Lateralizing Characteristics of Morphometric Changes to Hippocampus and Amygdala in Unilateral Temporal Lobe Epilepsy with Hippocampal Sclerosis
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. MRI Acquisition
2.3. MRI Analysis
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Participant Characteristics
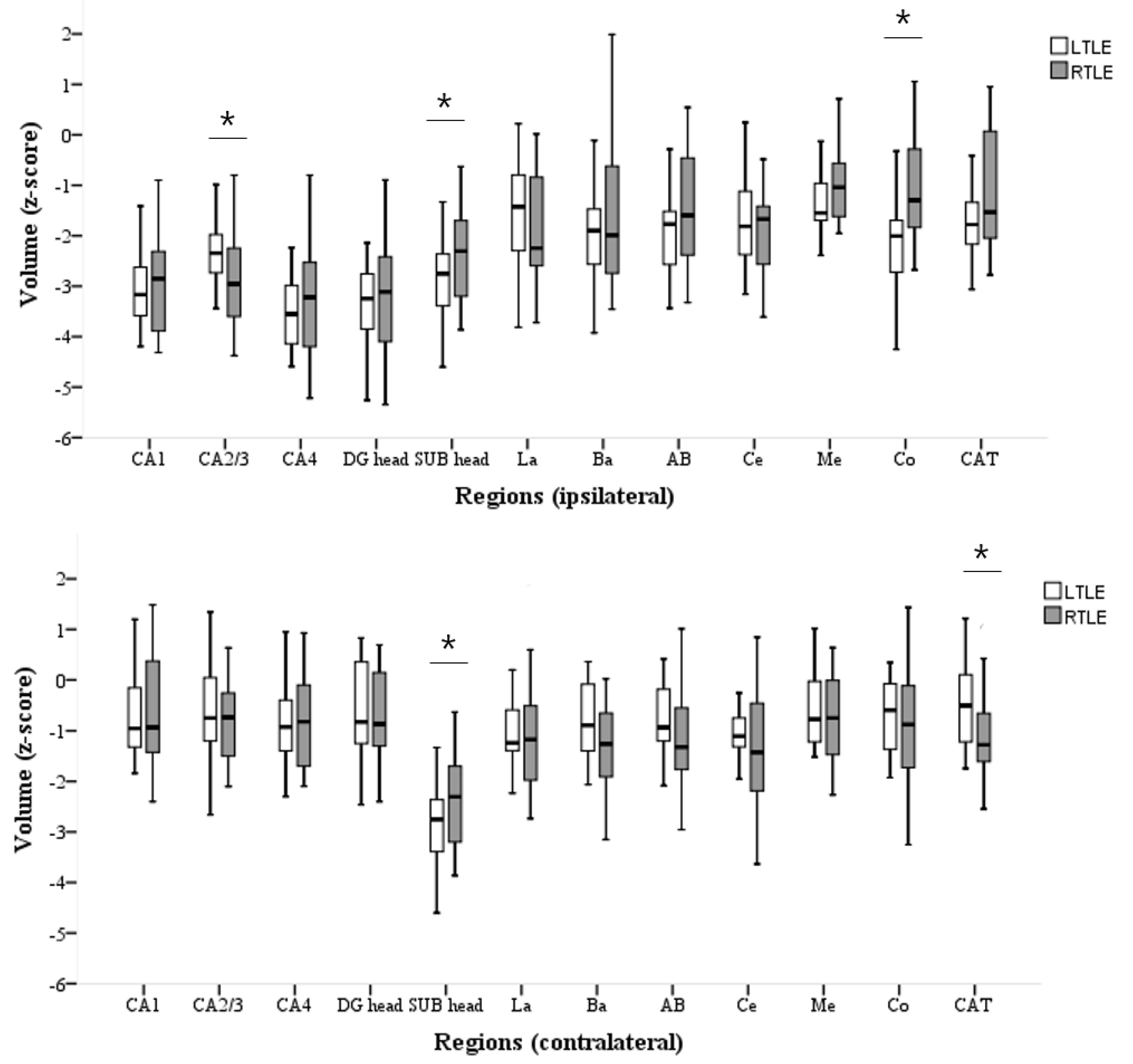
3.2. Volumetric Analyses of HC and AMG Subfields
3.3. Hippocampal Subfield Volumes and Clinical Correlations
3.4. AMG Subfield Volumes and Clinical Correlations
4. Discussion
4.1. Inhomogeneity of LTLE-HS and RTLE-HS Volume Losses
4.2. Inhomogeneous Clinical and MRI Features between LTLE-HS and RTLE-HS
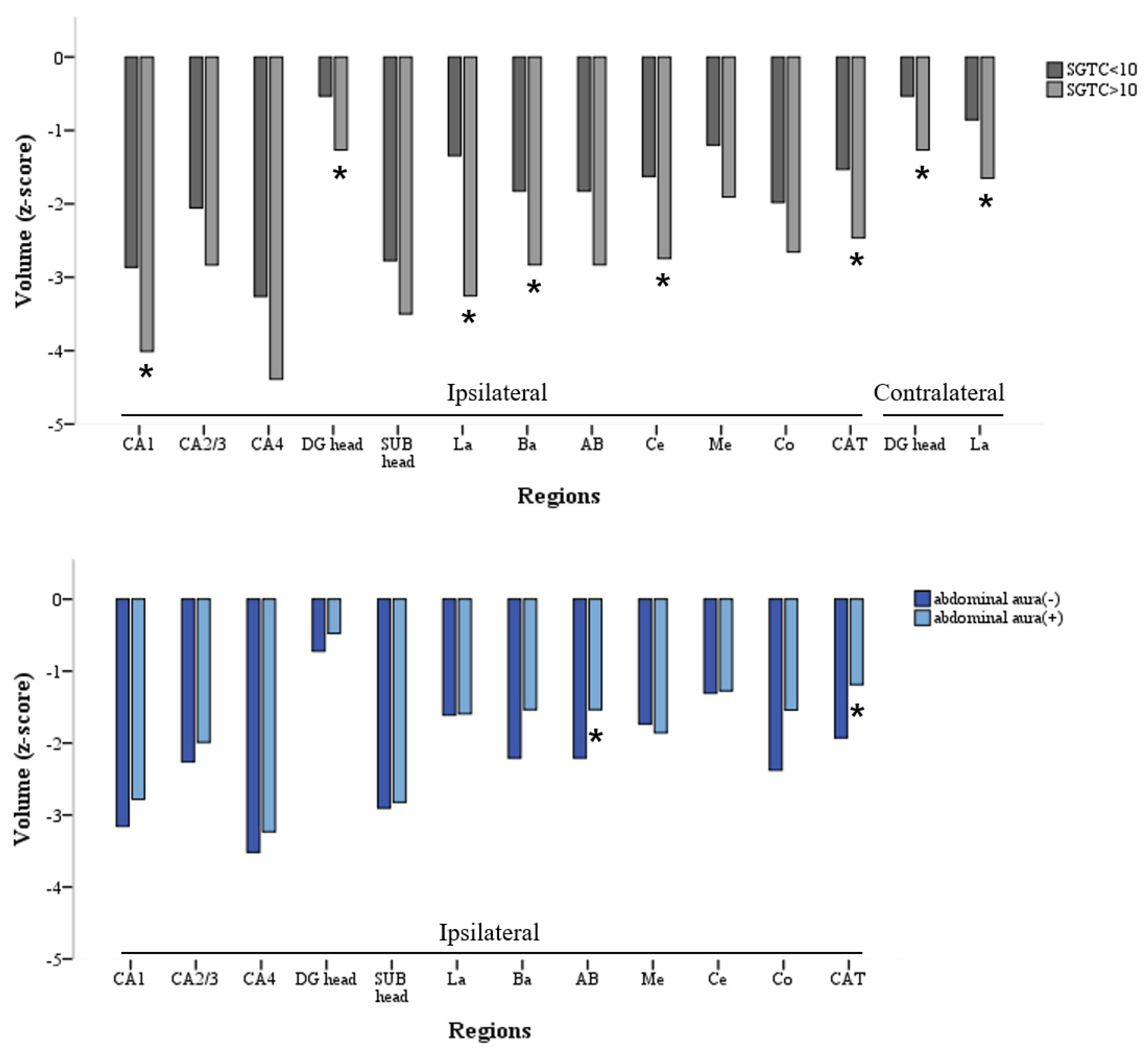
4.3. Bilateral and Widespread Damage from FTBTCS in LTLE-HS
4.4. Relationship between Abdominal FAS and Subfield Volume Change of AMG in LTLE-HS
4.5. Limitations of the Present Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Blumcke, I.; Spreafico, R.; Haaker, G.; Coras, R.; Kobow, K.; Bien, C.G.; Pfäfflin, M.; Elger, C.; Widman, G.; Schramm, J.; et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017, 377, 1648–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, C.R., Jr.; Rydberg, C.H.; Krecke, K.N.; Trenerry, M.R.; Parisi, J.E.; Rydberg, J.N.; Cascino, G.D.; Riederer, S.J. Mesial temporal sclerosis: Diagnosis with fluid-attenuated inversion-recovery versus spin-echo MR imaging. Radiology 1996, 199, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Berkovic, S.F.; Andermann, F.; Olivier, A.; Ethier, R.; Melanson, D.; Robitaille, Y.; Kuzniecky, R.; Peter, T.; Feindel, W. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann. Neurol. 1991, 29, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.J.; Bentley, M.D.; Twomey, C.K.; Zinsmeister, A.R. MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe: Validation studies. Radiology 1990, 176, 205–209. [Google Scholar] [CrossRef]
- Jackson, G.D.; Berkovic, S.F.; Tress, B.M.; Kalnins, R.M.; Fabinyi, G.C.; Bladin, P.F. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology 1990, 40, 1869–1875. [Google Scholar] [CrossRef]
- Malmgren, K.; Thom, M. Hippocampal sclerosis—Origins and imaging. Epilepsia 2012, 53, 19–33. [Google Scholar] [CrossRef]
- Mueller, C.A.; Scorzin, J.; von Lehe, M.; Fimmers, R.; Helmstaedter, C.; Zentner, J.; Lehman, T.-N.; Meenckle, H.-J.; Schulze-Bonhage, A.; Schramm, J. Seizure outcome 1 year after temporal lobe epilepsy: An analysis of MR volumetric and clinical parameters. Acta Neurochir. 2012, 154, 1327–1336. [Google Scholar] [CrossRef]
