Bone Fusion Morphology after Circumferential Minimally Invasive Spine Surgery Using Lateral Lumbar Interbody Fusion and Percutaneous Pedicle Screws without Bone Grafting in the Thoracic Spine: A Retrospective Study
Abstract
:1. Introduction
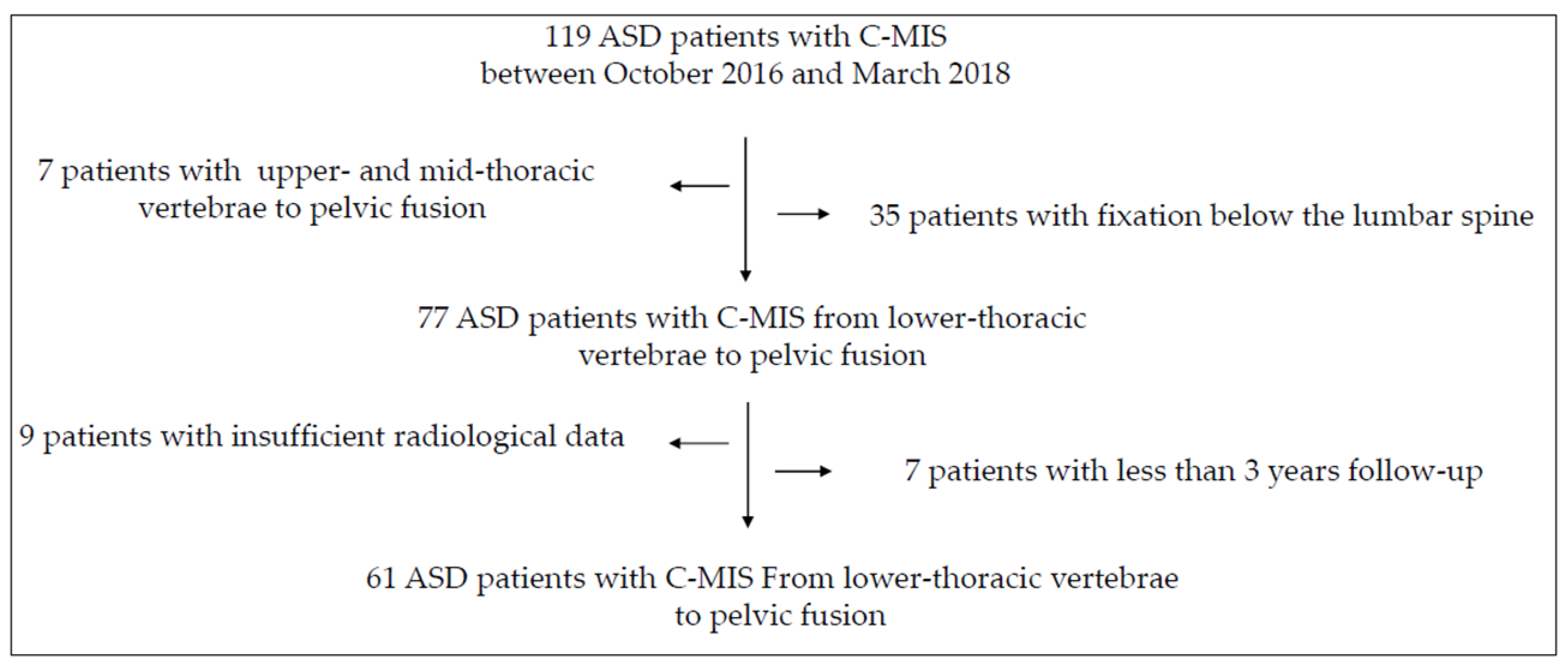
2. Materials and Methods
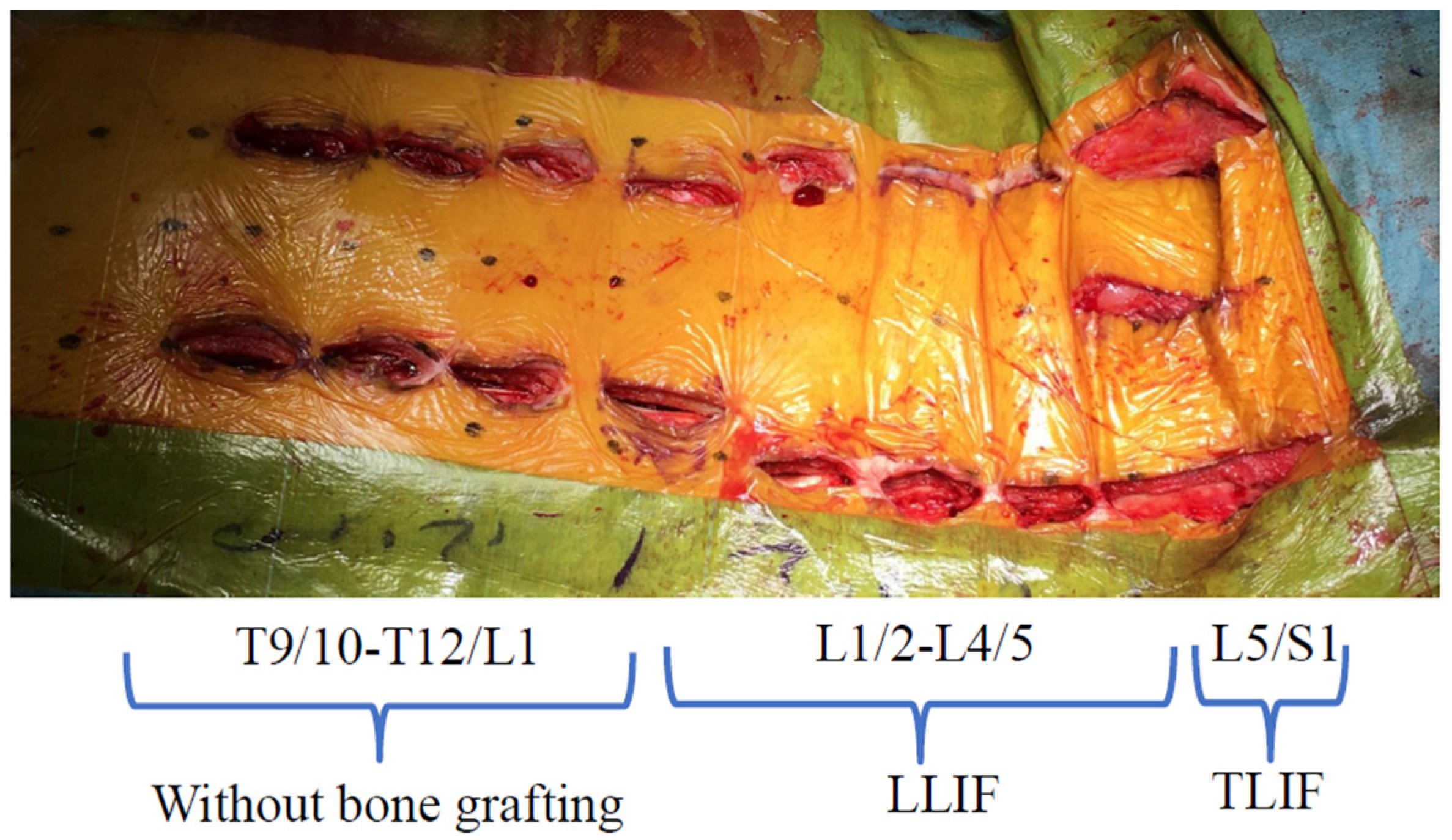
2.1. Surgical Technique
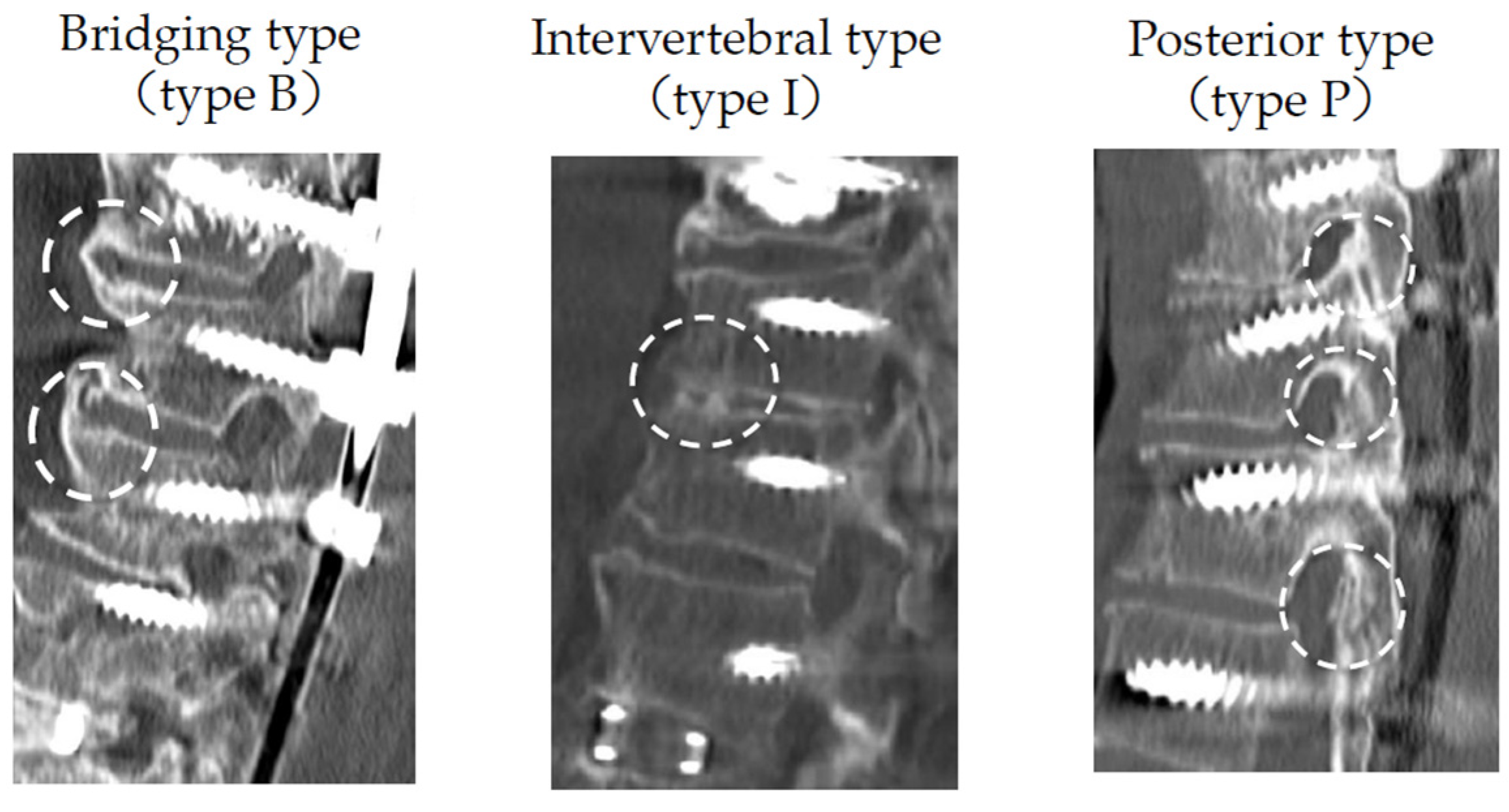
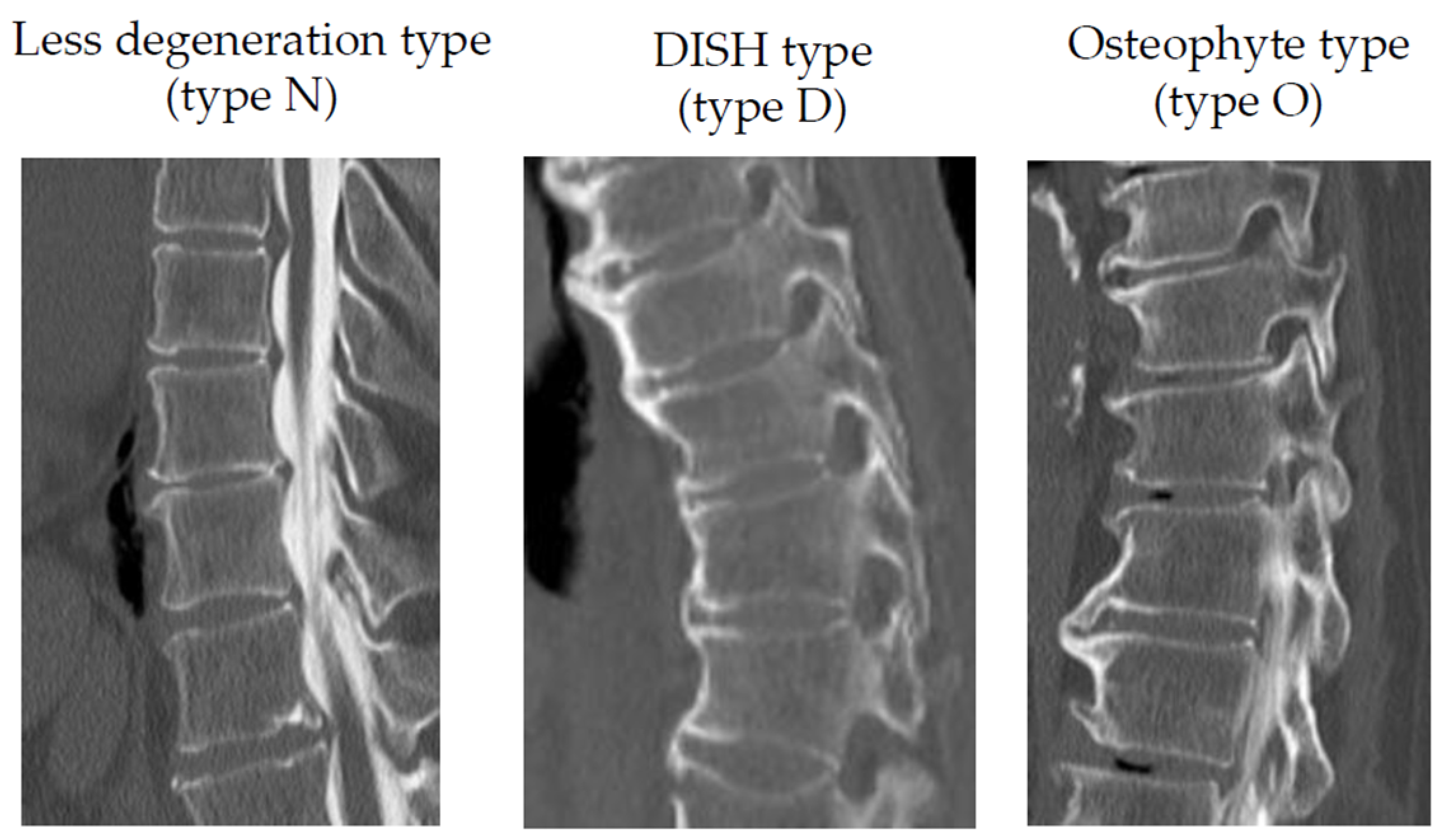
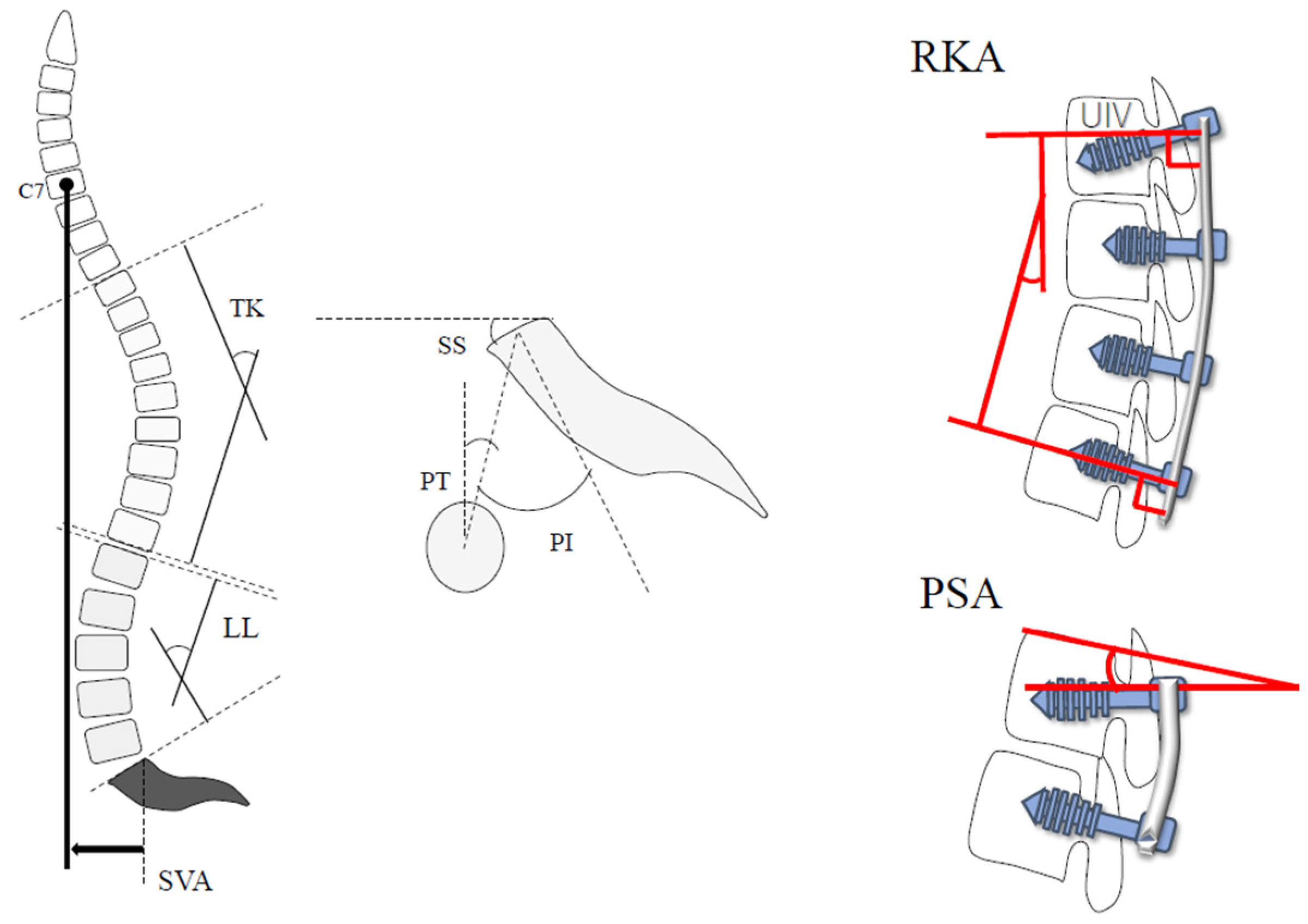
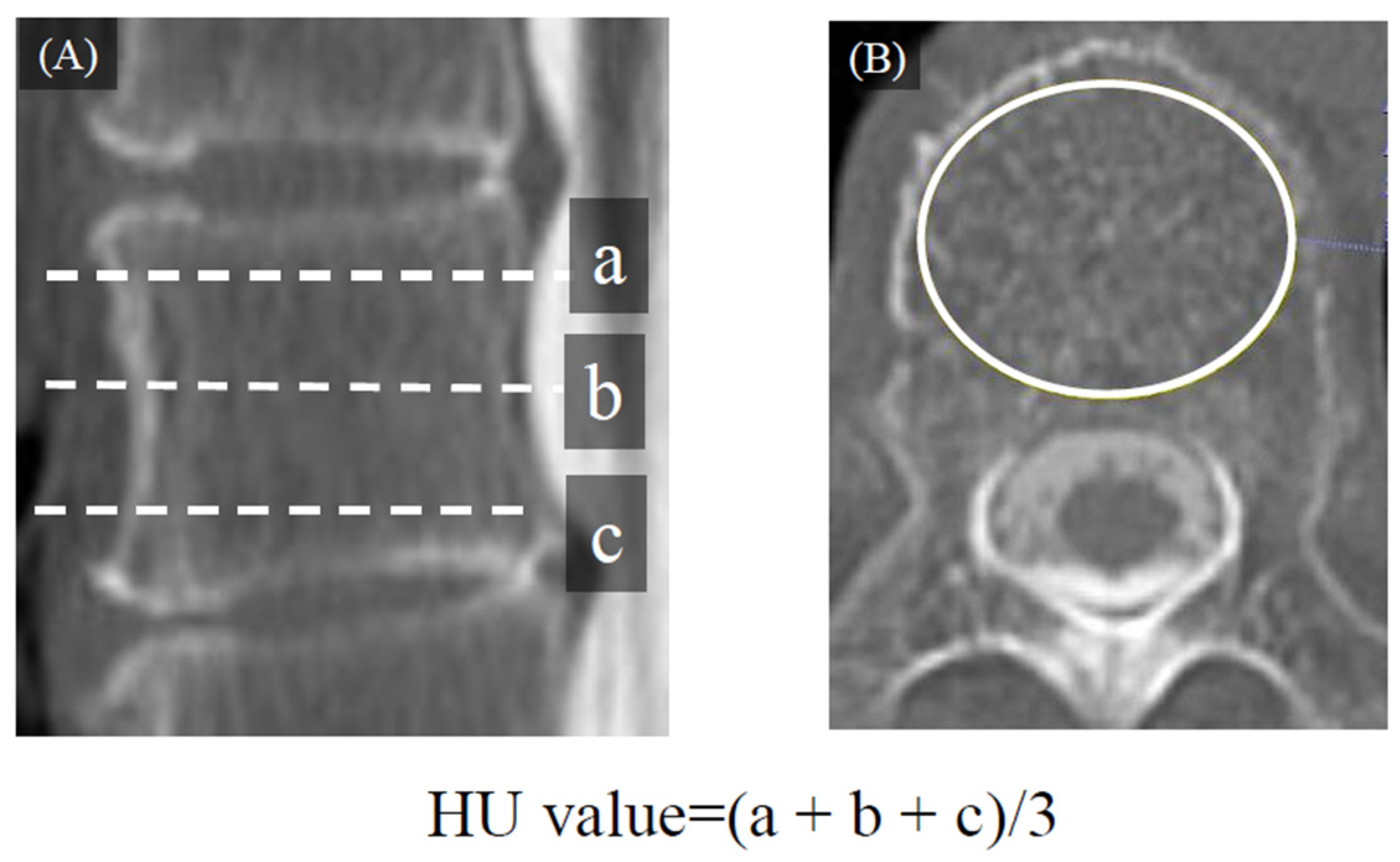
2.2. Radiological Evaluation
2.3. Statistical Analysis
3. Results
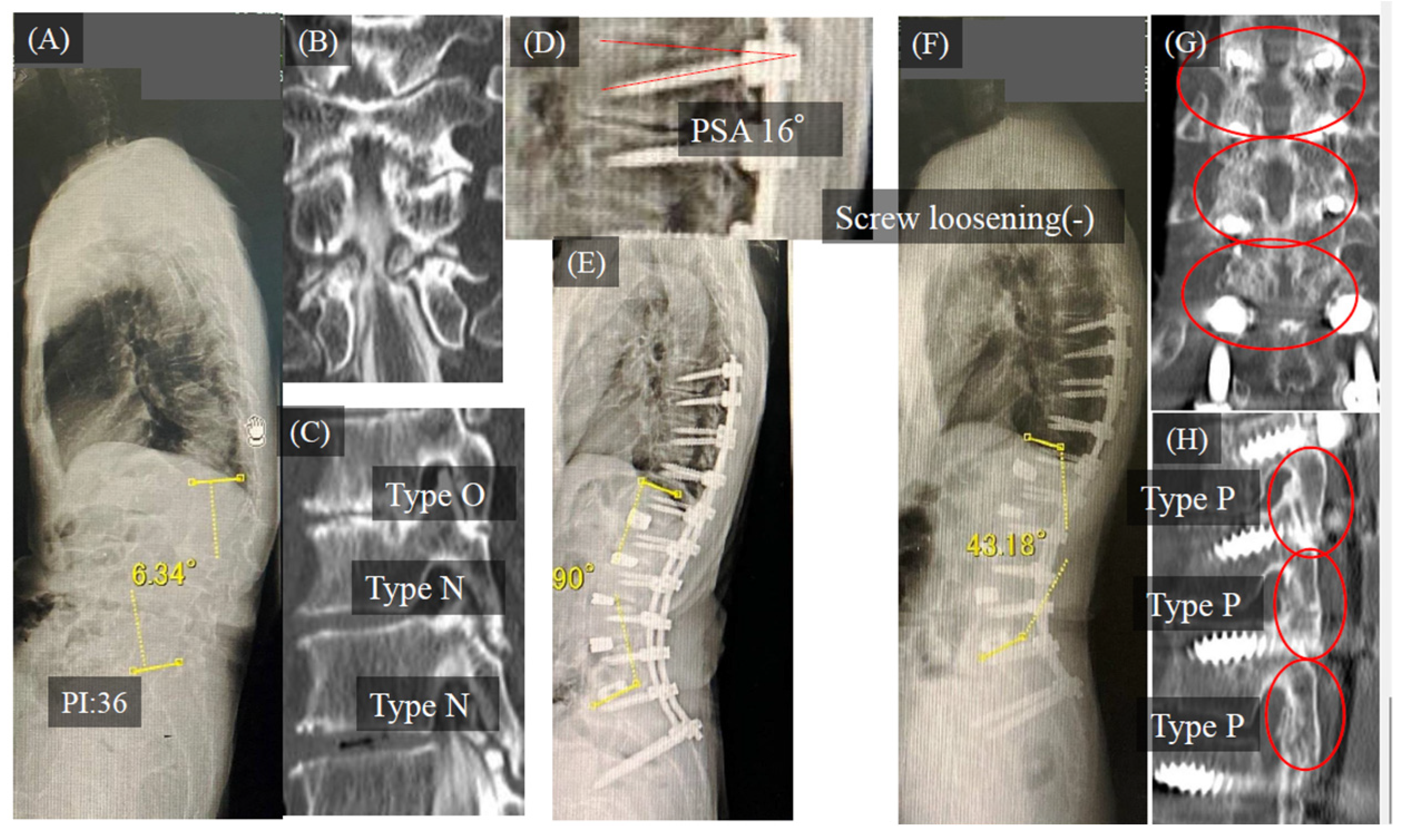
3.1. Case Study
Case Study 1
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwab, F.J.; Blondel, B.; Bess, S.; Hostin, R.; Shaffrey, C.I.; Smith, J.S.; Boachie-Adjei, O.; Burton, D.C.; Akbarnia, B.A.; Mundis, G.M.; et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: A prospective multicenter analysis. Spine 2013, 38, E803–E812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.S.; Klineberg, E.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Lafage, R.; Hostin, R.; Mundis, G.M.; Errico, T.J.; Kim, H.J.; et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J. Neurosurg. Spine 2016, 25, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassman, S.D.; Hamill, C.L.; Bridwell, K.H.; Schwab, F.J.; Dimar, J.R.; Lowe, T.G. