Strategies to Reduce Post-Hemorrhoidectomy Pain: A Systematic Review
Abstract
:1. Introduction
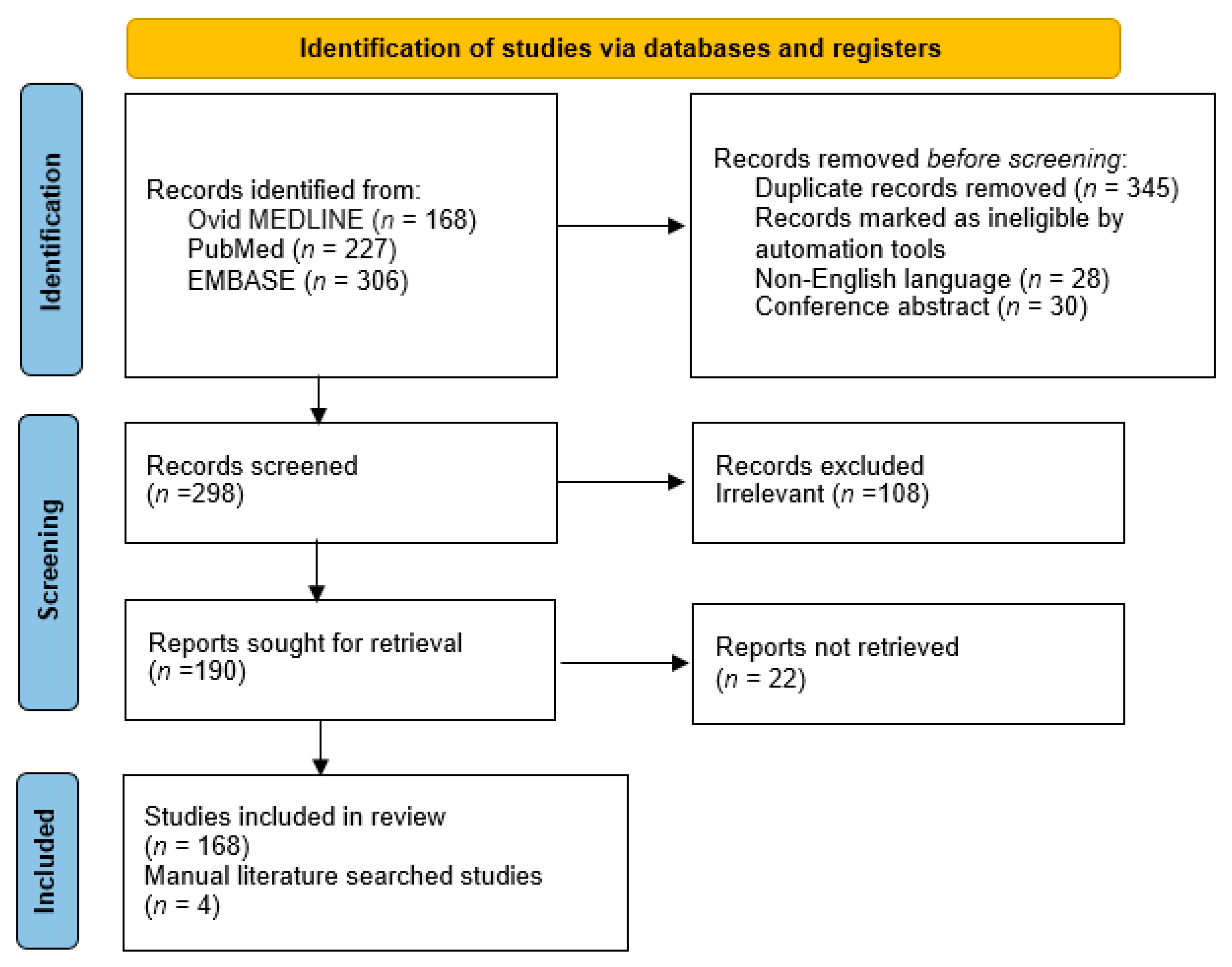
2. Materials and Methods
3. Results
3.1. Anesthetic Methods
3.2. Surgical Techniques
3.2.1. Closed versus Open Technique
3.2.2. Scissors, Diathermy or Other Instruments
3.2.3. Hemorrhoidectomy Combined with Lateral Internal Anal Sphincterotomy
3.3. Intraoperative Adjuncts
3.3.1. Injection of Botulinum Toxin
3.3.2. Intradermal Injection of Methylene Blue
3.3.3. Intrasphincteric Injection of Ketorolac
3.4. Postoperative Interventions
3.4.1. Topical Calcium Channel Blockers and Glyceryl Trinitrate
3.4.2. Topical Anesthetic Cream
3.4.3. Other Topical Medications
3.4.4. Oral Metronidazole
3.4.5. Flavonoids
3.4.6. Laxatives
3.4.7. Mesoglycan
3.4.8. Warm Sitz Bath
3.4.9. Avoidance of Spicy Foods
3.4.10. Transcutaneous Electrical Nerve Stimulation and Acupuncture
3.4.11. Patient’s Checklist for Analgesic Consumption
3.5. Limitations
3.6. Areas for Future Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, R.L.; Abcarian, H.; Davis, F.G.; Persky, V. Prevalence of benign anorectal disease in a randomly selected population. Dis. Colon Rectum 1995, 38, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V. Treatment of hemorrhoids: A coloproctologist’s view. World J. Gastroenterol. 2015, 21, 9245–9252. [Google Scholar] [CrossRef]
- Godeberge, P.; Sheikh, P.; Zagriadskiĭ, E.; Lohsiriwat, V.; Montaño, A.J.; Košorok, P.; De Schepper, H. Hemorrhoidal disease and chronic venous insufficiency: Concomitance or coincidence; results of the CHORUS study (Chronic venous and HemORrhoidal diseases evalUation and Scientific research). J. Gastroenterol. Hepatol. 2019, 35, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Lohsiriwat, V. Approach to hemorrhoids. Curr. Gastroenterol. Rep. 2013, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Picciariello, A.; Tsarkov, P.V.; Papagni, V.; Efetov, S.; Markaryan, D.R.; Tulina, I.; Altomare, D.F. Classifications and Clinical Assessment of Haemorrhoids: The Proctologist’s Corner. Rev. Recent Clin. Trials 2021, 16, 10–16. [Google Scholar] [CrossRef]
- Simillis, C.; Thoukididou, S.N.; Slesser, A.A.P.; Rasheed, S.; Tan, E.; Tekkis, P.P. Systematic review and network meta-analysis comparing clinical outcomes and effectiveness of surgical treatments for haemorrhoids. Br. J. Surg. 2015, 102, 1603–1618. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V. Hemorrhoids: From basic pathophysiology to clinical management. World J. Gastroenterol. 2012, 18, 2009–2017. [Google Scholar] [CrossRef]
- Gallo, G.; Martellucci, J.; Sturiale, A.; Clerico, G.; Milito, G.; Marino, F.; Cocorullo, G.; Giordano, P.; Mistrangelo, M.; Trompetto, M. Consensus statement of the Italian society of colorectal surgery (SICCR): Management and treatment of hemorrhoidal disease. Tech. Coloproctol. 2020, 24, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Lohsiriwat, D.; Lohsiriwat, V. Outpatient hemorrhoidectomy under perianal anesthetics infiltration. J. Med. Assoc. Thail. 2005, 88, 1821–1824. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Boonnithi, W.; Lohsiriwat, V. Towards ‘zero’ postoperative pain following common anal operations by effective anesthesia and non-opioid multimodal analgesia. J. Med. Assoc. Thail. 2020, 103, 103–108. [Google Scholar]
