Rosuvastatin Induces Renal HO-1 Activity and Expression Levels as a Main Protective Mechanism against STZ-Induced Diabetic Nephropathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animal Protocol and Experimental Design
2.3. Biochemical Analysis
2.4. Renal Histopathology
2.5. Western Blot Assay
2.6. HO-1 Activity Assay Method
2.7. Statistical Analysis
3. Results
3.1. The Effect on Weight and Blood Glucose Levels
3.2. The Effect on Biochemical Kidney Function Parameters and Serum Lipid Profile
3.3. The Effect on Renal Histopathology
3.4. The Effect on Renal Oxidative Stress Markers
3.5. The Effect on Renal HO-1 Expression and Activity
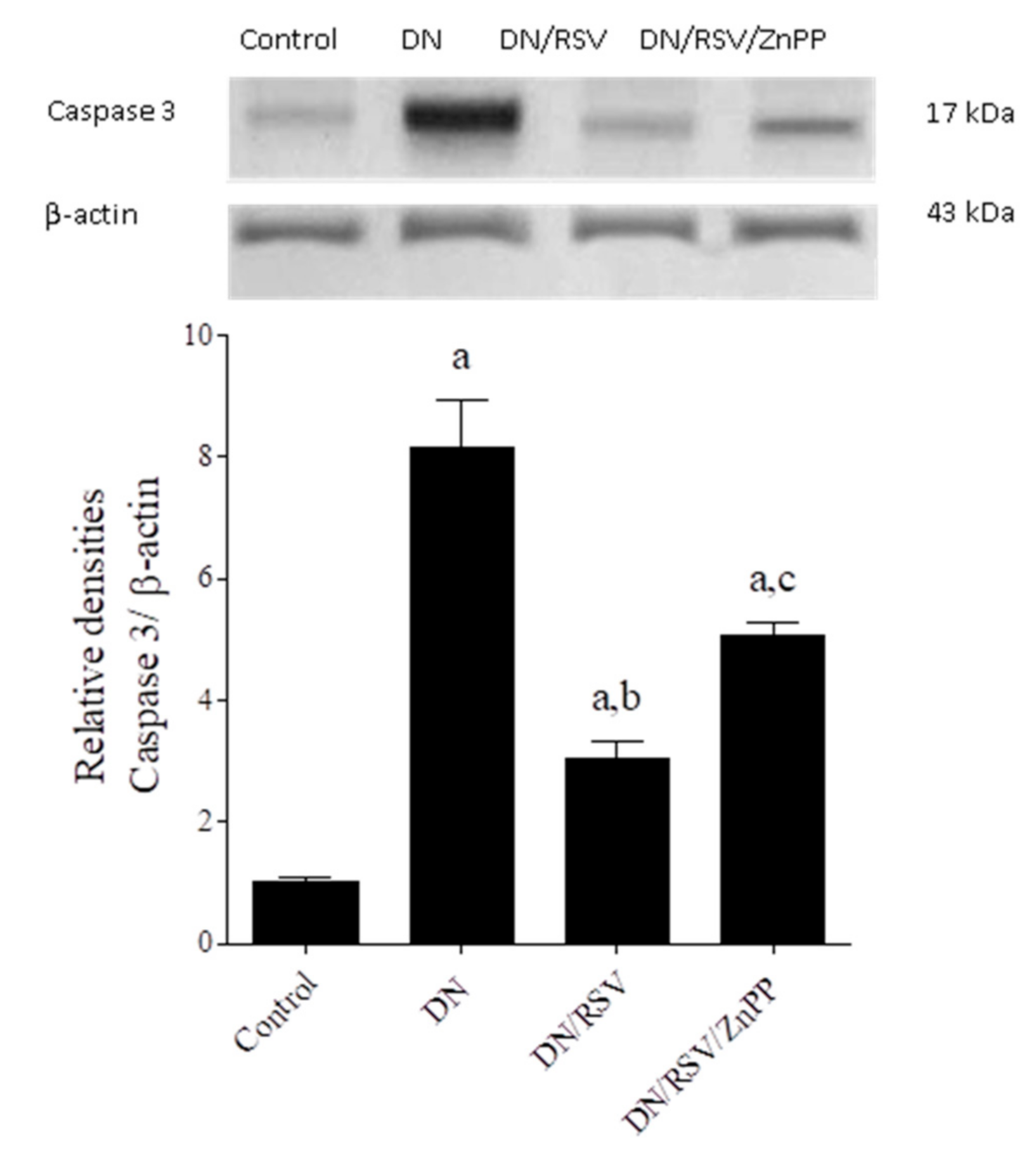
3.6. The Effect on Renal TNF-α and Caspase 3 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mohsen, M.; Elberry, A.A.; Mohamed, R.A.; Abdelrahim, M.E.A.; Hussein, R.R.S. Recent therapeutic targets in diabetic nephropathy. Int. J. Clin. Pract. 2021, 75, e14650. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.K. Development of Biomarkers and Molecular Therapy Based on Inflammatory Genes in Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 9985. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, T.A.; Friederich-Persson, M. Mitochondrial Reactive Oxygen Species and Kidney Hypoxia in the Development of Diabetic Nephropathy. Front. Physiol. 2017, 8, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef] [PubMed]
- Taye, A.; El-Sheikh, A.A. Lectin-like oxidized low-density lipoprotein receptor 1 pathways. Eur. J. Clin. Investig. 2013, 43, 740–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Liang, X.; He, W.; Liang, M.; Jin, J.; He, Q. ATF4-dependent heme-oxygenase-1 attenuates diabetic nephropathy by inducing autophagy and inhibiting apoptosis in podocyte. Ren. Fail. 2021, 43, 968–979. [Google Scholar] [CrossRef]
- Tesch, G.H. Diabetic nephropathy—Is this an immune disorder? Clin. Sci. 2017, 131, 2183–2199. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Liu, X.; Nie, Y.; Zhao, Z. Urinary heme oxygenase-1 as a potential biomarker for early diabetic nephropathy. Nephrology 2017, 22, 58–64. [Google Scholar] [CrossRef]
- Ali, M.A.M.; Heeba, G.H.; El-Sheikh, A.A.K. Modulation of heme oxygenase-1 expression and activity affects streptozotocin-induced diabetic nephropathy in rats. Fundam. Clin. Pharmacol. 2017, 31, 546–557. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, Y.; Li, C.; Liu, Y.; Zhang, L.; Wang, Y.; Che, K. HDL cholesterol and risk of diabetic nephropathy in patient with type 1 diabetes: A meta-analysis of cohort studies. Diabetes Res. Clin. Pract. 2016, 122, 84–91. [Google Scholar] [CrossRef]