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine 2007, 32, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.M.; Mundis, G.M.; Ahmed, Y.; El Ahmadieh, T.Y.; Wang, M.Y.; Mummaneni, P.V.; Uribe, J.S.; Okonkwo, D.O.; Eastlack, R.K.; Anand, N.; et al. Comparison of radiographic results after minimally invasive, hybrid, and open surgery for adult spinal deformity: A multicenter study of 184 patients. Neurosurg. Focus 2014, 36, E13. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Wang, M.Y.; Lafage, V.; Nguyen, S.; Ziewacz, J.; Okonkwo, D.O.; Uribe, J.S.; Eastlack, R.K.; Anand, N.; Haque, R.; et al. Comparison of two minimally invasive surgery strategies to treat adult spinal deformity. J. Neurosurg. Spine 2015, 22, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Mummaneni, P.V.; Park, P.; Fu, K.M.; Wang, M.Y.; Nguyen, S.; Lafage, V.; Uribe, J.S.; Ziewacz, J.; Terran, J.; Okonkwo, D.O.; et al. Does minimally invasive percutaneous posterior instrumentation reduce risk of proximal junctional kyphosis in adult spinal deformity surgery? A propensity-matched cohort analysis. Neurosurgery 2016, 78, 101–108. [Google Scholar]
- Cahill, P.J.; Wang, W.; Asghar, J.; Booker, R.; Betz, R.R.; Ramsey, C.; Baran, G. The use of a transition rod may prevent proximal junctional kyphosis in the thoracic spine after scoliosis surgery: A finite element analysis. Spine 2012, 37, E687–E695. [Google Scholar] [CrossRef]