- Castellví, J.; Sueiras, A.; Espinosa, J.; Vallet, J.; Gil, V.; Pi, F. Ligasure™ versus diathermy hemorrhoidectomy under spinal anesthesia or pudendal block with ropivacaine: A randomized prospective clinical study with 1-year follow-up. Int. J. Colorectal Dis. 2009, 24, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Hosseinpour, M.; Jalalvand, F.; Afshar, M.; Moosavi, S.S.; Behdad, S. Ischiorectal Block with Bupivacaine for Post Hemorrhoidectomy Pain. Korean J. Pain 2012, 25, 89–93. [Google Scholar] [CrossRef]
- Kim, B.G.; Kang, H. The Effect of Preemptive Perianal Ropivacaine and Ropivacaine with Dexmedetomidine on Pain after Hemorrhoidectomy: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Indian J. Surg. 2012, 76, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Brunat, G.; Pouzeratte, Y.; Mann, C.; Didelot, J.-M.; Rochon, J.-C.; Eledjam, J.-J. Posterior Perineal Block with Ropivacaine 0.75% for Pain Control during and after Hemorrhoidectomy. Reg. Anesth. Pain Med. 2003, 28, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Gorfine, S.R.; Onel, E.; Patou, G.; Krivokapic, Z.V. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: A multicenter, randomized, double-blind, placebo-controlled trial. Dis. Colon Rectum 2011, 54, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, H.; Masuda, J.; Fukushima, K.; Iwao, Y.; Suzuki, K.; Matsushima, M. Wound infiltration with lidocaine prolongs postoperative analgesia after haemorrhoidectomy with spinal anaesthesia. Can. J. Anaesth. 1996, 43, 914–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, H.; Moreira, J.P.; Isaac, R.R.; Alves-Neto, O.; Moreira, T.A.; Vieira, T.H.; Brasil, A.M. Morphine Spinal Block Anesthesia in Patients Who Undergo an Open Hemorrhoidectomy: A Prospective Analysis of Pain Control and Postoperative Complications. Ann. Coloproctol. 2014, 30, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Lee, Y. Intrathecal midazolam increases the analgesic effects of spinal blockade with bupivacaine in patients undergoing haemorrhoidectomy. Br. J. Anaesth. 2001, 86, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Mohamedahmed, A.Y.Y.; Stonelake, S.; Mohammed, S.S.S.; Zaman, S.; Ahmed, H.; Albarade, M.; Hajibandeh, S. Haemorrhoidectomy under local anaesthesia versus spinal anaesthesia: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2020, 35, 2171–2183. [Google Scholar] [CrossRef]
- Xia, W.; MacFater, H.S.; MacFater, W.S.; Otutaha, B.F.; Barazanchi, A.W.H.; Sammour, T.; Hill, A.G. Local Anaesthesia Alone Versus Regional or General Anaesthesia in Excisional Haemorrhoidectomy: A Systematic Review and Meta-Analysis. World J. Surg. 2020, 44, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Reis Neto, J.A.; Quilici, F.A.; Cordeiro, F.; Reis Junior, J.A. Open versus semi-open hemorrhoidectomy: A random trial. Int. Surg. 1992, 77, 84–90. [Google Scholar] [PubMed]
