (34.1) | 0.409 |
| Limited range of motion | 22 (42.3) | 1 (12.5) | 21 (47.7) | 0.118 |
| Joint involved | 0.823 | |||
| Hip | 21 (40.4) | 4 (50.0) | 17 (38.6) | |
| Knee | 18 (34.6) | 2 (25.0) | 16 (36.4) | |
| Shoulder | 9 (17.3) | 1 (12.5) | 8 (18.2) | |
| Ankle | 3 (5.8) | 1 (12.5) | 2 (4.5) | |
| Elbow | 1 (1.9) | 0 (0.0) | 1 (2.3) | |
| Comorbidities | ||||
| Hypertension | 36 (69.2) | 3 (37.5) | 33 (75.0) | 0.089 |
| Diabetes mellitus | 29 (55.8) | 2 (25.0) | 27 (61.4) | 0.118 |
| CAD | 13 (25.0) | 1 (12.5) | 12 (27.3) | 0.662 |
| HFpEF or HFrEF | 13 (25.0) | 2 (25.0) | 11 (25.0) | 1.000 |
| Malignancy | 3 (5.8) | 0 (0.0) | 3 (6.8) | 1.000 |
| Gout | 4 (7.7) | 0 (0.0) | 4 (9.1) | 1.000 |
| Rheumatic arthritis | 2 (3.8) | 0 (0.0) | 2 (4.5) | 1.000 |
| Osteoarthritis | 5 (9.6) | 0 (0.0) | 5 (11.4) | 1.000 |
| Prosthetic joint | 10 (19.2) | 0 (0.0) | 10 (22.7) | 0.328 |
| Liver cirrhosis | 11 (21.2) | 2 (25.0) | 9 (20.5) | 1.000 |
| Steroid use | 2 (3.8) | 0 (0.0) | 2 (4.5) | 1.000 |
| Blood test † | ||||
| White blood count (103/µL) n = 48 | 12.2 ± 5.8 | 14.6 ± 7.8 | 11.7 ± 5.3 | 0.168 |
| Leukocytosis ‡ | 24 (50.0) | 6 (75.0) | 18 (45.0) | 0.245 |
| Hemoglobin (g/dL) n = 48 | 9.5 ± 2.1 | 9.9 ± 2.2 | 9.4 ± 2.1 | 0.552 |
| Platelet (103/µL) n = 46 | 236.7 ± 120.4 | 264.6 ± 141.2 | 230.8 ± 116.9 | 0.477 |
| INR n = 18 | 1.2 ± 0.4 | 1.2 ± 0.0 | 1.2 ± 0.4 | 0.956 |
| C-reactive protein (mg/L) n = 41 | 160.5 ± 98.6 | 194.8 ± 100.0 | 152.2 ± 97.9 | 0.278 |
| Positive culture | 12 (23.1) | 5 (62.5) | 7 (15.9) | 0.004 * |
| Synovial fluid analyses § | ||||
| White blood count (103/mm3) | 69.2 ± 99.1 | 39.6 ± 43.6 | 79.1 ± 111.2 | 0.454 |
| PMN (%) | 85.2 ± 16.5 | 92.2 ± 5.4 | 83.2 ± 18.1 | 0.292 |
| Positive culture | 21 (65.6) | 2 (40.0) | 19 (70.4) | 0.189 |
| Microorganism | n | % |
|---|---|---|
| Blood cultures (n = 12) | ||
| Methicillin-resistant Staphylococcus aureus | 7 | 58.3 |