- Korkmaz, M.; Akgul, T.; Sariyilmaz, K.; Ozkunt, O.; Dikici, F.; Yazicioglu, O. Effectiveness of posterior structures in the development of proximal junctional kyphosis following posterior instrumentation: A biomechanical study in a sheep spine model. Acta Orthop. Traumatol. Turc. 2019, 53, 385–389. [Google Scholar] [CrossRef]
- Eastlack, R.K.; Srinivas, R.; Mundis, G.M.; Nguyen, S.; Mummaneni, P.V.; Okonkwo, D.O.; Kanter, A.S.; Anand, N.; Park, P.; Nunley, P.; et al. Early and late reoperation rates with various mis techniques for adult spinal deformity correction. Glob. Spine J. 2019, 9, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Taniguchi, S.; Adachi, T.; Kushida, T.; Paku, M.; Ando, M.; Saito, T.; Kotani, Y.; Tani, Y. Rod contour and overcorrection are risk factors of proximal junctional kyphosis after adult spinal deformity correction surgery. Eur. Spine J. 2021, 30, 1208–1214. [Google Scholar] [CrossRef]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J. Bone Joint Surg. Am. 2011, 93, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Fakurnejad, S.; Lau, D.; Daubs, M.D.; Coe, J.D.; Paonessa, K.J.; LaGrone, M.O.; Amaral, R.A.; Trobisch, P.D.; Lee, J.H.; et al. Results