- Cortese, F.; Gesualdo, M.; Cortese, A.; Carbonara, S.; Devito, F.; Zito, A.; Ricci, G.; Scicchitano, P.; Ciccone, M.M. Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol. Res. 2016, 107, 1–18. [Google Scholar] [CrossRef]
- Palaniswamy, C.; Selvaraj, D.R.; Selvaraj, T.; Sukhija, R. Mechanisms underlying pleiotropic effects of statins. Am. J. Ther. 2010, 17, 75–78. [Google Scholar] [CrossRef]
- Sharma, D.; Bhattacharya, P.; Kalia, K.; Tiwari, V. Diabetic nephropathy: New insights into established therapeutic paradigms and novel molecular targets. Diabetes Res. Clin. Pract. 2017, 128, 91–108. [Google Scholar] [CrossRef]
- Heeba, G.H.; Hamza, A.A. Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 2015, 141, 13–19. [Google Scholar] [CrossRef]
- Vaishya, R.; Singh, J.; Lal, H. Effect of irbesartan on streptozotocin induced diabetic nephropathy: An interventionary study. Indian J. Clin. Biochem. 2008, 23, 195–197. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Wu, Z.M.; Liu, Y.; Tan, Y.F.; Ren, F.; Wu, H. Upregulation of heme oxygenase-1 with hemin prevents D-galactosamine and lipopolysaccharide-induced acute hepatic injury in rats. Toxicology 2007, 237, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.V.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Hillegass, L.M.; Griswold, D.E.; Brickson, B.; Albrightson-Winslow, C. Assessment of myeloperoxidase activity in whole rat kidney. J. Pharmacol. Methods 1990, 24, 285–295. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Morsy, M.A.; Al-Taher, A.Y. Protective mechanisms of resveratrol against methotrexate-induced renal damage may involve BCRP/ABCG2. Fundam. Clin. Pharmacol. 2016, 30, 406–418. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Hamza, A.A.; Hassanin, S.O. Induction of heme oxygenase-1 with hemin alleviates cisplatin-induced reproductive toxicity in male rats and enhances its cytotoxicity in prostate cancer cell line. Toxicol. Lett. 2016, 264, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Zolotnik, I.A.; Figueroa, T.Y.; Yaspelkis, B.B., III. Insulin receptor and IRS-1 co-immunoprecipitation with SOCS-3, and IKKalpha/beta phosphorylation are increased in obese Zucker rat skeletal muscle. Life Sci. 2012, 91, 816–822. [Google Scholar] [CrossRef] [Green Version]
- Gezginci-Oktayoglu, S.; Tunali, S.; Yanardag, R.; Bolkent, S. Effects of Z-FA.FMK on D-galactosamine/tumor necrosis factor-alpha-induced kidney injury and oxidative stress in mice: Effects of Z-FA.FMK on TNF-alpha-mediated kidney injury. Mol. Cell Biochem. 2008, 309, 9–20. [Google Scholar] [CrossRef]