- Bonilha, L.; Keller, S.S. Quantitative MRI in refractory temporal lobe epilepsy: Relationship with surgical outcomes. Quant. Imaging. Med. Surg. 2015, 5, 204–224. [Google Scholar]
- Du, F.; Whetsell, W.O.; Jr Abou-Khalil, B.; Blumenkopf, B.; Lothman, E.W.; Schwarcz, R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993, 16, 223–233. [Google Scholar] [CrossRef]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Bartolomei, F.; Bernasconi, A.; Bernasconi, N.; Bien, C.G.; Cendes, F.; Coras, R. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013, 54, 1315–1329. [Google Scholar] [CrossRef]
- Van Leemput, K.; Bakkour, A.; Benner, T.; Wiggins, G.; Wald, L.L.; Augustinack, J.; Dickerson, B.C.; Golland, P.; Fischl, B. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus 2009, 19, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pipitone, J.; Park, M.T.; Winterburn, J.; Lett, T.A.; Lerch, J.P.; Pruessner, J.C.; Lepage, M.; Voineskos, A.N.; Chakravarty, M.M. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage 2014, 101, 494–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilha, L.; Kobayashi, E.; Rorden, C.; Cendes, F.; Li, L.M. Medial temporal lobe atrophy in patients with refractory temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1627–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernasconi, N.; Natsume, J.; Bernasconi, A. Progression in temporal lobe epilepsy: Differential atrophy in mesial temporal structures. Neurology 2005, 65, 223–228. [Google Scholar] [CrossRef]
- Guerreiro, C.; Cendes, F.; Li, L.M.; Jones-Gotman, M.; Andermann, F.; Dubeau, F.; Piazzini, A.; Feindel, W. Clinical patterns of patients with temporal lobe epilepsy and pure amygdalar atrophy. Epilepsia 1999, 40, 453–461. [Google Scholar] [CrossRef]
- Morey, R.A.; Petty, C.M.; Xu, Y.; Hayes, J.P.; Wagner, H.R., 2nd; Lewis, D.V.; LaBar, K.S.; Styner, M.; McCarthy, G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 2009, 45, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Pardoe, H.R.; Pell, G.S.; Abbott, D.F.; Jackson, G.D. Hippocampal volume assessment in temporal lobe epilepsy: How good is automated segmentation? Epilepsia 2009, 50, 2586–2592. [Google Scholar] [CrossRef] [Green Version]
- Sone, D.; Sato, N.; Maikusa, N.; Ota, M.; Sumida, K.; Yokoyama, K.; Kimura, Y.; Imabayashi, E.; Watanabe, Y.; Watanabe, M. Automated subfield volumetric analysis of hippocampus in temporal lobe epilepsy using high-resolution T2-weighed MR imaging. Neuroimage Clin. 2016, 12, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Kreilkamp, B.A.K.; Weber, B.; Elkommos, S.B.; Richardson, M.P.; Keller, S.S. Hippocampal subfield segmentation in temporal lobe epilepsy: Relation to outcomes. Acta. Neurol. Scand. 2018, 137, 598–608. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.S.; Richardson, M.P.; Schoene-Bake, J.C.; O’Muircheartaigh, J.; Elkommos, S.; Kreilkamp, B.; Goh, Y.Y.; Marson, A.G.; Elger, C.; Weber, B. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann. Neurol. 2015, 77, 760–774. [Google Scholar] [CrossRef] [Green Version]