of the 2014 SRS Survey on PJK/PJF: A report on variation of select srs member practice patterns, treatment indications, and opinions on classification development. Spine 2015, 40, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Isogai, N.; Yoshida, K.; Shiono, Y.; Sasao, Y.; Funao, H.; Ishi, K. Respective Correction Rates of Lateral Lumbar interbody Fusion and Percutaneous Pedicle Screw Fixation for Lumbar Degenerative Spindylolisthesis. Medicina 2022, 58, 169. [Google Scholar] [CrossRef]
- Nakanishi, K.; Uchino, K.; Watanabe, S.; Misaki, K.; Iba, H. Effect of Minimally Invasive Spine Stabilization in Metastatic Spinal Tumors. Medicina 2022, 58, 358. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Taniguchi, S.; Adachi, T.; Tani, Y.; Paku, M.; Ando, M.; Saito, T. Conditions for Achieving Postoperative Pelvic Incidence-Lumbar Lordosis <10° in Circumferential Minimally Invasive Surgery for Adult Spinal Deformity. J. Clin. Med. 2022, 11, 1586. [Google Scholar]
- Strom, R.G.; Bae, J.; Mizutani, J.; Valone, F.; Ames, C.P.; Deviren, V. Lateral interbody fusion combined with open posterior surgery for adult spinal deformity. J. Neurosurg. Spine 2016, 25, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, P.J.; Kelleher, J.P.; Ailon, T.; Heller, J.E.; Kasliwal, M.K.; Shaffrey, C.I.; Smith, J.S. Long-segment fusion for adult spinal deformity correction using low-dose recombinant human bone morphogenetic protein-2: A retrospective review of fusion rates. Neurosurgery 2016, 79, 212–221. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bridwell, K.H.; Lenke, L.G.; Rhim, S.; Cheh, G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: Prevalence and risk factor analysis of 144 cases. Spine 2006, 31, 2329–2336. [Google Scholar] [CrossRef]
- Hamilton, D.K.; Kanter, A.S.; Bolinger, B.D.; Mundis, G.M.; Nguyen, S.; Mummaneni, P.V.; Anand, N.; Fessler, R.G.; Passias, P.G.; Park, P.; et al. Reoperation rates in minimally invasive, hybrid and open surgical treatment for adult spinal deformity with minimum 2-year follow-up. Eur. Spine J. 2016, 25, 2605–2611. [Google Scholar] [CrossRef]
- Izeki, M.; Fujio, K.; Ota, S.; Soga, S.; Matsuda, S. Radiological follow-up of the degenerated facet joints after lateral lumbar interbody fusion with percutaneous pedicle screw fixation: Focus on spontaneous facet joint fusion. J. Orthop. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Maruo, K.; Ha, Y.; Inoue, S.; Samuel, S.; Okada, E.; Hu, S.S.; Deviren, V.; Burch, S.; William, S.; Ames, C.P.; et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine 2013, 38, E1469–E1476. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Iyer, S.; Zebala, L.P.; Kelly, M.P.; Sciubba, D.; Protopsaltis, T.S.; Gupta, M.; Neuman, B.J.; Mundis, G.M.; Ames, C.P.; et al. Perioperative neurologic complications in adult spinal deformity surgery: Incidence and risk factors in 564 patients. Spine 2017, 42, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Muheremu, A.; Sun, Z.; Zhong, W.; Jiang, S.; Li, W. Computed tomography Hounsfield unit-based prediction of pedicle screw loosening after surgery for degenerative lumbar spine disease. J. Neurosurg. Spine 2020, 32, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.C.; Elysee, J.; Lafage, R.; McCarthy, M.; Louie, P.K.; Alshabab, B.S.; Weissmann, K.; Lafage, V.; Schwab, F.; Kim, H.J. Preoperative hounsfield units at the planned upper instrumented vertebrae may predict proximal junctional kyphosis in adult spinal deformity. Spine 2021, 46, E174–E180. [Google Scholar] [CrossRef]
- Oe, S.; Narita, K.; Hasegawa, K.; Natarajan, R.N.; Yamato, Y.; Hasegawa, T.; Yoshida, G.; Banno, T.; Arima, H.; Mihara, Y.; et al. Longer screws can reduce the stress on the upper instrumented vertebra with long spinal fusion surgery: A finite element analysis study. Glob. Spine J. 2021, 21925682211018468. [Google Scholar] [CrossRef]