- Ho, Y.H.; Seow-Choen, F.; Tan, M.; Leong, A.F. Randomized controlled trial of open and closed haemorrhoidectomy. Br. J. Surg. 1997, 84, 1729–1730. [Google Scholar] [PubMed]
- Arbman, G.; Krook, H.; Haapaniemi, S. Closed vs. open hemorrhoidectomy—Is there any difference? Dis. Colon Rectum 2000, 43, 31–34. [Google Scholar] [CrossRef]
- You, S.Y.; Kim, S.H.; Chung, C.S.; Lee, D.K. Open vs. closed hemorrhoidectomy. Dis. Colon Rectum 2005, 48, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Perez, F.; Miranda, E.; Serrano, P.; Candela, F.; Lacueva, J.; Hernández, H.; Calpena, R. Open versus closed day-case haemorrhoidectomy: Is there any difference? Results of a prospective randomised study. Int. J. Colorectal Dis. 2004, 19, 370–373. [Google Scholar] [PubMed]
- Pokharel, N.; Chhetri, R.K.; Malla, B.; Joshi, H.N.; Shrestha, R.K. Haemorrhoidectomy: Ferguson’s (closed) vs. Milligan Morgan’s technique (open). Nepal Med. Coll. J. 2009, 11, 136–137. [Google Scholar] [PubMed]
- Bhatti, M.I.; Sajid, M.S.; Baig, M.K. Milligan–Morgan (Open) Versus Ferguson Haemorrhoidectomy (Closed): A Systematic Review and Meta-Analysis of Published Randomized, Controlled Trials. World J. Surg. 2016, 40, 1509–1519. [Google Scholar] [CrossRef]
- Andrews, B.T.; Layer, G.T.; Jackson, B.T.; Nicholls, R.J. Randomized trial comparing diathermy hemorrhoidectomy with the scissor dissection Milligan-Morgan operation. Dis. Colon Rectum 1993, 36, 580–583. [Google Scholar] [CrossRef]
- Ibrahim, S.; Tsang, C.; Lee, Y.L.; Eu, K.W.; Seow-Choen, F. Prospective, randomized trial comparing pain and complications between diathermy and scissors for closed hemorrhoidectomy. Dis. Colon Rectum 1998, 41, 1418–1420. [Google Scholar] [CrossRef]
- Mastakov, M.Y.; Buettner, P.G.; Ho, Y.-H. Updated meta-analysis of randomized controlled trials comparing conventional excisional haemorrhoidectomy with LigaSure for haemorrhoids. Tech. Coloproctol. 2008, 12, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Milito, G.; Cadeddu, F.; Muzi, M.G.; Nigro, C.; Farinon, A.M. Haemorrhoidectomy with Ligasure vs conventional excisional techniques: Meta-analysis of randomized controlled trials. Colorectal Dis. 2010, 12, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Nienhuijs, S.; de Hingh, I. Pain after conventional versus Ligasure haemorrhoidectomy. A meta-analysis. Int. J. Surg. 2010, 8, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushaya, C.D.; Caleo, P.J.; Bartlett, L.; Buettner, P.G.; Ho, Y.H. Harmonic scalpel compared with conventional excisional haemorrhoidectomy: A meta-analysis of randomized controlled trials. Tech. Coloproctol. 2014, 18, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Youssef, M.; Elfeki, H.; Thabet, W.; El-Hamed, T.M.A.; Farid, M. Literature review of the role of lateral internal sphincterotomy (LIS) when combined with excisional hemorrhoidectomy. Int. J. Colorectal Dis. 2016, 31, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavalu, S.; Rajkumar, S. The Role of Lateral Internal Sphincterotomy in Haemorrhoidectomy: A Study in a Tertiary Care Center. Cureus 2021, 13, e15630. [Google Scholar] [CrossRef]