- Wieser, H.G.; Blume, W.T.; Fish, D.; Goldensohn, E.; Hufnagel, A.; King, D.; Sperling, M.R.; Lüders, H.; Pedley, T.A. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 2001, 42, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Suh, S.; Joo, E.Y.; Hong, S.B. Morphological alterations in amygdalo-hippocampal substructures in narcolepsy patients with cataplexy. Brain Imaging Behav. 2016, 10, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, G.; Pievani, M.; Jovicich, J.; Aiello, M.; Bargallo, N.; Barkhof, F.; Bartres-Faz, D.; Betramello, A.; Pizinni, F.B.; Blin, O. Amygdalar nuclei and hippocampal subfields on MRI: Test-retest reliability of automated volumetry across different MRI sites and vendors. Neuroimage 2020, 218, 116932. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.S.; Cresswell, P.; Denby, C.; Wieshmann, U.; Eldridge, P.; Baker, G.; Roberts, N. Persistent seizures following left temporal lobe surgery are associated with posterior and bilateral structural and functional brain abnormalities. Epilepsy Res. 2007, 74, 131–139. [Google Scholar] [CrossRef]
- Keller, S.S.; Roberts, N. Voxel-based morphometry of temporal lobe epilepsy: An introduction and review of the literature. Epilepsia 2008, 49, 741–757. [Google Scholar] [CrossRef]
- Costa, B.S.; Santos, M.C.V.; Rosa, D.V.; Schutze, M.; Miranda, D.M.; Romano-Silva, M.A. Automated evaluation of hippocampal subfields volumes in mesial temporal lobe epilepsy and its relationship to the surgical outcome. Epilepsy Res. 2019, 154, 152–156. [Google Scholar] [CrossRef]
- Bernhardt, B.C.; Kim, H.; Bernasconi, N. Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology 2013, 81, 1840–1847. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.S.; Schoene-Bake, J.C.; Gerdes, J.S.; Weber, B.; Deppe, M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: Cross-sectional evidence for progressive neurologic injury. PLoS ONE 2012, 7, e46791. [Google Scholar]
- Coan, A.C.; Appenzeller, S.; Bonilha, L.; Li, L.M.; Cendes, F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 2009, 73, 834–842. [Google Scholar] [CrossRef]
- García-Fiñana, M.; Denby, C.E.; Keller, S.S.; Wieshmann, U.C.; Roberts, N. Degree of Hippocampal Atrophy Is Related to Side of Seizure Onset in Temporal Lobe Epilepsy. Am. J. Neuroradiol. 2006, 27, 1046–1052. [Google Scholar]