- Okuyama, K.; Abe, E.; Suzuki, T.; Tamura, Y.; Chiba, M.; Sato, K. Influence of bone mineral density on pedicle screw fixation: A study of pedicle screw fixation augmenting posterior lumbar interbody fusion in elderly patients. Spine J. 2001, 1, 402–407. [Google Scholar] [CrossRef]
- Sawakami, K.; Watanabe, K.; Hasegawa, K.; Yamamoto, N.; Shimakura, T.; Ohashi, M.; Shoji, H.; Mizouchi, T.; Tanaka, Y.; Segawa, H.; et al. Neoadjuvant teriparatide therapy targeting the osteoporotic spine: Influence of administration period from the perspective of bone histomorphometry. J. Neurosurg. Spine 2021, 36, 429–439. [Google Scholar] [CrossRef]
- Kawabata, A.; Yoshii, T.; Hirai, T.; Ushio, S.; Kaito, T.; Yamashita, T.; Fujiwara, H.; Nagamoto, Y.; Matsuoka, Y.; Suzuki, H.; et al. Effect of bisphosphonates or teriparatide on mechanical complications after posterior instrumented fusion for osteoporotic vertebral fracture: A multi-center retrospective study. BMC Musculoskelet. Disord. 2020, 21, 420. [Google Scholar] [CrossRef]
- Yagi, M.; Ohne, H.; Konomi, T.; Fujiyoshi, K.; Kaneko, S.; Komiyama, T.; Takemitsu, M.; Yato, Y.; Machida, M.; Asazuma, T. Teriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformity. Osteoporos. Int. 2016, 27, 3495–3502. [Google Scholar] [CrossRef]