- Davies, J.; Duffy, D.; Boyt, N.; Aghahoseini, A.; Alexander, D.; Leveson, S. Botulinum toxin (botox) reduces pain after hemorrhoidectomy: Results of a double-blind, randomized study. Dis. Colon Rectum 2003, 46, 1097–1102. [Google Scholar] [CrossRef]
- Patti, R.; Almasio, P.L.; Muggeo, V.; Buscemi, S.; Arcara, M.; Matranga, S.; Di Vita, G. Improvement of Wound Healing After Hemorrhoidectomy: A Double-Blind, Randomized Study of Botulinum Toxin Injection. Dis. Colon Rectum 2005, 48, 2173–2179. [Google Scholar] [CrossRef]
- Singh, B.; Box, B.; Lindsey, I.; George, B.; Mortensen, N.; Cunningham, C. Botulinum toxin reduces anal spasm but has no effect on pain after haemorrhoidectomy. Colorectal Dis. 2009, 11, 203–207. [Google Scholar] [CrossRef]
- Patti, R.; Almasio, P.L.; Arcara, M.; Sammartano, S.; Romano, P.; Fede, C.; Di Vita, G. Botulinum Toxin vs. Topical Glyceryl Trinitrate Ointment for Pain Control in Patients Undergoing Hemorrhoidectomy: A Randomized Trial. Dis. Colon Rectum 2006, 49, 1741–1748. [Google Scholar] [CrossRef]
- Sim, H.-L.; Tan, K.-Y. Randomized single-blind clinical trial of intradermal methylene blue on pain reduction after open diathermy haemorrhoidectomy. Colorectal Dis. 2014, 16, O283–O287. [Google Scholar] [CrossRef]
- O’Donovan, S.; Ferrara, A.; Larach, S.; Williamson, P. Intraoperative use of toradol® facilitates outpatient hemorrhoidectomy. Dis. Colon Rectum 1994, 37, 793–799. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Chen, C.-Y.; Chen, R.-J.; Kang, Y.-N.; Wei, P.-L. Topical diltiazem ointment in post-hemorrhoidectomy pain relief: A meta-analysis of randomized controlled trials. Asian J. Surg. 2017, 41, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.S.; Eu, K.W.; Heah, S.M.; Seow-Choen, F.; Chan, Y.W. Randomized clinical trial of haemorrhoidectomy under a mixture of local anaesthesia versus general anaesthesia. Br. J. Surg. 2000, 87, 410–413. [Google Scholar] [CrossRef]
- Rahimi, M.; Kazemeini, A.R.; Pourtabatabaei, N.; Honarmand, A.R. Comparison of topical anesthetic cream (EMLA) and diclofenac suppository for pain relief after hemorrhoidectomy: A randomized clinical trial. Surg. Today 2012, 42, 1201–1205. [Google Scholar] [CrossRef]
- Shiau, J.M.; Su, H.P.; Chen, H.S.; Hung, K.C.; Lin, S.E.; Tseng, C.C. Use of a topical anesthetic cream (EMLA) to reduce pain after hemorrhoidectomy. Reg. Anesth. Pain Med. 2008, 33, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.J.R.; Cornille, J.B.; Pathak, S.; Charters, P.; Daniels, I.; Smart, N.J. Systematic review and meta-analysis of the role of metronidazole in post-haemorrhoidectomy pain relief. Colorectal Dis. 2017, 19, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Manning, J.P.R.; Barazanchi, A.W.H.; Su’A, B.; Hill, A.G. Metronidazole following excisional haemorrhoidectomy: A systematic review and meta-analysis. ANZ J. Surg. 2018, 88, 408–414. [Google Scholar] [CrossRef]
