Primary Lymphocutaneous Nocardia brasiliensis in an Immunocompetent Host: Case Report and Literature Review
Abstract
:1. Introduction
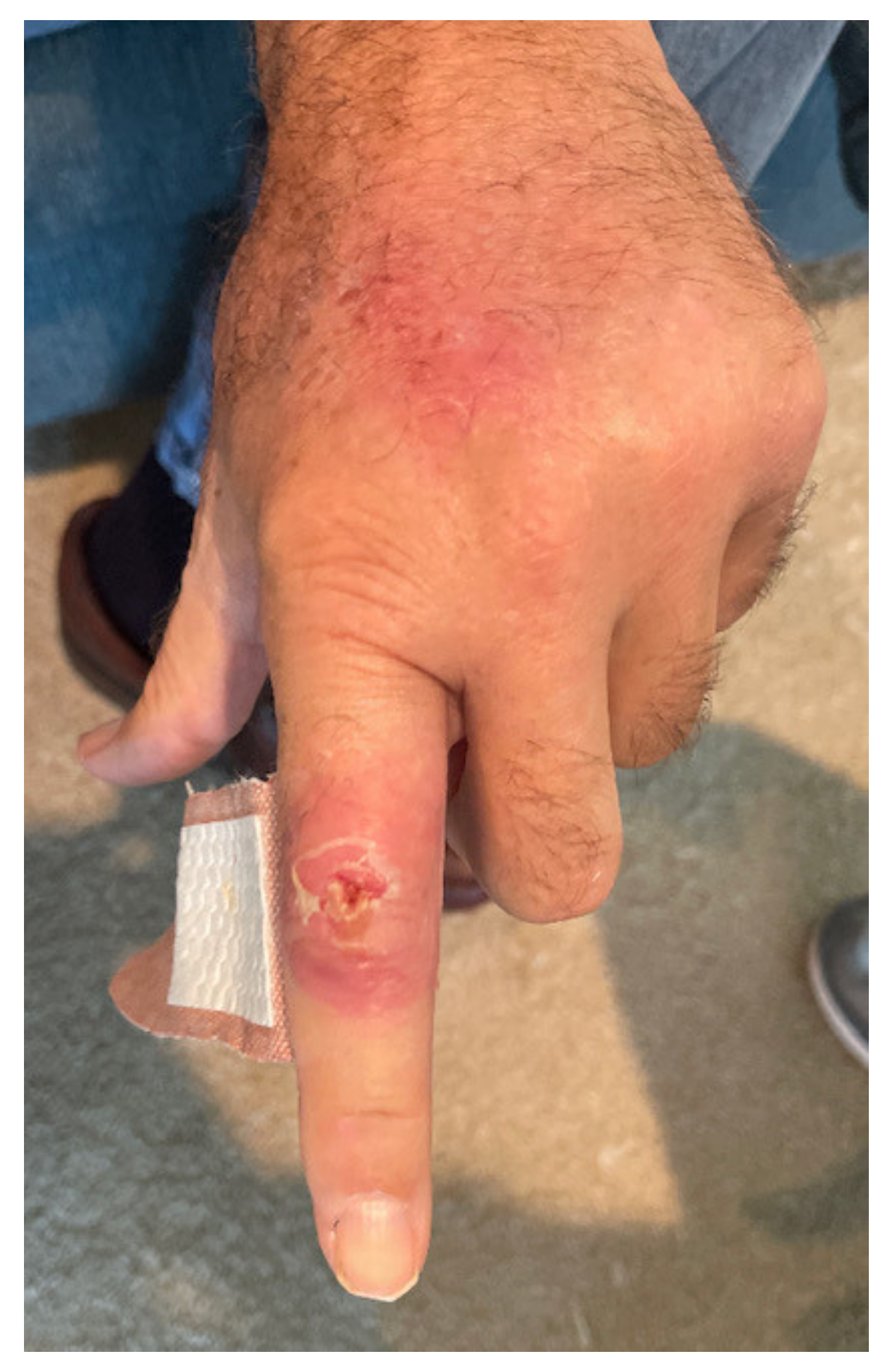
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nocard, E.I.E. Note sur la maladie des boeufs de la Guadeloupe, connue sous le nom de farcin. Ann. Inst. Pasteur. 1888, 2, 293–302. [Google Scholar]
- Eppinger, H. Ueber eine neue pathogenic Cladothrix und eine durch sie hervorgerufene Pseudotuberculosis. Wiener Klinische Wochenschrift 1890, 3, 321–323. [Google Scholar]
- Sharer, L.R.; Kapila, R. Neuropathologic observations in acquired immunodeficiency syndrome (AIDS). Acta Neuropathol. 1985, 66, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.L.; Barrio, J.L.; Pitchenik, A.E. Pulmonary nocardiosis in the acquired immunodeficiency syndrome: Diagnosis with bronchoalveolar lavage and treatment with non-sulphur containing drugs. Chest 1986, 90, 912–914. [Google Scholar] [CrossRef]
- Martínez-Barricarte, R. Isolated Nocardiosis, an Unrecognized Primary Immunodeficiency? Front. Immunol. 2020, 11, 590239. [Google Scholar] [CrossRef]
- Mehta, H.H.; Shamoo, Y. Pathogenic Nocardia: A diverse genus of emerging pathogens or just poorly recognized? PLoS Pathog. 2020, 16, e1008280. [Google Scholar] [CrossRef] [Green Version]
- Nocardiosis Information for Healthcare Workers. Available online: https://www.cdc.gov/nocardiosis/health-care-workers/index.html (accessed on 10 February 2022).
