Comparative Toxicity of Interferon Beta-1a Impurities of Heavy Metal Ions
Abstract
:1. Introduction
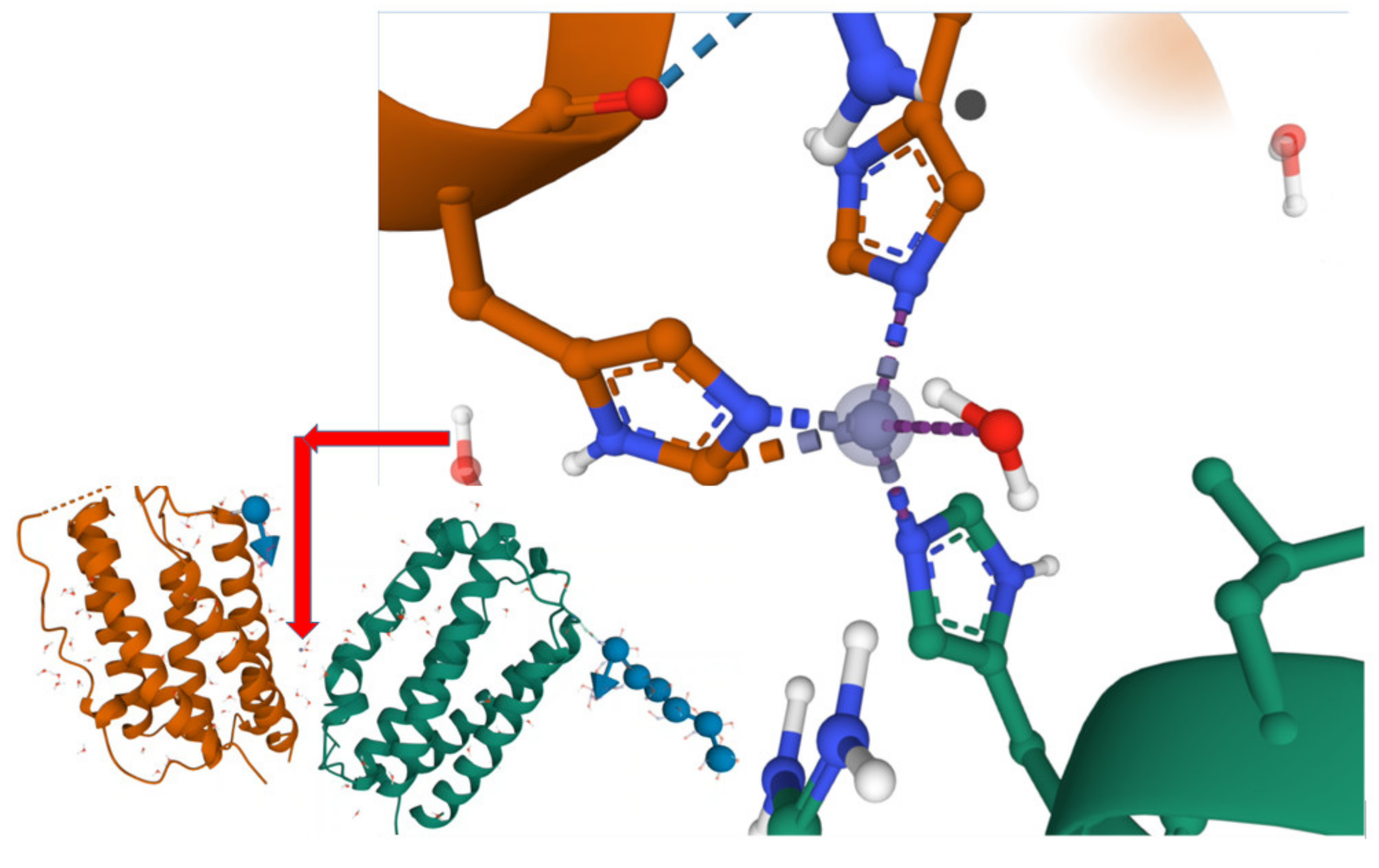
2. Results and Discussion
3. Materials and Methods
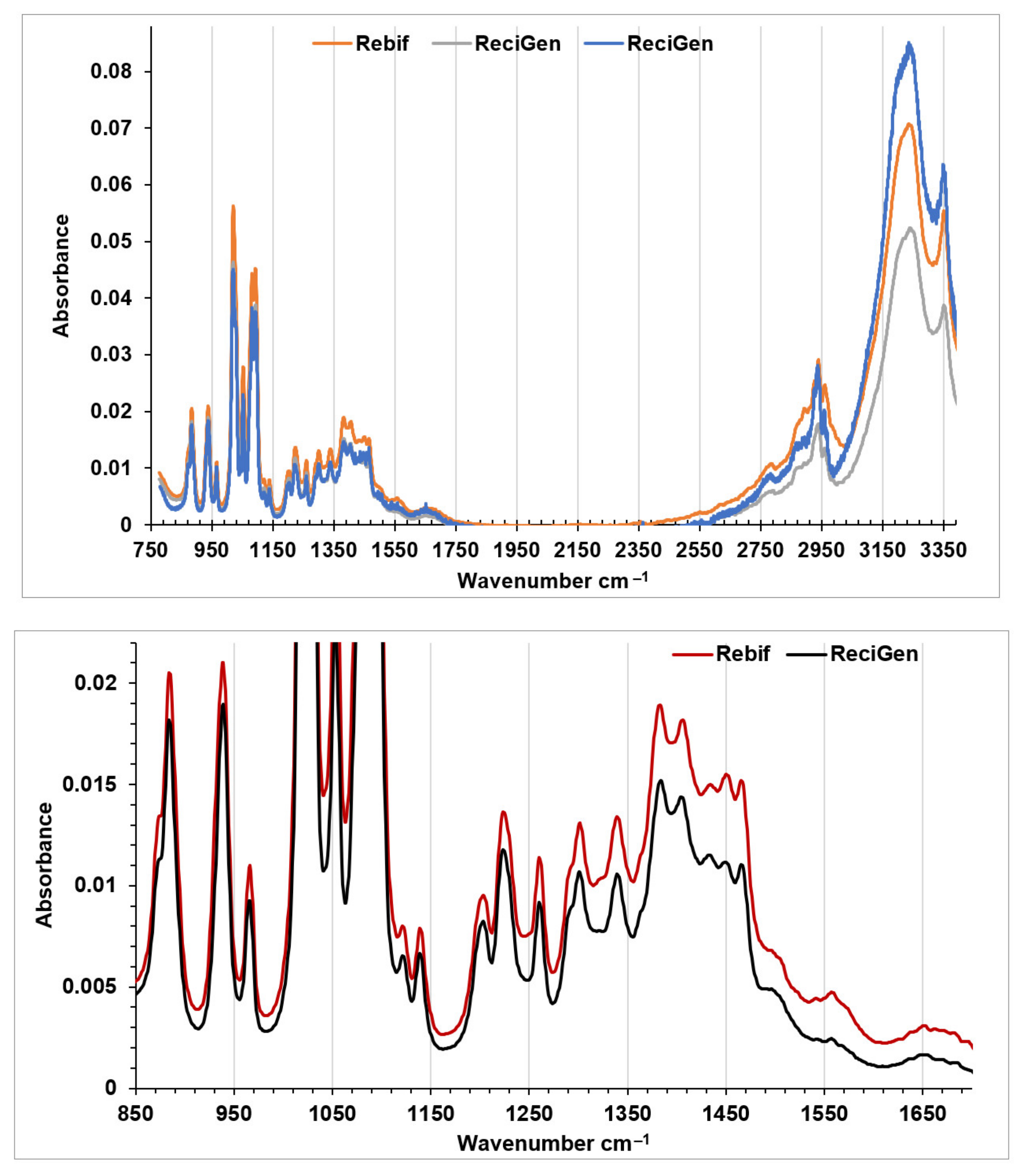
3.1. FTIR Spectroscopy
3.2. Gel Electrophoresis
3.3. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
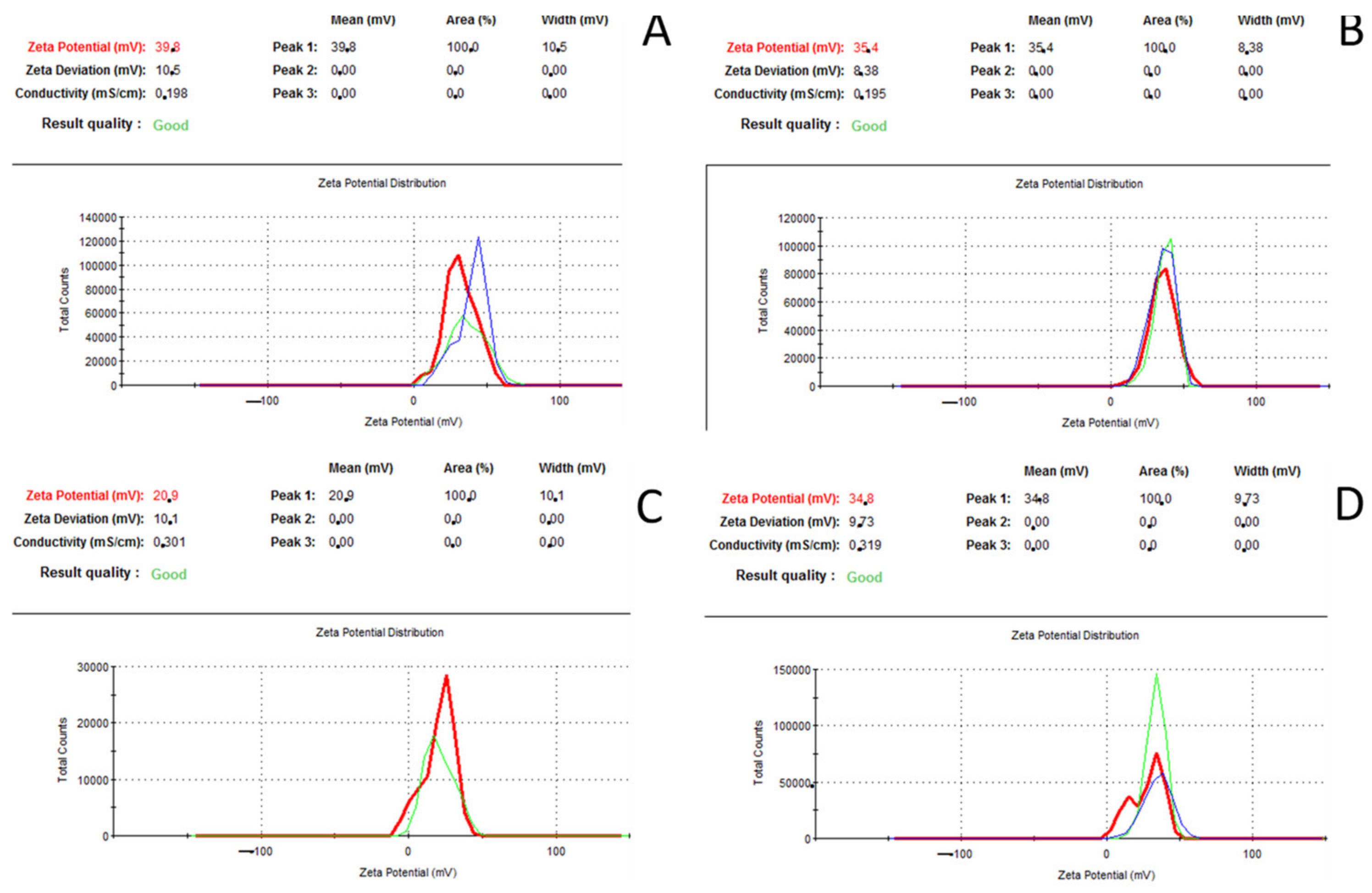
3.4. Zeta Potential Analysis
3.5. Calculation of Molar Components in the Pharmaceutical Preparation of IFNβ-1a
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobs, L.D.; Cookfair, D.L.; Rudick, R.A.; Herndon, R.M.; Richert, J.R.; Salazar, A.M.; Fischer, J.S.; Goodkin, D.E.; Granger, C.V.; Simon, J.H.; et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1996, 39, 285–294. [Google Scholar] [CrossRef]