| Methicillin-susceptible Staphylococcus aureus | 2 | 16.7 |
| Group B Streptococcus | 1 | 8.3 |
| Salmonella group D | 1 | 8.3 |
| Escherichia coli | 1 | 8.3 |
| Synovial fluid cultures (n = 21) | ||
| Methicillin-resistant Staphylococcus aureus | 10 | 47.6 |
| Oxacillin-susceptible Staphylococcus aureus | 2 | 9.5 |
| Oxacillin-susceptible Staphylococcus lugdunensis | 1 | 4.8 |
| Staphylococcus hominis | 1 | 4.8 |
| Group B Streptococcus | 2 | 9.5 |
| Salmonella group D | 1 | 4.8 |
| Escherichia coli | 1 | 4.8 |
| Vancomycin-resistant Enterococcus | 1 | 4.8 |
| Enterococcus faecalis | 1 | 4.8 |
| Candida famata | 1 | 4.8 |
| Case# | Age | Gender | Past Medical History | Joint | Dialysis Route | Surgical Debridement | Antibiotic Therapy | Length of Stay (Days) | In-Hospital Mortality |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | Female | HFpEF, HTN | Knee | Tunneled cuffed catheter | Y | D1-D7: Oxacillin, Ceftriaxone D8-D20: Ceftazidime, Vancomycin D21-D26: Imipenem/Cilastatin, Teicoplanin, Micafungin | 28 | Y |
| 2 | 67 | Male | DM, HTN, liver cirrhosis | Hip | AVG | N | D1-D13: Oxacillin | 12 | Y |
| 3 | 85 | Male | DM | Ankle | Tunneled cuffed catheter | N | D1-D5: Ertapenem, Vancomycin D6-D15: Daptomycin | 28 | Y |
| 4 | 60 | Male | DM, HTN, osteoarthritis | Hip | AVG | Y | D1-D7: Vancomycin | 13 | N |
| 5 | 73 | Male | HTN, HFPEF, DM, CAD | Hip | AVF | Y | D1-D2: Oxacillin D3-D4: Gentamycin D5-D12: Teicoplanin, Ceftriaxone D13-D30: Cotrimoxazole | 12 | N |
| Variable | Total n = 52 | Tunneled Cuffed Catheter (n = 8) | Non-Tunneled Cuffed Catheter (n = 44) | p Value |
|---|---|---|---|---|
| Treatment | ||||
| Surgical debridement | 26 (50) | 4 (50.0) | 22 (50.0) | 1.000 |
| Duration of antibiotic treatment (days) † | 29.6 ± 26.8 | 57.1 ± 45.0 | 24.9 ± 19.7 | 0.108 |
| In-hospital outcomes | ||||
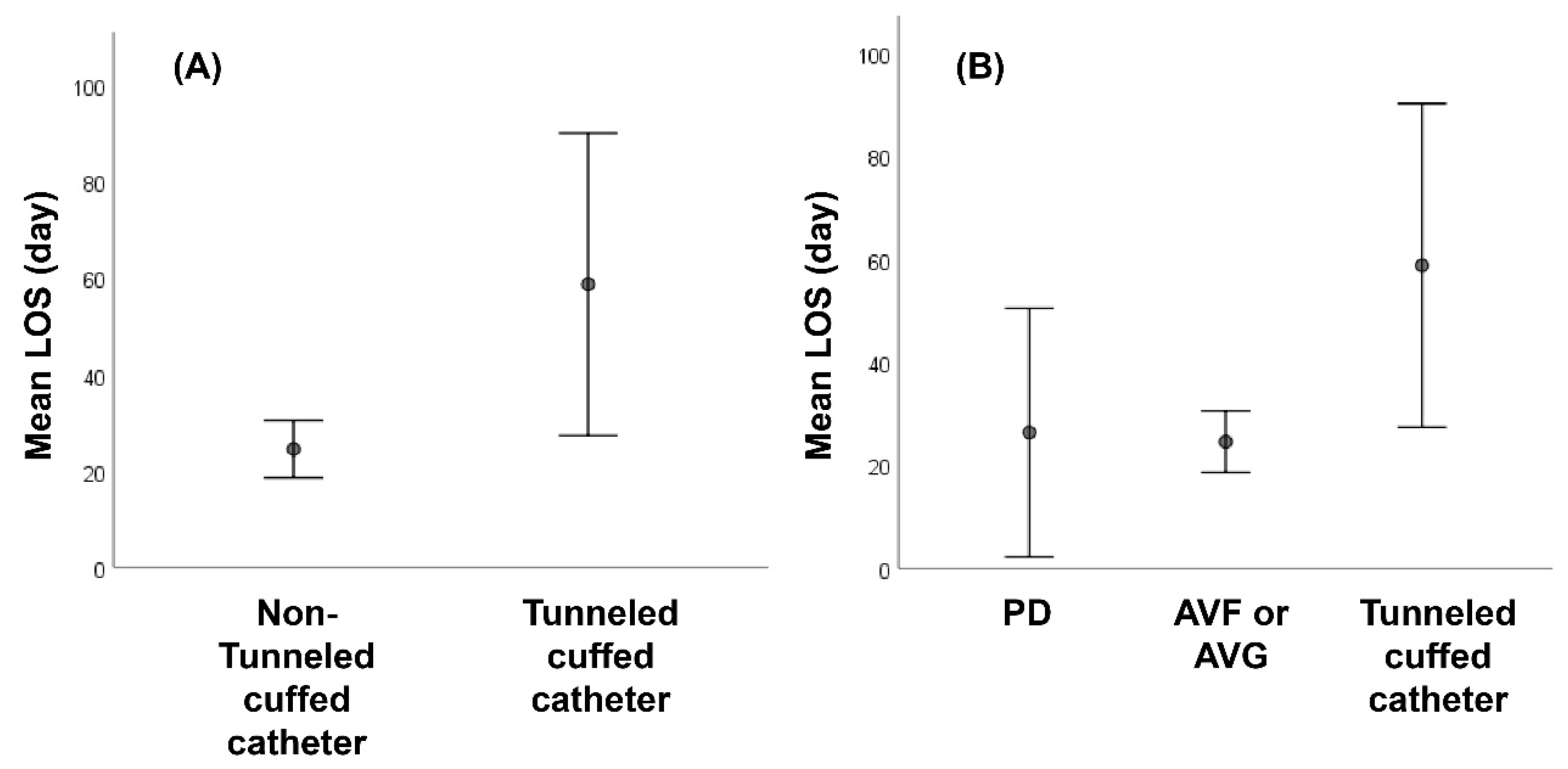