- Abraham, N.G.; Lutton, J.D.; Levere, R.D. Heme metabolism and erythropoiesis in abnormal iron states: Role of delta-aminolevulinic acid synthase and heme oxygenase. Exp. Hematol. 1985, 13, 838–843. [Google Scholar]
- Jiang, Z.; Zhang, J.; Lu, Y. Protective Effects and Mechanisms of Rosuvastatin on Acute Kidney Injury Induced by Contrast Media in Rats. Int. J. Nephrol. 2020, 2020, 3490641. [Google Scholar] [CrossRef]
- Lin, M.; Xu, T.; Zhang, W.; Li, D.; Li, Y.; Hong, X.; Luan, Y.; Zhang, W.; Wang, M. Effect of statins on post-contrast acute kidney injury: A multicenter retrospective observational study. Lipids Health Dis. 2021, 20, 63. [Google Scholar] [CrossRef]
- Rasheed, H.A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I. Rosuvastatin Attenuates acute nephrotoxicity through modulation of oxidative stress in Sprague Dawley rats. J. Pak. Med. Assoc. 2019, 69, S98–S102. [Google Scholar]
- Abdeen, A.; Aboubakr, M.; Elgazzar, D.; Abdo, M.; Abdelkader, A.; Ibrahim, S.; Elkomy, A. Rosuvastatin attenuates piroxicam-mediated gastric ulceration and hepato-renal toxicity in rats. Biomed. Pharmacother. 2019, 110, 895–905. [Google Scholar] [CrossRef]
- Jabarpour, M.; Rashtchizadeh, N.; Ghorbani, H.A.; Argani, H.; Nemati, M.; Dastmalchi, S.; Roshangar, L.; Ranjbarzadhag, M.; Mesgari-Abbasi, M.; Bargahi, N.; et al. Protection of renal damage by HMG-CoA inhibitors: A comparative study between atorvastatin and rosuvastatin. Iran J. Basic Med. Sci. 2020, 23, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, B.H.; Ku, S.K.; Park, J.H.; Oh, E.; Kwak, M.K. Beneficial Effects of Sarpogrelate and Rosuvastatin in High Fat Diet/Streptozotocin-Induced Nephropathy in Mice. PLoS ONE 2016, 11, e0153965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Wu, G.; Yang, C.; Li, Y.; Jing, Q.; Han, Y. Rosuvastatin attenuates contrast-induced nephropathy through modulation of nitric oxide, inflammatory responses, oxidative stress and apoptosis in diabetic male rats. J. Transl. Med. 2015, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Maruyama, N.; Okada, K.; Matsumoto, S.; Matsumoto, K.; Soma, M. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. J. Atheroscler. Thromb. 2011, 18, 1018–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodiya, H.; Jain, M.; Goswami, S.S. Renal toxicity of lisinopril and rosuvastatin, alone and in combination, in Wistar rats. Int. J. Toxicol. 2011, 30, 518–527. [Google Scholar] [CrossRef]
- Annigeri, R.A.; Mani, R.M. Acute interstitial nephritis due to statin and its class effect. Indian J. Nephrol. 2015, 25, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Vijayakanthi, N.; Felner, E.I.; Romero, R.; Daley, T.C. Rhabdomyolysis due to rosuvastatin in a patient with ROHHAD syndrome. J. Clin. Lipidol. 2021, 15, 789–792. [Google Scholar] [CrossRef]