- Nagashima, R. Mechanisms of action of sucralfate. J. Clin. Gastroenterol. 1981, 3, 117–127. [Google Scholar]
- Ala, S.; Saeedi, M.; Eshghi, F.; Rafati, M.; Hejazi, V.; Hadianamrei, R. Efficacy of 10% sucralfate ointment in the reduction of acute postoperative pain after open hemorrhoidectomy: A prospective, double-blind, randomized, placebo-controlled trial. World J. Surg. 2013, 37, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.J.; Heda, P.S.; Kalaskar, S.; Tamaskar, V.P. Topical Sucralfate Decreases Pain After Hemorrhoidectomy and Improves Healing: A Randomized, Blinded, Controlled Study. Dis. Colon Rectum 2008, 51, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Vejdan, A.K.; Khosravi, M.; Amirian, Z.; Daneshmand, M.; Babak, B.; Samira, K.; Azin, S.; Kosar, S.; Razie, K. Evaluation of the efficacy of topical sucralfate on healing haemorrhoidectomy incision wounds and reducing pain severity: A randomised clinical trial. Int. Wound J. 2020, 17, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H. Evidence-based reviewof methods used to reduce pain after excisional hemorrhoidectomy. J. Coloproctol. 2019, 39, 81–89. [Google Scholar]
- Ala, S.; Alvandipour, M.; Saeedi, M.; Mansourifar, M.; Monajati, M.; Shiva, A. Effect of Topical Baclofen 5% on Post-Hemorrhoidectomy Pain: Randomized Double Blind Placebo-Controlled Clinical Trial. J. Gastrointest. Surg. 2019, 24, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ala, S.; Eshghi, F.; Enayatifard, R.; Fazel, P.; Rezaei, B.; Hadianamrei, R. Efficacy of Cholestyramine Ointment in Reduction of Postoperative Pain and Pain during Defecation after Open Hemorrhoidectomy: Results of a Prospective, Single-center, Randomized, Double-blind, Placebo-controlled Trial. World J. Surg. 2012, 37, 657–662. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Eshghi, F.; Hosseinimehr, S.J.; Rahmani, N.; Khademloo, M.; Norozi, M.S.; Hojati, O. Effects of Aloe vera Cream on Posthemorrhoidectomy Pain and Wound Healing: Results of a Randomized, Blind, Placebo-Control Study. J. Altern. Complement. Med. 2010, 16, 647–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanlıöz, M.; Ekici, U. The Effects of Using Liposomal Bupivacaine and Aloe Vera Cream after Haemorrhoidectomy on Postoperative Pain, Need for Analgesics, Hospitalisation Period and Return to Work and Social Life. Turk. J. Colorectal Dis. 2020, 30, 184–190. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Duran, M.; Alias, D.; Manso, B.; Moreno, A.; Nevado, C.; Lopez, S.; Jimenez, M.; Garia-Olmo, D. Reduction of postoperative pain and improvement of patients’ comfort after Milligan-Morgan hemorrhoidectomy using topical application of vitamin E ointment. Int. J. Colorectal Dis. 2015, 31, 1371–1372. [Google Scholar] [CrossRef]
- Ho, Y.H.; Seow-Choen, F.; Low, J.Y.; Tan, M.; Leong, A.P. Randomized controlled trial of trimebutine (anal sphincter relaxant) for pain after haemorrhoidectomy. Br. J. Surg. 1997, 84, 377–379. [Google Scholar]