| Length of stay | 29.9 ± 25.1 | 58.8 ± 37.6 | 24.7 ± 18.3 | 0.038 * |
| Intensive care unit admission | 5 (9.6) | 2 (25.0) | 3 (6.8) | 0.164 |
| Death | 3 (5.8) | 2 (25.0) | 1 (2.3) | 0.011 * |
| 1-year complications | ||||
| Recurrence | 8 (15.4) | 1 (12.5) | 7 (15.9) | 1.000 |
| Joint replacement | 15 (28.8) | 1 (12.5) | 14 (31.8) | 0.412 |
| Amputation | 2 (3.8) | 0 (0.0) | 2 (4.5) | 1.000 |
| Osteomyelitis | 1 (1.9) | 0 (0.0) | 1 (2.3) | 1.000 |
| Abscess formation | 1 (1.9) | 0 (0.0) | 1 (2.3) | 1.000 |
| Univariate | ||
|---|---|---|
| Coef (95%CI) | p Value | |
| Age | −0.09 (−0.29, 0.14) | 0.408 |
| Male | −0.09 (−0.37, 0.20) | 0.536 |
| Fever | 0.07 (−0.22, 0.35) | 0.644 |
| Joint redness | −0.08 (−0.36, 0.21) | 0.592 |
| Tunneled cuffed catheter | 0.49 (0.25, 0.74) | <0.001 * |
| Diabetes mellitus | −0.26 (−0.53, 0.02) | 0.068 |
| Liver cirrhosis | 0.12 (−0.16, 0.40) | 0.385 |
| Congestive heart failure | 0.09 (−0.19, 0.37) | 0.527 |
| Gout | −0.02 (−0.30, 0.27) | 0.893 |
| Rheumatic arthritis | −0.14 (−0.42, 0.14) | 0.323 |
| Osteoarthritis | −0.06 (−0.35, 0.22) | 0.664 |
| Prosthetic joint | −0.13 (−0.41, 0.15) | 0.360 |
| Surgical debridement | 0.03 (−0.25, 0.32) | 0.824 |
| Leukocytosis | 0.25 (−0.05, 0.51) | 0.098 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Age | 1.00 (0.95,1.05) | 0.850 | ||
| Male | 0.79 (0.21,2.91) | 0.723 | ||
| Fever | 5.60 (1.40,22.36) | 0.015 | 4.91 (1.10,21.83) | 0.037 * |
| Joint redness | 1.88 (0.49,7.20) | 0.355 | ||
| Tunneled cuffed catheter | 8.81 (1.70,45.58) | 0.009 | 7.60 (1.31,44.02) | 0.024 * |
| Diabetes mellitus | 0.74 (0.20,2.70) | 0.647 | ||
| Liver cirrhosis | 0.69 (0.13,3.73) | 0.666 | ||
| Congestive heart failure | 1.00 (0.23,4.44) | 1.000 | ||
| Gout | 0.64 (0.07,6.05) | 0.694 | ||
| Rheumatic arthritis | 3.55 (0.21,61.38) | 0.384 | ||
| Osteoarthritis | 2.47 (0.36,16.84) | 0.357 | ||
| Prosthetic joint | 0.80 (0.15,4.40) | 0.797 | ||