- Pail, M.; Brázdil, M.; Mareček, R.; Mikl, M. An optimized voxel-based morphometric study of gray matter changes in patients with left-sided and right-sided mesial temporal lobe epilepsy and hippocampal sclerosis (MTLE/HS). Epilepsia 2010, 51, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bernhardt, B.C.; Bernasconi, A.; Bernasconi, N. Gray matter structural compromise is equally distributed in left and right temporal lobe epilepsy. Hum. Brain Mapp. 2016, 37, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, D.; Shah, J.; Kupsky, W.J.; Johnson, R.; Shah, A.; Hayman-Abello, B.; Ergh, T.; Poore, Q.; Canady, A.; Watson, C. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology 2001, 57, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Seidenberg, M.; Kelly, K.G.; Parrish, J.; Geary, E.; Dow, C.; Rutecki, P.; Hermann, B. Ipsilateral and Contralateral MRI Volumetric Abnormalities in Chronic Unilateral Temporal Lobe Epilepsy and their Clinical Correlates. Epilepsia 2005, 46, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Theodore, W.H.; Bhatia, S.; Hatta, J.; Fazilat, S.; DeCarli, C.; Bookheimer, S.Y.; Gaillard, W.D. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology 1999, 52, 132. [Google Scholar] [CrossRef] [PubMed]
- Cendes, F.; Andermann, F.; Gloor, P.; Lopes-Cendes, I.; Andermann, E.; Melanson, D.; Jones-Gotman, M.; Robitaille, Y.; Evans, A.; Peters, T. Atrophy of mesial structures in patients with temporal lobe epilepsy: Cause or consequence of repeated seizures? Ann. Neurol. 1993, 34, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, D.; Shah, J.; Shah, A.; Watson, C. Hippocampal sclerosis is a progressive disorder: A longitudinal volumetric MRI study. Ann. Neurol. 2003, 53, 413–416. [Google Scholar] [CrossRef]
- Andrade-Valenca, L.P.; Valenca, M.M.; Ribeiro, L.T.; Matos, A.L.; Sales, L.V.; Velasco, T.R.; Santos, A.C.; Leite, J.P. Clinical and neuroimaging features of good and poor seizure control patients with mesial temporal lobe epilepsy and hippocampal atrophy. Epilepsia 2003, 44, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Tasch, E.; Cendes, F.; Li, L.M.; Dubeau, F.; Andermann, F.; Arnold, D.L. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann. Neurol. 1999, 45, 568–576. [Google Scholar] [CrossRef]
- Rektor, I.; Zakopcan, J.; Tyrlikova, I.; Kuba, R.; Brazdil, M.; Chrastina, J.; Novák, Z. Secondary generalization in seizures of temporal lobe origin: Ictal EEG pattern in a stereo-EEG study. Epilepsy Behav. 2009, 15, 235–239. [Google Scholar] [CrossRef]