- Spelman, D. Clinical Manifestations and Diagnosis of Nocardiosis. Available online: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-nocardiosis#H6 (accessed on 10 February 2022).
- Wilson, J.W. Nocardiosis: Updates and Clinical Overview. Mayo. Clin. Proc. 2012, 87, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Carmona, M.C.; Rosas-Taraco, A.G.; Welsh, O. Systemic increased immune response to Nocardia brasiliensis co-exists with local immunosuppressive microenvironment. Antonie Van Leeuwenhoek 2012, 102, 473–480. [Google Scholar] [CrossRef]
- Nouioui, I.; Cortés-Albayay, C.; Neumann-Schaal, M.; Vicente, D.; Cilla, G.; Klenk, H.-P.; Marimón, J.M.; Ercibengoa, M. Genomic Virulence Features of Two Novel Species Nocardia barduliensis sp. nov. and Nocardia gipuzkoensis sp. nov., Isolated from Patients with Chronic Pulmonary Diseases. Microorganisms 2020, 8, 1517. [Google Scholar] [CrossRef]
- Filice, G.A.; Beaman, B.L.; Krick, J.A.; Remington, J.S. Effects of Human Neutrophils and Monocytes on Nocardia asteroides: Failure of Killing despite Occurrence of the Oxidative Metabolic Burst. J. Infect. Dis. 1980, 142, 432–438. [Google Scholar] [CrossRef]
- Filice, G.A. Resistance of Nocardia asteroides to Oxygen-Dependent Killing by Neutrophils. J. Infect. Dis. 1983, 148, 861–867. [Google Scholar] [CrossRef]
- Beaman, B.L.; Beaman, L. Nocardia species: Host-parasite relationships. Clin. Microbiol. Rev. 1994, 7, 213–264. [Google Scholar] [CrossRef]
- Riviere, F.; Billhot, M.; Soler, C.; Vaylet, F.; Margery, J. Pulmonary nocardiosis in immunocompetent patients: Can COPD be the only risk factor? Eur. Respir. Rev. 2011, 20, 210–212. [Google Scholar] [CrossRef] [Green Version]
- Smego, R.A.; Gallis, H.A. The Clinical Spectrum of Nocardia brasiliensis Infection in the United States. Clin. Infect. Dis. 1984, 6, 164–180. [Google Scholar] [CrossRef]
- Margalit, I.; Lebeaux, D.; Tishler, O.; Goldberg, E.; Bishara, J.; Yahav, D.; Coussement, J. How do I manage nocardiosis? Clin. Microbiol. Infect. 2021, 27, 550–558. [Google Scholar] [CrossRef]
- Colaneri, M.; Lombardi, A.; Morea, A.; Monzillo, V.; Mariani, B.; Marone, M.; Sciarra, M.; Sambo, M.; Brunetti, E.; Bruno, R.; et al. An eight-year experience of Nocardia infection in Italy: Does immunosuppression matter? New Microbiol. 2021, 44, 111–116. [Google Scholar]
- Maraki, S.; Scoulica, E.; Alpantaki, K.; Dialynas, M.; Tselentis, Y. Lymphocutaneous nocardiosis due to Nocardia brasiliensis. Diagn. Microbiol. Infect. Dis. 2003, 47, 341–344. [Google Scholar] [CrossRef]
- Tarchini, G.; Ross, F.S. Primary lymphocutaneous nocardiosis associated with gardening: A case series. World J. Clin.Infect. Dis. 2013, 3, 86–89. [Google Scholar] [CrossRef]
- Maraki, S.; Chochlidakis, S.; Nioti, E.; Tselentis, Y. Primary lymphocutaneous nocardiosis in an immunocompetent patient. Ann. Clin. Microbiol. Antimicrob. 2004, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xu, X.; Wu, M.; Zhu, J.; Cen, P.; Ding, J.; Wu, S.; Jin, J. Lymphocutaneous nocardiosis caused by Nocardia brasiliensis in an immunocompetent patient: A case report. J. Int. Med. Res. 2020, 48, 300060519897690. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.W.; Brodell, L.A.; Lambert, E.; Menegus, M.; Scott, G.A.; Tu, J.H. Primary cutaneous Nocardia brasiliensis infection isolated in an immunosuppressed patient: A case report. Cutis 2012, 89, 75–77. [Google Scholar]