- Nikalji, R.; Sen, S. Rosuvastatin-Induced Rhabdomyolysis: A Case Report. Indian J. Nephrol. 2021, 31, 190–193. [Google Scholar] [CrossRef]
- Petreski, T.; Piko, N.; Petrijan, T.; Dvoršak, B.; Hojs, R.; Bevc, S. Statin-Associated Necrotizing Myopathy Leading to Acute Kidney Injury: A Case Report. Case Rep. Nephrol. Dial. 2021, 11, 129–135. [Google Scholar] [CrossRef]
- Sabanis, N.; Paschou, E.; Drylli, A.; Papanikolaou, P.; Zagkotsis, G. Rosuvastatin and Colchicine combined myotoxicity: Lessons to be learnt. CEN Case Rep. 2021, 10, 570–575. [Google Scholar] [CrossRef]
- Sibley, R.A.; Katz, A.; Papadopoulos, J. The Interaction between Rosuvastatin and Ticagrelor Leading to Rhabdomyolysis: A Case Report and Narrative Review. Hosp. Pharm. 2021, 56, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Mitaritonno, M.; Lupo, M.; Greco, I.; Mazza, A.; Cervellin, G. Severe rhabdomyolysis induced by co-administration of cocaine and heroin in a 45 years old man treated with rosuvastatin: A case report. Acta Biomed. 2021, 92, e2021089. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Barma, S.; Konwar, N.; Dewanjee, S.; Manna, P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur. J. Pharmacol. 2016, 791, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Mahfouz, M.K. Effect of resveratrol and rosuvastatin on experimental diabetic nephropathy in rats. Biomed. Pharmacother. 2016, 82, 685–692. [Google Scholar] [CrossRef]
- El-Sawaf, E.S.; Saleh, S.; Abdallah, D.M.; Ahmed, K.A.; El-Abhar, H.S. Vitamin D and rosuvastatin obliterate peripheral neuropathy in a type-2 diabetes model through modulating Notch1, Wnt-10α, TGF-β and NRF-1 crosstalk. Life Sci. 2021, 279, 119697. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhou, Q.; Lu, M.; Wang, H. Rosuvastatin corrects oxidative stress and inflammation induced by LPS to attenuate cardiac injury by inhibiting the NLRP3/TLR4 pathway. Can. J. Physiol. Pharmacol. 2021, 99, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.; Alamgeer, K.S.; Shahzad, M.; Bukhari, I.A.; Vohra, F.; Afzal, S. Rosuvastatin Attenuates Rheumatoid Arthritis-Associated Manifestations via Modulation of the Pro- and Anti-inflammatory Cytokine Network: A Combination of In Vitro and In Vivo Studies. ACS Omega 2021, 6, 2074–2084. [Google Scholar] [CrossRef]
