Do Gastric Signet Ring Cell Carcinomas and ECL-Cell Neuroendocrine Tumours Have a Common Origin?
Abstract
:1. Introduction
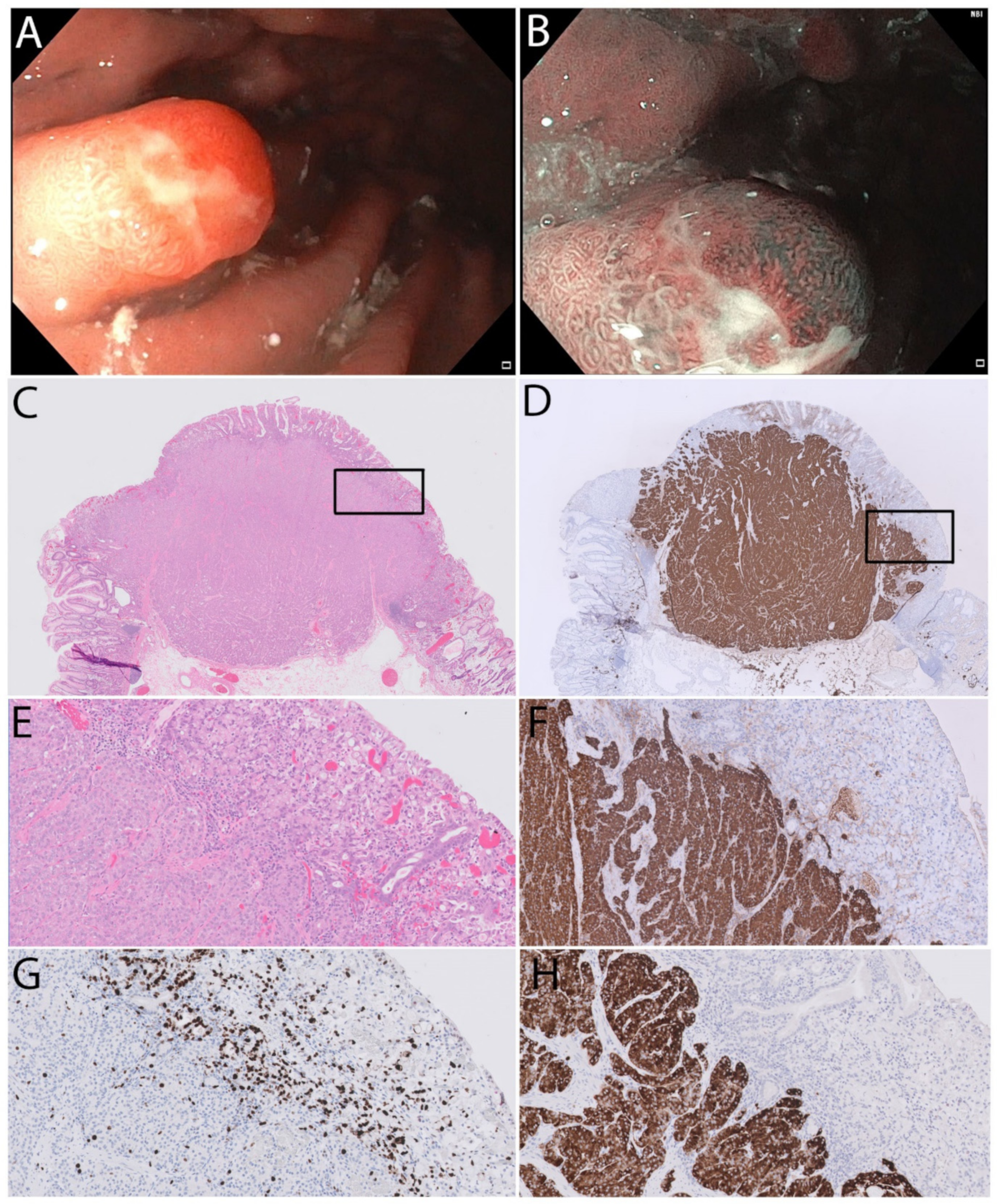
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fossmark, R.; Sordal, O.; Jianu, C.S.; Qvigstad, G.; Nordrum, I.S.; Boyce, M.; Waldum, H.L. Treatment of gastric carcinoids type 1 with the gastrin receptor antagonist netazepide (YF476) results in regression of tumours and normalisation of serum chromogranin A. Aliment. Pharmacol. Ther. 2012, 36, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Boyce, M.; Steele, I.A.; Campbell, F.; Varro, A.; Pritchard, D.M. Netazepide, a gastrin receptor antagonist, normalises tumour biomarkers and causes regression of type 1 gastric neuroendocrine tumours in a nonrandomised trial of patients with chronic atrophic gastritis. PLoS ONE 2013, 8, e76462. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Qvigstad, G.; Qvigstad, T.; Westre, B.; Sandvik, A.K.; Brenna, E.; Waldum, H.L. Neuroendocrine differentiation in gastric adenocarcinomas associated with severe hypergastrinemia and/or pernicious anemia. APMIS 2002, 110, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Bakkelund, K.; Fossmark, R.; Nordrum, I.; Waldum, H. Signet ring cells in gastric carcinomas are derived from neuroendocrine cells. J. Histochem. Cytochem. 2006, 54, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Kanomata, N. Neuroendocrine and mucinous differentiation in signet ring cell carcinoma of the stomach: Evidence for a common cell of origin in composite tumors. Hum. Pathol. 2012, 43, 1344. [Google Scholar] [CrossRef]
- Bartley, A.N.; Rashid, A.; Fournier, K.F.; Abraham, S.C. Neuroendocrine and mucinous differentiation in signet ring cell carcinoma of the stomach: Evidence for a common cell of origin in composite tumors. Hum. Pathol. 2011, 42, 1420–1429. [Google Scholar] [CrossRef]
- Fujiyoshi, Y.; Eimoto, T. Chromogranin A expression correlates with tumour cell type and prognosis in signet ring cell carcinoma of the stomach. Histopathology 2008, 52, 305–313. [Google Scholar] [CrossRef]
- Sjöblom, S.M.; Sipponen, P.; Miettinen, M.; Karonen, S.L.; Jrvinen, H.J. Gastroscopic screening for gastric carcinoids and carcinoma in pernicious anemia. Endoscopy 1988, 20, 52–56. [Google Scholar] [CrossRef]
- Solcia, E.; Bordi, C.; Creutzfeldt, W.; Dayal, Y.; Dayan, A.D.; Falkmer, S.; Grimelius, L.; Havu, N. Histopathological classification of nonantral gastric endocrine growths in man. Digestion 1988, 41, 185–200. [Google Scholar] [CrossRef]
- Bosman, F.T. World Health Organization Classification of Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Uğraş, N.; Sarkut, P.; Yerci, Ö.; Öztürk, E. Coexistence of gastric multiple neuroendocrine tumors with unusual morphological features and gastric signet-ring cell carcinoma. Turk. J. Surg. 2018, 34, 152–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness-Jensen, E.; Bringeland, E.A.; Mattsson, F.; Mjønes, P.; Lagergren, J.; Grønbech, J.E.; Waldum, H.L.; Fossmark, R. Hypergastrinemia is associated with an increased risk of gastric adenocarcinoma with proximal location: A prospective population-based nested case-control study. Int. J. Cancer 2021, 148, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Sandvik, A.K.; Brenna, E. Gastrin, the enterochromaffinlike cell, and gastric tumors. Gastroenterology 1993, 105, 1264–1266. [Google Scholar] [CrossRef]