- Di Re, A.; Toh, J.W.T.; Iredell, J.; Ctercteko, G. Metronidazole in the Management of Post-Open Haemorrhoidectomy Pain: Systematic Review. Ann. Coloproctol. 2020, 36, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Wilkie, B.D.; Chandra, R.; Chua, J.; Lam, D.C.S.; Paratz, E.D.; An, V.; Keck, J.O. Efficacy of postoperative oral metronidazole for haemorrhoidectomy pain: A randomized double-blind, placebo-controlled trial. Colorectal Dis. 2021, 23, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A.; Perry, C.M. Micronised purified flavonoid fraction: A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003, 63, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Colak, T.; Akca, T.; Dirlik, M.; Kanik, A.; Dag, A.; Aydin, S. Micronized Flavonoids in Pain Control After Hemorrhoidectomy: A Prospective Randomized Controlled Study. Surg. Today 2003, 33, 828–832. [Google Scholar] [CrossRef] [PubMed]
- La Torre, F.; Nicolai, A.P. Clinical use of micronized purified flavonoid fraction for treatment of symptoms after hemorrhoidectomy: Results of a randomized, controlled, clinical trial. Dis. Colon Rectum 2004, 47, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, W.Y.; Chun, H.K. Clinical effects of Venitol on complications after hemorrhoidectomy prospective randomized and placebo-controlled trial. J. Korean Soc. Coloproctol. 1998, 14, 761–766. [Google Scholar]
- Perera, N.; Liolitsa, D.; Iype, S.; Croxford, A.; Yassin, M.; Lang, P.; Ukaegbu, O.; van Issum, C. Phlebotonics for haemorrhoids. Cochrane Database Syst. Rev. 2012, 8, CD004322. [Google Scholar] [CrossRef]
- Sheikh, P.; Lohsiriwat, V.; Shelygin, Y. Micronized Purified Flavonoid Fraction in Hemorrhoid Disease: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 2792–2812. [Google Scholar] [CrossRef] [PubMed]
- Nessim, A.; Wexner, S.D.; Agachan, F.; Alabaz, O.; Weiss, E.G.; Nogueras, J.J.; Daniel, N.; Lee Billotti, V. Is bowel confinement necessary after anorectal reconstructive surgery? A prospective, randomized, surgeon-blinded trial. Dis. Colon Rectum 1999, 42, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Mahony, R.; Behan, M.; O’Herlihy, C.; O’Connell, P.R. Randomized, Clinical Trial of Bowel Confinement vs. Laxative Use After Primary Repair of a Third-Degree Obstetric Anal Sphincter Tear. Dis. Colon Rectum 2004, 47, 12–17. [Google Scholar] [CrossRef]
- Johnson, C.D.; Chir, M.; Budd, J.; Ward, A.J. Laxatives after hemorrhoidectomy. Dis. Colon Rectum 1987, 30, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Kecmanovic, D.M.; Pavlov, M.J.; Ceranic, M.; Kerkez, M.D.; Rankovic, V.I.; Masirevic, V.P. Bulk agentPlantago ovata after Milligan-Morgan hemorrhoidectomy with Ligasure™. Phytother. Res. 2006, 20, 655–658. [Google Scholar] [CrossRef]