- Bao, W.; Song, F.; Li, X.; Rong, S.; Yang, W.; Wang, D.; Xu, J.; Fu, J.; Zhao, Y.; Liu, L. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: A HuGE review and meta-analysis. Am. J. Epidemiol. 2010, 172, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Bolisetty, S.; Zarjou, A.; Agarwal, A. Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury. Am. J. Kidney Dis. 2017, 69, 531–545. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Hu, B.; Xu, G.; Chen, F.; Ma, R.; Zhang, N.; Liu, J.; Ma, X.; Zhu, J.; Wu, Y.; et al. Activation of Nrf2/HO-1 Pathway by Glycogen Synthase Kinase-3beta Inhibition Attenuates Renal Ischemia/Reperfusion Injury in Diabetic Rats. Kidney Blood Press Res. 2017, 42, 369–378. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Huang, Q.; Liu, R.; Liu, J.; Zhang, F.; Liu, S.; Jiang, Y. Moringa oleifera Lam. seed extract protects kidney function in rats with diabetic nephropathy by increasing GSK-3β activity and activating the Nrf2/HO-1 pathway. Phytomedicine 2021, 95, 153856. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, Q.; Peng, J.; Jin, L.; Zhu, X.; Zheng, D.; Zhang, Y.; Wang, R.; Song, Y.; Hu, W.; et al. Fucoxanthin regulates Nrf2 signaling to decrease oxidative stress and improves renal fibrosis depending on Sirt1 in HG-induced GMCs and STZ-induced diabetic rats. Eur. J. Pharmacol. 2021, 913, 174629. [Google Scholar] [CrossRef]
- Chen, N.; Song, S.; Yang, Z.; Wu, M.; Mu, L.; Zhou, T.; Shi, Y. ChREBP deficiency alleviates apoptosis by inhibiting TXNIP/oxidative stress in diabetic nephropathy. J. Diabetes Complicat. 2021, 35, 108050. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, B.H.; Ku, S.K.; Kim, D.H.; Jung, K.A.; Oh, E.; Kwak, M.K. Amelioration of high fat diet-induced nephropathy by cilostazol and rosuvastatin. Arch. Pharm. Res. 2017, 40, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Jeng, J.C.; Hsieh, M.W.; Chen, Y.C.; Lu, T.H.; Rau, C.S.; Jeng, S.F. Involvement of the p38 pathway in the differential induction of heme oxygenase-1 by statins in Neuro-2A cells exposed to lipopolysaccharide. Drug Chem. Toxicol. 2011, 34, 8–19. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Kuo, C.T.; Chang, G.J.; Chen, Y.H.; Lai, Y.J.; Cheng, M.L.; Chen, W.J. Rosuvastatin suppresses atrial tachycardia-induced cellular remodeling via Akt/Nrf2/heme oxygenase-1 pathway. J. Mol. Cell Cardiol. 2015, 82, 84–92. [Google Scholar] [CrossRef]
- Azuma, J.; Wong, R.J.; Morisawa, T.; Hsu, M.; Maegdefessel, L.; Zhao, H.; Kalish, F.; Kayama, Y.; Wallenstein, M.B.; Deng, A.C.; et al. Heme Oxygenase-1 Expression Affects Murine Abdominal Aortic Aneurysm Progression. PLoS ONE 2016, 11, e0149288. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Tong, S.; Hannan, N.J.; Hastie, R.; Cannon, P.; Kaitu’u-Lino, T.J. Effects of simvastatin, rosuvastatin and pravastatin on soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sENG) secretion from human umbilical vein endothelial cells, primary trophoblast cells and placenta. BMC Pregnancy Childbirth 2016, 16, 117. [Google Scholar] [CrossRef]