- Durelli, L.; Verdun, E.; Barbero, P.; Bergui, M.; Versino, E.; Ghezzi, A.; Montanari, E.; Zaffaroni, M. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: Results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002, 359, 1453–1460. [Google Scholar] [CrossRef]
- Shalhoub, S. Interferon beta-1b for COVID-19. Lancet 2020, 395, 1670–1671. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.-P.; et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef]
- Dorgham, K.; Neumann, A.U.; Decavele, M.; Luyt, C.-E.; Yssel, H.; Gorochov, G. Considering Personalized Interferon Beta Therapy for COVID-19. Antimicrob. Agents Chemother. 2021, 65, e00065-21. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.M.; Braz, M.; Llorens-Rico, V.; Wauters, J.; Van Weyenbergh, J. Endogenous interferon-beta but not interferon-alpha or interferon-lambda levels in nasal mucosa predict clinical outcome in critical COVID-19 patients independent of viral load. medRxiv 2021. [Google Scholar] [CrossRef]
- Kim, H.J.; Jo, A.; Jeon, Y.J.; An, S.; Lee, K.-M.; Yoon, S.S.; Choi, J.Y. Nasal commensal Staphylococcus epidermidis enhances interferon-λ-dependent immunity against influenza virus. Microbiome 2019, 7, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, E.U.; Hohlfeld, R. Multiple sclerosis: Side effects of interferon beta therapy and their management. Neurology 1999, 53, 1622–1627. [Google Scholar] [CrossRef]
- Ich Harmonised Guideline Guideline for Elemental Impurities Q3d(R1). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-32.pdf (accessed on 12 January 2022).
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis: Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2014; pp. 1–18. [Google Scholar] [CrossRef]
- Karpusas, M.; Nolte, M.; Benton, C.B.; Meier, W.; Lipscomb, W.N.; Goelz, S. The crystal structure of human interferon β at 2.2-Å resolution. Proc. Natl. Acad. Sci. USA 1997, 94, 11813–11818. [Google Scholar] [CrossRef] [Green Version]
- Gamble, A.J.; Peacock, A.F.A. De Novo Design of Peptide Scaffolds as Novel Preorganized Ligands for Metal-Ion Coordination. Protein Des. 2014, 1216, 211–231. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Database (Search by “Interferon”). Available online: https://clinicaltrials.gov (accessed on 12 January 2022).