- Cook, M.J.; Fish, D.R.; Shorvon, S.D.; Straughan, K.; Stevens, J.M. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain 1992, 115, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.F.; Lemieux, L.; Kitchen, N.D.; Fish, D.R.; Shorvon, S.D. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain 2001, 124, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmazer-Hanke, D.M.; Wolf, H.K.; Schramm, J.; Elger, C.E.; Wiestler, O.D.; Blümcke, I. Subregional Pathology of the Amygdala Complex and Entorhinal Region in Surgical Specimens from Patients with Pharmacoresistant Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2000, 59, 907–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avery, R.A.; Zubal, I.G.; Studholme, C.; Slawski, J.; Corsi, M.; Spencer, D.D.; Spences, S.S. Interictal 99mTc-HMPAO SPECT in temporal lobe epilepsy: Relation to clinical variables. Epilepsia 2001, 42, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Blume, W.T. Clinical and electroencephalographic correlates of the multiple independent spike foci pattern in children. Ann. Neurol. 1978, 4, 541–547. [Google Scholar] [CrossRef]
- Savic, I.; Altshuler, L.; Baxter, L.; Engel, J., Jr. Pattern of interictal hypometabolism in PET scans with fludeoxyglucose F 18 reflects prior seizure types in patients with mesial temporal lobe seizures. Arch. Neurol. 1997, 54, 129–136. [Google Scholar] [CrossRef]
- Njiokiktjien, C. Differences in vulnerability between the hemispheres in early childhood and adulthood. Fiziol. Cheloveka 2006, 32, 45–50. [Google Scholar] [CrossRef]
- Kemmotsu, N.; Girard, H.M.; Bernhardt, B.C.; Bonilha, L.; Lin, J.J.; Tecoma, E.S.; Iragui, V.J.; Hagler, D.J., Jr.; Halgren, E.; McDonald, C.R. MRI analysis in temporal lobe epilepsy: Cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 2011, 52, 2257–2266. [Google Scholar] [CrossRef] [Green Version]
- Fakhoury, T.; Abou-Khalil, B.; Peguero, E. Differentiating Clinical Features of Right and Left Temporal Lobe Seizures. Epilepsia 1994, 35, 1038–1044. [Google Scholar] [CrossRef]
- Moon, H.J.; Chung, C.K.; Lee, S.K. Surgical Prognostic Value of Epileptic Aura Based on History and Electrical Stimulation. J. Epilepsy Res. 2019, 9, 111–118. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nei, M.; Sharan, A.; Sperling, M.R. Type of preoperative aura may predict postsurgical outcome in patients with temporal lobe epilepsy and mesial temporal sclerosis. Epilepsy Behav. 2015, 50, 98–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan, Y.-C.; Shih, Y.-H.; Chen, C.; Yu, H.-Y.; Yiu, C.-H.; Lin, Y.-Y.; Kwan, S.-Y.; Yen, D.-J. Abdominal auras in patients with mesial temporal sclerosis. Epilepsy Behav. 