- Bryant, E.; Davis, C.L.; Kucenic, M.J.; Mark, L.A. Lymphocutaneous nocardiosis: A case report and review of the literature. Cutis 2010, 85, 73–76. [Google Scholar]
- Schwartz, J.G.; McGough, D.A.; Thorner, R.E.; Fetchick, R.J.; Tio, F.O.; Rinaldi, M.G. Primary lymphocutaneous Nocardia brasiliensis infection: Three case reports and a review of the literature. Diagn. Microbiol. Infect. Dis. 1988, 10, 113–120. [Google Scholar] [CrossRef]
- Secchin, P.; Trope, B.M.; Fernandes, L.A.; Barreiros, G.; Ramos-E-Silva, M. Cutaneous Nocardiosis Simulating Cutaneous Lymphatic Sporotrichosis. Case Rep. Dermatol. 2017, 9, 119–129. [Google Scholar] [CrossRef]
- Palmieri, J.R.; Santo, A.; Johnson, S.E. Soil-acquired cutaneous nocardiosis on the forearm of a healthy male contracted in a swamp in rural eastern Virginia. Int. Med. Case Rep. J. 2014, 7, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Sheffer, S.; Shreberk-Hassidim, R.; Olshtain, K.; Maly, A.; Zlotogorski, A.; Ramot, Y. Lymphocutaneous nocardiosis caused by Nocardia brasiliensis in an immunocompetent elderly woman. Int. J. Derm. 2016, 55, e45–e47. [Google Scholar] [CrossRef]
- Williams, P.C.M.; Bartlett, A.W.; Palasanthiran, P.; McMullan, B. A not so innocuous playground fall: Lymphocutaneous nocardiosis in an immunocompetent boy. Arch. Dis. Child. 2021, 107, 257–258. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Ding, X.J.; Chen, Y.J.; Wang, L.; Zhang, Z.Z. Diagnosis and treatment of lymphocutaneous dermatosis caused by Nocardia brasiliensis: A case report. Ann. Palliat. Med. 2020, 9, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Xu, X.-G.; Zhang, M.; Jiang, P.; Zhou, X.-Y.; Li, Z.-Z.; Zhang, M.-F. Sporotrichosis: Clinical and Histopathological Manifestations. Am. J. Dermatopathol. 2011, 33, 296–302. [Google Scholar] [CrossRef]
- Marshall, K.R.; Walton, S.A.; Boyd, M.; Bishop, B.; Wellehan, J.; Craft, W.; Santoro, D. Erysipeloid lesions caused by Erysipelothrix rhusiopathiae in a dog: Clinical and histopathological findings, molecular diagnosis and treatment. Vet. Dermatol. 2019, 30, 434-e134. [Google Scholar] [CrossRef]
- Lamps, L.W.; Havens, J.M.; Sjöstedt, A.; Page, D.L.; Scott, M.A. Histologic and molecular diagnosis of tularemia: A potential bioterrorism agent endemic to North America. Mod. Pathol. 2004, 17, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, H.; Gunathilaka, N.; Semege, S.; Pathirana, N.; Manamperi, N.; De Silva, C.; Fernando, D. Histopathology of Cutaneous Leishmaniasis Caused by Leishmania donovani in Sri Lanka. Biomed. Res. Int. 2020, 2020, 4926819. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, S.; Ahmed, F. Histopathological Approach in Diagnosis of Mycetoma Causative Agents: A Mini Review. J. Cytol. Histol. 2017, 8, 466. [Google Scholar]
- Li, J.J.; Beresford, R.; Fyfe, J.; Henderson, C. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: A review of 13 cases. J. Cutan. Pathol. 2017, 44, 433–443. [Google Scholar] [CrossRef]
- Van de Sande Wendy, W.J. Global burden of human mycetoma: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2013, 7, e2550. [Google Scholar] [CrossRef] [Green Version]
- Palmer, D.L.; Harvey, R.L.; Wheeler, J.K. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine 1974, 53, 391–401. [Google Scholar] [CrossRef]
- Muñoz, J.; Mirelis, B.; Aragón, L.M.; Gutiérrez, N.; Sánchez, F.; Español, M.; Esparcia, O.; Gurguí, M.; Domingo, P.; Coll, P. Clinical and microbiological features of nocardiosis 1997–2003. J. Med. Microbiol. 2007, 56, 545–550. [Google Scholar] [CrossRef] [Green Version]