- Tufano, A.; Arturo, C.; Cimino, E.; Di Minno, M.; Di Capua, M.; Cerbone, A.M.; Di Minno, G. Mesoglycan: Clinical Evidences for Use in Vascular Diseases. Int. J. Vasc. Med. 2010, 2010, 390643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, G.; Mistrangelo, M.; Passera, R.; Testa, V.; Pozzo, M.; Perinotti, R.; Lanati, I.; Lazzari, I.; Tonello, P.; Ugliono, E.; et al. Efficacy of Mesoglycan in Pain Control after Excisional Hemorrhoidectomy: A Pilot Comparative Prospective Multicenter Study. Gastroenterol. Res. Pract. 2018, 2018, 6423895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, G.; Di Saverio, S.; Clerico, G.; Sturiale, A.; Manigrasso, M.; Luc, A.; Trompetto, M.; Sammarco, G. Mesoglycan for pain control after open excisional HAEMOrrhoidectomy (MeHAEMO): An observational multicentre study on behalf of the Italian Society of Colorectal Surgery (SICCR). BMC Surg. 2020, 20, 251. [Google Scholar]
- Dodi, G.; Bogoni, F.; Infantino, A.; Pianon, P.; Mortellaro, L.M.; Lise, M. Hot or cold in anal pain? A study of the changes in internal anal sphincter pressure profiles. Dis. Colon Rectum 1986, 29, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A. Role of Warm-Water Bath in Anorectal Conditions. The “thermosphincteric reflex”. J. Clin. Gastroenterol. 1993, 16, 304–308. [Google Scholar] [CrossRef]
- Lohsiriwat, V.; Lohsiriwat, D. Ambulatory anorectal surgery under perianal anesthetics infiltration: Analysis of 222 cases. J. Med. Assoc. Thail. 2007, 90, 278–281. [Google Scholar]
- Lang, D.S.; Tho, P.C.; Ang, E.N. Effectiveness of the Sitz bath in managing adult patients with anorectal disorders. Jpn. J. Nurs. Sci. 2011, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Siew Ping, D.L.; Chi, T.P.; Li, G.M.; Nk, E.A. The effectiveness of sitz bath in managing adult patients with anorectal disorders: A systematic review. JBI Libr. Syst. Rev. 2010, 8, 447–469. [Google Scholar] [CrossRef]
- Tejirian, T.; Abbas, M.A. Sitz Bath: Where Is the Evidence? Scientific Basis of a Common Practice. Dis. Colon Rectum 2005, 48, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.J. Warm Sitz Bath Does Not Reduce Symptoms in Posthaemorrhoidectomy Period: A Randomized, Controlled Study. ANZ J. Surg. 2008, 78, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Balta, A.Z.; Ozdemir, Y.; Sucullu, I.; Filiz, A.I.; Yucel, E.; Akin, M.L. The effect of early warm plastic bag application on postoperative pain after hemorrhoidectomy: A prospective randomized controlled trial. Am. Surg. 2015, 81, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.M.; Ciotu, C.I.; Szallasi, A. The Mysteries of Capsaicin-Sensitive Afferents. Front. Physiol. 2020, 11, 554195. [Google Scholar] [CrossRef]
- Gupta, P.J. Effect of red chili consumption on postoperative symptoms during the post-hemorrhoidectomy period: Randomized, double-blind, controlled study. World J. Surg. 2007, 31, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Kocot-Kępska, M.; Zajączkowska, R.; Zhao, J.; Wordliczek, J.; Tomasik, P.; Przeklasa-Muszyńska, A. The role of complementary and alternative methods in the treatment of pain in patients with cancer—Current evidence and clinical practice: A narrative review. Contemp. Oncol. 2021, 25, 88–94. [Google Scholar] [CrossRef]