- Calvete, O.; Reyes, J.; Zuniga, S.; Paumard-Hernandez, B.; Fernandez, V.; Bujanda, L.; Rodriguez-Pinilla, M.S.; Palacios, J.; Heine-Suner, D.; Banka, S.; et al. Exome sequencing identifies ATP4A gene as responsible of an atypical familial type I gastric neuroendocrine tumour. Hum. Mol. Genet. 2015, 24, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.; Calvete, O.; Mjønes, P.; Benitez, J.; Waldum, H.L. ECL-cell carcinoids and carcinoma in patients homozygous for an inactivating mutation in the gastric H(+) K(+) ATPase alpha subunit. APMIS 2016, 124, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, J.; Honda, S.; Kodama, M.; Sato, R.; Murakami, K.; Fujioka, T. Enterocromaffin-like cell tumor induced by Helicobacter pylori infection in Mongolian gerbils. Helicobacter 2002, 7, 390–397. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Digestive System Tumors. In WHO Classification of Tumors, 5th ed.; International Agency for Research on Cancer Press: Lyon, France, 2019. [Google Scholar]
- de Mestier, L.; Cros, J.; Neuzillet, C.; Hentic, O.; Egal, A.; Muller, N.; Bouché, O.; Cadiot, G.; Ruszniewski, P.; Couvelard, A.; et al. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology 2017, 105, 412–425. [Google Scholar] [CrossRef]
- Solcia, E.; Fiocca, R.; Villani, L.; Luinetti, O.; Capella, C. Hyperplastic, dysplastic, and neoplastic enterochromaffin-like-cell proliferations of the gastric mucosa. Classification and histogenesis. Am. J. Surg. Pathol. 1995, 19 (Suppl. 1), S1–S7. [Google Scholar]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef]
- Nikaido, M.; Kakiuchi, N.; Miyamoto, S.; Hirano, T.; Takeuchi, Y.; Funakoshi, T.; Yokoyama, A.; Ogasawara, T.; Yamamoto, Y.; Yamada, A.; et al. Indolent feature of Helicobacter pylori-uninfected intramucosal signet ring cell carcinomas with CDH1 mutations. Gastric Cancer 2021, 24, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Ringnes, E.; Nordbo, H.; Sordal, O.; Nordrum, I.S.; Hauso, O. The normal neuroendocrine cells of the upper gastrointestinal tract lack E-cadherin. Scand. J. Gastroenterol. 2014, 49, 974–978. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fossmark, R.; Johannessen, R.; Qvigstad, G.; Mjønes, P. Do Gastric Signet Ring Cell Carcinomas and ECL-Cell Neuroendocrine Tumours Have a Common Origin? Medicina 2022, 58, 470. https://doi.org/10.3390/medicina58040470
Fossmark R, Johannessen R, Qvigstad G, Mjønes P. Do Gastric Signet Ring Cell Carcinomas and ECL-Cell Neuroendocrine Tumours Have a Common Origin? Medicina. 2022; 58(4):470. https://doi.org/10.3390/medicina58040470
Chicago/Turabian StyleFossmark, Reidar, Rune Johannessen, Gunnar Qvigstad, and Patricia Mjønes. 2022. "Do Gastric Signet Ring Cell Carcinomas and ECL-Cell Neuroendocrine Tumours Have a Common Origin?" Medicina 58, no. 4: 470. https://doi.org/10.3390/medicina58040470
APA StyleFossmark, R., Johannessen, R., Qvigstad, G., & Mjønes, P. (2022). Do Gastric Signet Ring Cell Carcinomas and ECL-Cell Neuroendocrine Tumours Have a Common Origin? Medicina, 58(4), 470. https://doi.org/10.3390/medicina58040470