2012, 25, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage 2015, 115, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Santos, J.E.; de Carvalho, L.E.D.; Kandratavicius, L.; Diniz, P.R.B.; Scandiuzzi, R.C.; Coras, R.; Blümcke, I.; Assirati, J.A.; Carlotti, C.G.; Matias, C.C.M.S. Manual Hippocampal Subfield Segmentation Using High-Field MRI: Impact of Different Subfields in Hippocampal Volume Loss of Temporal Lobe Epilepsy Patients. Front. Neurol. 2018, 9, 927. [Google Scholar] [CrossRef]
| LTLE (n = 22) | RTLE (n = 26) | HC (n = 28) | p-Value | |
|---|---|---|---|---|
| Age at MRI (years) | 28.7 ± 10.6 | 34.2 ± 12.1 | 38.4 ± 6.4 | 0.084 a |
| Male, n (%) | 5 (22.7%) | 10 (38.5%) | 12 (42.9%) | 0.293 b |
| Age at epilepsy onset (years) | 14.8 ± 10.4 | 17.4 ± 12.8 | 0.576 c | |
| Duration of epilepsy (years) | 16.7 ± 10.4 | 16.7 ± 12.9 | 0.780 c | |
| Frequency of FAS (/month) | 4.4 ± 5.3 | 12.2 ± 35.0 | 0.552 c | |
| Frequency of FIAS (/month) | 7.8 ± 18.6 | 10.2 ± 29.2 | 0.362 c | |
| History of febrile seizure, n (%) | 10 (45.5%) | 13 (50.0%) | 0.753 b | |
| SGTCS > 10, n (%) | 3 (13.6%) | 4 (15.4%) | 0.864 b | |
| Abdominal FAS, n (%) | 8 (36.4%) | 18 (69.2%) | 0.023 b | |
| Psychic FAS, n (%) | 12 (54.5%) | 7 (26.9%) | 0.051 b | |
| Surgical resection, n (%) | 17 (77.3%) | 20 (76.9%) |
| Age at Epilepsy Onset | Duration of Epilepsy | Frequency of FAS | Frequency of FIAS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Side | Region | LTLE | RTLE | LTLE | RTLE | LTLE | RTLE | LTLE | RTLE | |
| Ipsilateral | Hippocampus | CA1 head | 0.204 | 0.066 | −0.499 * | −0.453 * | 0.207 | 0.290 | 0.151 | 0.367 |
| CA1 | 0.246 | 0.127 | −0.484 * | −0.442 * | 0.165 | 0.291 | 0.120 | 0.374 | ||
| CA2/3 head | 0.079 | −0.021 | −0.438 | −0.421 * | 0.310 | 0.477 | 0.254 | 0.261 | ||
| CA2/3 | 0.046 | 0.101 | −0.385 | −0.468 * | 0.209 | 0.343 | 0.154 | 0.329 | ||
| CA4 head | 0.144 | 0.017 | −0.600 * | −0.485 * | 0.142 | 0.396 | 0.046 | 0.323 | ||
| CA4 | 0.380 | 0.269 | −0.316 | −0.475 * | 0.078 | 0.238 | −0.148 | 0.332 | ||
| DG head | 0.150 | 0.030 | −0.587 * | −0.464 * | 0.217 | 0.385 | 0.153 | 0.309 | ||
| DG body | 0.348 | 0.280 | −0.360 | −0.506 * | 0.111 | 0.263 | −0.171 | 0.318 | ||
| SUB head | 0.419 | 0.159 | −0.165 | −0.423 * | −0.091 | 0.202 | −0.076 | 0.238 | ||
| SUB body | 0.164 | 0.358 | −0.143 | −0.592 * | 0.031 | 0.196 | −0.132 | 0.305 | ||
| Amygdala | La | −0.055 | 0.107 | −0.185 | −0.388 | −0.234 | 0.072 | −0.114 | 0.082 | |
| Ba | −0.020 | 0.107 | −0.250 | −0.375 | −0.055 | 0.108 | 0.042 | 0.285 | ||
| AB | 0.020 | 0.065 | −0.189 | −0.313 | −0.054 | 0.114 | 0.164 | 0.317 | ||