- McNeil, M.M.; Brown, J.M.; Hutwagner, L.C.; Schiff, T.A. Evaluation of therapy for Nocardia asteroides complex infecton: CDN/NCID report. Infect. Dis. Clin. Pract. 1995, 4, 287–292. [Google Scholar] [CrossRef]
- Lerner, P.I. Nocardiosis. Clin. Infect. Dis. 1996, 22, 891–905. [Google Scholar] [CrossRef] [Green Version]





| Case/Ref | Age | Sex | Country | Comorbidities | Immuno-suppression | Nocardia spp. | Type of Infection | Treatment (Duration) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 [19] | 64 | M | Greece | None | No | NB | LC | 2 months | Successful |
| 2 [20] | 49 | M | USA | DM | No | NB | LC | 6 months | Successful |
| 3 [20] | 59 | M | USA | HTN, Metastatic Esophageal Cancer | Yes | NB | LC | 6 months | Successful |
| 4 [20] | 78 | M | USA | CAD, Prostate Cancer | Yes | NB | LC | 6 months | Successful |
| 5 [20] | 62 | M | USA | Ulcerative colitis | Yes | NB | LC | Indefinite Bactrim due to immuno-suppressive regimen | Successful |
| 6 [21] | 32 | M | Unknown | None | No | NB | LC | 3 months | Successful |
| 7 [22] | 34 | M | Chinese | None | No | NB | LC | 6 weeks | Successful |
| 8 [23] | 65 | M | Unknown | PKD s/p renal transplant | Yes | NB | LC | 1 year | Successful |
| 9 [24] | 65 | M | Unknown | Myasthenia gravis | Yes | NB | LC | 6 months | Successful |
| 10 [25] | 37 | M | USA | None | None | NB | LC | 2 months | Successful |
| 11 [25] | 69 | F | USA | DM, Temporal arteritis | Yes | NB | LC | 3 months | Successful |
| 12 [25] | 70 | M | USA | CAD, PVD, HTN | No | NB | LC | 2 months | Successful |
| 13 [26] | 61 | M | Brazil | None reported | No | NB | LC | 14 days, pt refused longer treatment. | Successful |
| 14 [27] | 45 | M | USA | None reported | No | NB | LC | 3 months | Successful |
| 15 [28] | 82 | F | Israel | HTN, HLD, CVA, Osteoporosis | No | NB | LC | Bactrim discontinued due to allergic reaction. Doxycycline prescribed, unknown duration. | Successful |
| 16 [29] | 9 | M | Australia | None | No | NB | LC | 6 months due to relapsing of the infection | Successful |
| 17 [30] | 87 | M | China | CAD, asthma | No | NB | LC | 4 weeks | Successful |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumic, I.; Brown, A.; Magee, K.; Elwasila, S.; Kaljevic, M.; Antic, M.; Igandan, O.; Cardozo, M.; Rueda Prada, L.; Paulson, M. Primary Lymphocutaneous Nocardia brasiliensis in an Immunocompetent Host: Case Report and Literature Review. Medicina 2022, 58, 488. https://doi.org/10.3390/medicina58040488
Dumic I, Brown A, Magee K, Elwasila S, Kaljevic M, Antic M, Igandan O, Cardozo M, Rueda Prada L, Paulson M. Primary Lymphocutaneous Nocardia brasiliensis in an Immunocompetent Host: Case Report and Literature Review. Medicina. 2022; 58(4):488. https://doi.org/10.3390/medicina58040488
Chicago/Turabian StyleDumic, Igor, Alethea Brown, Kyle Magee, Sammer Elwasila, Marija Kaljevic, Marina Antic, Oladapo Igandan, Milena Cardozo, Libardo Rueda Prada, and Margaret Paulson. 2022. "Primary Lymphocutaneous Nocardia brasiliensis in an Immunocompetent Host: Case Report and Literature Review" Medicina 58, no. 4: 488. https://doi.org/10.3390/medicina58040488
APA StyleDumic, I., Brown, A., Magee, K., Elwasila, S., Kaljevic, M., Antic, M., Igandan, O., Cardozo, M., Rueda Prada, L., & Paulson, M. (2022). Primary Lymphocutaneous Nocardia brasiliensis in an Immunocompetent Host: Case Report and Literature Review. Medicina, 58(4), 488. https://doi.org/10.3390/medicina58040488