| Leukocytosis | 4.20 (0.97,18.18) | 0.055 | ||
| Univariate | ||
|---|---|---|
| OR (95%CI) | p Value | |
| Age | 1.04 (0.94,1.16) | 0.403 |
| Male | 2.46 (0.21,28.89) | 0.475 |
| Fever | 5.54 (0.46,66.32) | 0.177 |
| Joint redness | 1.10 (0.09,13.09) | 0.940 |
| Tunneled cuffed catheter | 14.33 (1.12,183.18) | 0.041 * |
| Diabetes mellitus | 1.63 (0.14,19.18) | 0.698 |
| Liver cirrhosis | 1.95 (0.16,23.73) | 0.600 |
| Congestive heart failure | 1.54 (0.13,18.54) | 0.733 |
| Gout | 5.63 (0.41,76.43) | 0.194 |
| Rheumatic arthritis | 7.68 (0.53,110.65) | 0.135 |
| Osteoarthritis | 3.58 (0.28,45.80) | 0.326 |
| Prosthetic joint | 2.22 (0.18,27.26) | 0.532 |
| Surgical debridement | 0.48 (0.04,5.65) | 0.559 |
| Leukocytosis | 2.09 (0.18,1.00) | 0.502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, H.-T.; Liau, S.-K.; Niu, K.-Y.; Hsiao, C.-H.; Yeh, C.-C.; Lu, J.-X.; Ng, C.-J.; Yen, C.-C. Clinical Characteristics and In-Hospital Outcomes in Dialysis Patients with Septic Arthritis. Medicina 2022, 58, 401. https://doi.org/10.3390/medicina58030401
Yeh H-T, Liau S-K, Niu K-Y, Hsiao C-H, Yeh C-C, Lu J-X, Ng C-J, Yen C-C. Clinical Characteristics and In-Hospital Outcomes in Dialysis Patients with Septic Arthritis. Medicina. 2022; 58(3):401. https://doi.org/10.3390/medicina58030401
Chicago/Turabian StyleYeh, Hsin-Tzu, Shuh-Kuan Liau, Kuang-Yu Niu, Chien-Han Hsiao, Chung-Cheng Yeh, Jian-Xun Lu, Chip-Jin Ng, and Chieh-Ching Yen. 2022. "Clinical Characteristics and In-Hospital Outcomes in Dialysis Patients with Septic Arthritis" Medicina 58, no. 3: 401. https://doi.org/10.3390/medicina58030401
APA StyleYeh, H.-T., Liau, S.-K., Niu, K.-Y., Hsiao, C.-H., Yeh, C.-C., Lu, J.-X., Ng, C.-J., & Yen, C.-C. (2022). Clinical Characteristics and In-Hospital Outcomes in Dialysis Patients with Septic Arthritis. Medicina, 58(3), 401. https://doi.org/10.3390/medicina58030401