- Kannappan, S.; Darrow, J.J.; Kesselheim, A.S.; Beall, R.F. The timing of 30-month stay expirations and generic entry: A cohort study of first generics, 2013–2020. Clin. Transl. Sci. 2021, 14, 1917–1923. [Google Scholar] [CrossRef]
- Kaplan, W.A.; Cárdenas, J.; Mansilla, C.; Tobar, T.; Wirtz, V.J. The implementation of the bioequivalence certification policy in Chile: An analysis of market authorization data. PLoS ONE 2019, 14, e0217334. [Google Scholar] [CrossRef]
- Castro, L.S.; Lobo, G.S.; Pereira, P.; Freire, M.G.; Neves, M.C.; Pedro, A.Q. Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation. Vaccines 2021, 9, 328. [Google Scholar] [CrossRef]
- Berillo, D.; Zharkinbekov, Z.; Kim, Y.; Raziyeva, K.; Temirkhanova, K.; Saparov, A. Stimuli-Responsive Polymers for Transdermal, Transmucosal and Ocular Drug Delivery. Pharmaceutics 2021, 13, 2050. [Google Scholar] [CrossRef]
- Berillo, D.; Yeskendir, A.; Zharkinbekov, Z.; Raziyeva, K.; Saparov, A. Peptide-Based Drug Delivery Systems. Medicina 2021, 57, 1209. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Yu, Z. Study on the anti-herpes simplex virus activity of a suppository or ointment form of Astragalus membranaceus combined with interferon alpha 2b in human diploid cell culture. Chin. J. Exp. Clin. Virol. 1998, 12, 269–271. [Google Scholar]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids as a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Schiraldi, M.; Monestier, M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009, 30, 502–509. [Google Scholar] [CrossRef]
- Kieseier, B.C. The Mechanism of Action of Interferon-β in Relapsing Multiple Sclerosis. CNS Drugs 2011, 25, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Goodin, D.S. The Use of Interferon Beta in the Treatment of Multiple Sclerosis. In Handbook of Multiple Sclerosis; CRC Press: Boca Raton, FL, USA, 2006; pp. 359–376. ISBN 9780429191251. [Google Scholar] [CrossRef]
- Impurities—Procedures, Elemental Impurities—Procedures/Chemical Tests Second Supplement to USP 38–NF 33. Available online: https://www.usp.org/sites/default/files/usp/document/our-work/chemical-medicines/key-issues/c233.pdf (accessed on 12 January 2022).
- Sánchez-Alarcón, J.; Milić, M.; Bustamante-Montes, L.P.; Isaac-Olivé, K.; Valencia-Quintana, R.; Ramírez-Durán, N. Genotoxicity of Mercury and Its Derivatives Demonstrated In Vitro and In Vivo in Human Populations Studies. Systematic Review. Toxics 2021, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Lohren, H.; Blagojevic, L.; Fitkau, R.; Ebert, F.; Schildknecht, S.; Leist, M.; Schwerdtle, T. Toxicity of organic and inorganic mercury species in differentiated human neurons and human astrocytes. J. Trace Elements Med. Biol. 2015, 32, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.; Priya, S.; Sharma, S.K.; Andersson, S.; Jakobsson, S.; Tanghe, R.; Ashouri, A.; Rauch, S.; Goloubinoff, P.; Christen, P.; et al. Cadmium Causes Misfolding and Aggregation of Cytosolic Proteins in Yeast. Mol. Cell. Biol. 2017, 37, e00490-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, R.; Roccheri, M.C. Heavy Metals and Metalloids as Autophagy Inducing Agents: Focus on Cadmium and Arsenic. Cells 2012, 1, 597–616. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; Walter, L.J.; Hruza, A.; Reichert, P.; Trotta, P.P.; Nagabhushan, T.L.; Walter, M.R. Zinc mediated dimer of human interferon-α2b revealed by X-ray crystallography. Structure 1996, 4, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Derrick, T.S.; Kashi, R.S.; Durrani, M.; Jhingan, A.; Middaugh, C. Effect of metal cations on the conformation and inactivation of recombinant human factor VIII. J. Pharm. Sci. 2004, 93, 2549–2557. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Bertolasi, V.; Remelli, M.; Faa, G. Chelating agents for human diseases related to aluminium overload. Coord. Chem. Rev. 2012, 256, 89–104. [Google Scholar] [CrossRef]