- Wang, P.; Luo, L.; Shen, Q.; Shi, G.; Mohammed, A.; Ni, S.; Wu, X. Rosuvastatin improves myocardial hypertrophy after hemodynamic pressure overload via regulating the crosstalk of Nrf2/ARE and TGF-β/smads pathways in rat heart. Eur. J. Pharmacol. 2018, 820, 173–182. [Google Scholar] [CrossRef]
- Dolkart, O.; Amar, E.; Shapira, S.; Marmor, S.; Steinberg, E.L.; Weinbroum, A.A. Protective effects of rosuvastatin in a rat model of lung contusion: Stimulation of the cyclooxygenase 2-prostaglandin E-2 pathway. Surgery 2015, 157, 944–953. [Google Scholar] [CrossRef]
- Hsu, M.; Muchova, L.; Morioka, I.; Wong, R.J.; Schröder, H.; Stevenson, D.K. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem. Biophys. Res. Commun. 2006, 343, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Nakkina, V.; Kamble, P.; Sharma, S.S. Heme oxygenase-1, a novel target for the treatment of diabetic complications: Focus on diabetic peripheral neuropathy. Pharmacol. Res. 2015, 102, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017, 93, 646–653. [Google Scholar] [CrossRef]
- Potočnjak, I.; Broznić, D.; Kindl, M.; Kropek, M.; Vladimir-Knežević, S.; Domitrović, R. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of ERK1/2, STAT3, and NF-kappaB activation. Food Chem. Toxicol. 2017, 107, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Han, S.H.; Li, J.J.; Lee, S.H.; Jung, D.S.; Kwak, S.J.; Kim, S.H.; Kim, D.K.; Yoo, T.H.; Kim, J.H.; et al. Induction of heme oxygenase-1 protects against podocyte apoptosis under diabetic conditions. Kidney Int. 2009, 76, 838–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| % ∆ Body Weight | Kidney Index | Blood Glucose at 0-Time (mg/dL) | Blood Glucose at Day 60 (mg/dL) | |
|---|---|---|---|---|
| Control | 134 ± 3 | 0.52 ± 0.02 | 126 ± 3 | 148 ± 6 |
| RSV | 135 ± 2 | 0.5 ± 0.04 | 128 ± 8 | 112 ± 10 |
| DN | 85 ± 1 a | 1.0 ± 0.05 a | 542 ± 63 a | 464 ± 70 a |
| DN/RSV | 103 ± 1 a,b | 0.59 ± 0.03 b | 504 ± 41 a | 462 ± 49 a |
| DN/RSV/ZnPP | 88 ± 2 a,c | 0.85 ± 0.05 a,c | 458 ± 79 a | 538 ± 29 a |
| Control | RSV | DN | DN/RSV | DN/RSV/ZnPP | |
|---|---|---|---|---|---|
| Serum Urea (mg/dL) | 11 ± 1 | 10 ± 1 | 24 ± 1 a | 12 ± 2 b | 28 ± 1 a,c |
| Serum Creatinine (mg/dL) | 0.89 ± 0.10 | 0.98 ± 0.08 | 4.00 ± 0.13 a | 1.2 ± 0.06 b | 3.6 ± 0.29 a,c |