- Chiu, J.H.; Chen, W.S.; Chen, C.H.; Jiang, J.K.; Tang, G.J.; Lui, W.Y.; Lin, J.K. Effect of transcutaneous electrical nerve stimulation for pain relief on patients undergoing hemorrhoidectomy: Prospective, randomized, controlled trial. Dis. Colon Rectum 1999, 42, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, A.-M.; Chen, M.; Tang, T.-C.; Du, Y.-J.; Zheng, H. Acupuncture and related techniques for postoperative pain after hemorrhoidectomy: A systematic review and network meta-analysis. Eur. J. Integr. Med. 2020, 37, 101112. [Google Scholar] [CrossRef]
- Zhang, A.M.; Chen, M.; Tang, T.C.; Qin, D.; Yue, L.; Zheng, H. Somatosensory stimulation treatments for postoperative analgesia of mixed hemorrhoids: Protocol for a systematic review and network meta-analysis. Medicine 2019, 98, e14441. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Rickard, M.J.F.X.; Keshava, A.; Suen, M.K.L. Impact of post-haemorrhoidectomy pain relief checklists on pain outcomes: A randomized controlled trial. ANZ J. Surg. 2020, 90, 580–584. [Google Scholar] [CrossRef]
- Joshi, G.P.; Neugebauer, E.A.M. Evidence-based management of pain after haemorrhoidectomy surgery. Br. J. Surg. 2010, 97, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Sammour, T.; Barazanchi, A.W.H.; Hill, A.G.; On Behalf of the PROSPECT Group (Collaborators). Evidence-Based Management of Pain After Excisional Haemorrhoidectomy Surgery: A PROSPECT Review Update. World J. Surg. 2016, 41, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, F.E.F.; Lacerda-Filho, A.; Mansur, E.S.; de Oliveira, F.H.; de Queiroz, F.L.; França-Neto, P.R.; Misson, N. Benefits of flavonoid and metronidazole use after excisional hemorrhoidectomy: A randomized double-blind clinical trial. Tech. Coloproctol. 2021, 25, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V. High Compliance with Surgical Site Infection (SSI) Prevention Bundle Reduces Incisional SSI after Colorectal Surgery. Ann. Coloproctol. 2021, 37, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V.; Lertbannaphong, S.; Polakla, B.; Riansuwan, W. Implementation of enhanced recovery after surgery and its increasing compliance improved 5-year overall survival in resectable stage III colorectal cancer. Updates Surg. 2021, 73, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
| Anesthetic methods |
|
| Surgical techniques |
|
| Intraoperative adjunct |
|
| Postoperative interventions |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohsiriwat, V.; Jitmungngan, R. Strategies to Reduce Post-Hemorrhoidectomy Pain: A Systematic Review. Medicina 2022, 58, 418. https://doi.org/10.3390/medicina58030418
Lohsiriwat V, Jitmungngan R. Strategies to Reduce Post-Hemorrhoidectomy Pain: A Systematic Review. Medicina. 2022; 58(3):418. https://doi.org/10.3390/medicina58030418
Chicago/Turabian StyleLohsiriwat, Varut, and Romyen Jitmungngan. 2022. "Strategies to Reduce Post-Hemorrhoidectomy Pain: A Systematic Review" Medicina 58, no. 3: 418. https://doi.org/10.3390/medicina58030418