- Armstrong, R.; Winsper, S.; Blair, J. Hypothesis: Is Alzheimer’s Disease a Metal-induced Immune Disorder? Neurodegeneration 1995, 4, 107–111. [Google Scholar] [CrossRef]
- Fan, H.; Ralston, J.; Dibiase, M.; Faulkner, E.; Middaugh, C.R. Solution Behavior of IFN-β-1a: An Empirical Phase Diagram Based Approach. J. Pharm. Sci. 2005, 94, 1893–1911. [Google Scholar] [CrossRef]
- Kozlowski, H.; Potocki, S.; Remelli, M.; Rowinska-Zyrek, M.; Valensin, D. Specific metal ion binding sites in unstructured regions of proteins. Coord. Chem. Rev. 2013, 257, 2625–2638. [Google Scholar] [CrossRef]
- McConnell, K.D.; Fitzkee, N.C.; Emerson, J.P. Metal Ion Binding Induces Local Protein Unfolding and Destabilizes Human Carbonic Anhydrase II. Inorg. Chem. 2022, 61, 1249–1253. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Meager, A.; Dolman, C.; Dilger, P.; Bird, C.; Giovannoni, G.; Schellekens, H.; Thorpe, R.; Wadhwa, M. An Assessment of Biological Potency and Molecular Characteristics of Different Innovator and Noninnovator Interferon-Beta Products. J. Interf. Cytokine Res. 2011, 31, 383–392. [Google Scholar] [CrossRef]
- Lewen, N.; Mathew, S.; Schenkenberger, M.; Raglione, T. A rapid ICP-MS screen for heavy metals in pharmaceutical compounds. J. Pharm. Biomed. Anal. 2004, 35, 739–752. [Google Scholar] [CrossRef]

| Metal Ion | Content of IFNβ-1a | ICH Q3D (R1)PDE, μg/Day | |||||
|---|---|---|---|---|---|---|---|
| ReciGen, μg/L | ReciGen, in One Syringe, Weight of IFNβ-1a, ng | Accumulated Expose Dosage after ReciGen Application for One Year, μg | Rebif, μg/L | Rebif in One Syringe, Weight of IFNβ-1a, ng | Accumulated Expose Dosage after Rebif Application for One Year, μg | ||
| Al | 7.40 ± 0.17 | 3.70 | 0.58 | ND | ND | ND | - |
| Cr | 13.80± 0.32 | 6.90 | 1.08 | 7.11 ± 0.16 | 3.7 | 0.58 | 15.0 |
| Mn | 1.39 ± 0.03 | 0.70 | 0.11 | ND | ND | ND | - |
| Fe | 75.8 ±1.7 | 38.0 | 5.93 | ND | ND | ND | - |
| Ni | 4.63 ± 0.10 | 2.30 | 0.36 | 1.70 ± 0.04 | 0.85 | 0.13 | 20.0 |
| Zn | 2.88 ± 0.06 | 0.001 | 0.22 | ND | ND | ND | - |
| As | 2.69 ± 0.062 | 1.29 | 0.20 | ND | ND | ND | 15.0 |
| Hg | 1.05 ± 0.024 | 0.50 | 0.08 | ND | ND | ND | 3.0 |
| Σ metal ion, nmol | 0.8785 | 0.0857 | |||||
| Pharmaceutical Active Ingredient | ||
| IFNβ-1a recombinant human | 22.0 μg (6 million units) (19.8–24.2 μg) | 44.0 μg (12 million units) (39.6–48.4 μg) |
| Supplementary Substances (EurPh, USP) | ||
| Benzyl alhocol | 2.5 mg | 2.5 mg |
| Mannitol | 22.5 mg | 22.5 mg |
| Methionine | 0.06 mg ± 0.01 | 0.06 mg ± 0.01 |
| Poloxamer 188 | 0.25 mg | 0.25 mg |
| 0.01 M acetate buffer pH 4.2 | 0.5 mL | 0.5 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berillo, D. Comparative Toxicity of Interferon Beta-1a Impurities of Heavy Metal Ions. Medicina 2022, 58, 463. https://doi.org/10.3390/medicina58040463
Berillo D. Comparative Toxicity of Interferon Beta-1a Impurities of Heavy Metal Ions. Medicina. 2022; 58(4):463. https://doi.org/10.3390/medicina58040463
Chicago/Turabian StyleBerillo, Dmitriy. 2022. "Comparative Toxicity of Interferon Beta-1a Impurities of Heavy Metal Ions" Medicina 58, no. 4: 463. https://doi.org/10.3390/medicina58040463
APA StyleBerillo, D. (2022). Comparative Toxicity of Interferon Beta-1a Impurities of Heavy Metal Ions. Medicina, 58(4), 463. https://doi.org/10.3390/medicina58040463