| Serum Albumin (g/dL) | 3.4 ± 0.3 | 3.1 ± 0.2 | 2.0 ± 0.1 a | 3.2 ± 0.2 b | 2.2 ± 0.1 a,c |
| ACR (mg/g) | 17 ± 1 | 13 ± 1 | 193 ± 23 a | 69 ± 10 b | 187 ± 18 a,c |
| Serum Na+ (mEq/L) | 134 ± 2 | 133 ± 12 | 210 ± 5 a | 145 ± 6 b | 230 ± 18 a,c |
| Urine Na+ (mEq/L) | 937 ± 24 | 1063 ± 46 | 393 ± 23 a | 863 ± 75 b | 532 ± 6 a,c |
| Serum K+ (mEq/L) | 3.4 ± 0.2 | 4.1 ± 0.2 | 7.6 ± 0.5 a | 4.6 ± 0.3 b | 6.3 ± 0.3 a |
| Urine K+ (mEq/L) | 89 ± 6 | 83 ± 5 | 22 ± 1 a | 44 ± 5 a,b | 27.8 ± 1.9 a |
| Total Cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | |
|---|---|---|---|---|
| Control | 70 ± 1 | 36.6 ± 0.5 | 50 ± 3 | 14 ± 2 |
| RSV | 70 ± 3 | 36.0 ± 0.3 | 51 ± 3 | 14 ± 1 |
| DN | 79 ± 2 a | 49.3 ± 1.9 a | 22 ± 3 a | 45 ± 2 a |
| DN/RSV | 63 ± 2 b | 36.1 ± 1.6 b | 50 ± 2 b | 23 ± 2 b |
| DN/RSV/ZnPP | 64 ± 2 b | 38.9 ± 1.7 b | 45 ± 4 b | 22 ± 2 b |
| Tubular Degeneration | Tubular Dilation | Congested Blood Vessels | Leukocytic Infiltration | Vacuolation of Lining Epithelium | Congestion of Glomerular Tuft | |
|---|---|---|---|---|---|---|
| Control | - | - | - | - | - | - |
| RSV | - | - | - | - | - | - |
| DN | ++ | ++ | +++ | +++ | +++ | +++ |
| DN/RSV | - | + | + | + | + | + |
| DN/RSV/ZNPP | ++ | + | ++ | +++ | ++ | ++ |
| GSH (mmol/g Tissue) | SOD (U/g Tissue) | Catalase (U/g Tissue) | MDA (nmol/g Tissue) | MPO (U/g Tissue) | NO (nmol/g Tissue) | |
|---|---|---|---|---|---|---|
| Control | 56 ± 3 | 1199 ± 62 | 433 ± 11 | 200 ± 3 | 0.12 ± 0.02 | 112 ± 3 |
| RSV | 50 ± 4 | 1138 ± 80 | 397 ± 14 | 189 ± 4 | 0.11 ± 0.02 | 124 ± 10 |
| DN | 29 ± 2 a | 629 ± 70 a | 135 ± 19 a | 352 ± 8 a | 0.32 ± 0.04 a | 264 ± 8 a |
| DN/RSV | 48 ± 2 b | 1131 ± 73 b | 320 ± 21 a,b | 305 ± 10 a,b | 0.17 ± 0.01 b | 131 ± 9 b |
| DN/RSV/ZNPP | 32 ± 2 a,c | 451 ± 52 a,c | 198 ± 20 a,c | 361 ± 8 a,c | 0.30 ± 0.04 a,c | 248 ± 24 a,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heeba, G.H.; Ali, M.A.M.; El-Sheikh, A.A.K. Rosuvastatin Induces Renal HO-1 Activity and Expression Levels as a Main Protective Mechanism against STZ-Induced Diabetic Nephropathy. Medicina 2022, 58, 425. https://doi.org/10.3390/medicina58030425
Heeba GH, Ali MAM, El-Sheikh AAK. Rosuvastatin Induces Renal HO-1 Activity and Expression Levels as a Main Protective Mechanism against STZ-Induced Diabetic Nephropathy. Medicina. 2022; 58(3):425. https://doi.org/10.3390/medicina58030425
Chicago/Turabian StyleHeeba, Gehan H., Marwa A. M. Ali, and Azza A. K. El-Sheikh. 2022. "Rosuvastatin Induces Renal HO-1 Activity and Expression Levels as a Main Protective Mechanism against STZ-Induced Diabetic Nephropathy" Medicina 58, no. 3: 425. https://doi.org/10.3390/medicina58030425