| Ce | 0.442 * | 0.298 | −0.535 * | −0.310 | −0.378 | −0.020 | −0.218 | 0.322 | ||
| Me | 0.483 * | 0.381 | −0.535 * | −0.392 * | −0.074 | 0.476 * | 0.112 | 0.264 | ||
| Co | −0.001 | 0.306 | −0.006 | −0.407 * | 0.106 | 0.195 | 0.210 | 0.276 | ||
| CAT | −0.035 | 0.024 | −0.155 | −0.273 | −0.045 | 0.210 | 0.123 | 0.327 | ||
| Contralateral | Hippocampus | CA1 head | −0.123 | 0.197 | −0.287 | −0.031 | 0.292 | 0.064 | −0.015 | 0.001 |
| CA1 | −0.233 | 0.280 | −0.205 | −0.121 | 0.270 | 0.029 | 0.088 | −0.014 | ||
| CA2/3 head | −0.417 | 0.256 | −0.173 | −0.192 | 0.211 | 0.036 | 0.124 | −0.080 | ||
| CA2/3 | −0.445 | 0.305 | −0.097 | −0.128 | 0.226 | −0.033 | 0.124 | −0.183 | ||
| CA4 head | −0.353 | 0.239 | −0.282 | −0.222 | 0.226 | 0.096 | 0.002 | −0.005 | ||
| CA4 | −0.263 | 0.221 | −0.022 | −0.196 | 0.254 | 0.020 | −0.007 | −0.328 | ||
| DG head | −0.266 | 0.162 | −0.331 | −0.135 | 0.289 | 0.053 | 0.052 | −0.026 | ||
| DG body | −0.199 | 0.148 | −0.027 | −0.188 | 0.221 | 0.083 | −0.050 | −0.280 | ||
| SUB head | −0.059 | 0.146 | 0.035 | 0.000 | 0.015 | 0.096 | 0.008 | 0.054 | ||
| SUB body | 0.052 | 0.272 | 0.122 | −0.273 | 0.177 | −0.235 | −0.113 | −0.136 | ||
| Amygdala | La | −0.046 | 0.300 | −0.323 | −0.234 | 0.071 | 0.264 | −0.123 | 0.014 | |
| Ba | −0.177 | 0.196 | −0.322 | −0.120 | 0.227 | 0.213 | 0.020 | 0.108 | ||
| AB | −0.187 | 0.208 | −0.278 | −0.020 | 0.381 | 0.057 | 0.239 | −0.038 | ||
| Ce | 0.069 | 0.116 | −0.306 | −0.048 | 0.052 | 0.125 | −0.007 | 0.126 | ||
| Me | 0.153 | 0.074 | −0.406 | 0.027 | −0.092 | 0.225 | 0.285 | 0.320 | ||
| Co | −0.073 | 0.234 | −0.301 | −0.021 | 0.221 | 0.060 | 0.342 | −0.076 | ||
| CAT | −0.341 | 0.183 | −0.170 | 0.092 | 0.364 | 0.125 | 0.221 | 0.013 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Kim, J.; Kim, D.; Hwang, Y.; Seo, D.; Hong, S.; Shon, Y.-M. Lateralizing Characteristics of Morphometric Changes to Hippocampus and Amygdala in Unilateral Temporal Lobe Epilepsy with Hippocampal Sclerosis. Medicina 2022, 58, 480. https://doi.org/10.3390/medicina58040480
Jo H, Kim J, Kim D, Hwang Y, Seo D, Hong S, Shon Y-M. Lateralizing Characteristics of Morphometric Changes to Hippocampus and Amygdala in Unilateral Temporal Lobe Epilepsy with Hippocampal Sclerosis. Medicina. 2022; 58(4):480. https://doi.org/10.3390/medicina58040480
Chicago/Turabian StyleJo, Hyunjin, Jeongsik Kim, Dongyeop Kim, Yoonha Hwang, Daewon Seo, Seungbong Hong, and Young-Min Shon. 2022. "Lateralizing Characteristics of Morphometric Changes to Hippocampus and Amygdala in Unilateral Temporal Lobe Epilepsy with Hippocampal Sclerosis" Medicina 58, no. 4: 480. https://doi.org/10.3390/medicina58040480