- Yilgor, C.; Sogunmez, N.; Boissiere, L.; Yavuz, Y.; Obeid, I.; Kleinstück, F.; Pérez-Grueso, F.J.S.; Acaroglu, E.; Haddad, S.; Mannion, A.F.; et al. Global alignment and proportion (GAP) score: Development and validation of a new method of analyzing spinopelvic alignment to predict mechanical complications after adult spinal deformity surgery. Spine J. 2017, 17, S155–S156. [Google Scholar] [CrossRef]
| Parameters | Findings (n = 61) | Number of Cases |
|---|---|---|
| Age (years) | 73.2 ± 7.3 (52–82) | |
| Gender (male: female) | 15:46 | |
| Duration of follow-up (month) | 49.3 ± 5.5 (38–58) | |
| Number of fused levels (segment) | 10.3 ± 0.5 (10–13) | |
| Number of LLIF (segment) | 4.0 ± 0.5 (3–5) | |
| Number of corpectomy cases | 3% | 2 cases |
| UIV (case) | ||
| T9 | 30% | 18 cases |
| T10 | 70% | 43 cases |
| Operative time (min) | ||
| Anterior | 128.0 ± 30.4 (78–218) | |
| Posterior | 255.0 ± 44.1 (171–387) | |
| Total | 381.5 ± 59.6 (289–541) | |
| Estimated blood loss (mL) | ||
| Anterior | 103.4 ± 143.1 (5–605) | |
| Posterior | 612.7 ± 301.7 (79–1530) | |
| Total | 716.1 ± 358.8 (123–1610) | |
| Complications (%) | ||
| Screw loosening | 46% | 28 cases |
| UIV and UIV-1 | 13% | 9 cases |
| UIV | 33% | 20 cases |
| PJK | 20% | 12 cases |
| RF | ||
| Thoracic | 0% | 0 cases |
| Lumbar | 15% | 9 cases |
| Lumbosacral | 13% | 8 cases |
| Thoracic (n = 191, 61 Cases) | Lumbar (n = 242) | Lumbosacral (n = 61) | |||
|---|---|---|---|---|---|
| Bone fusion rate | 54% (104/191, 33 cases) | 95% (231/242) | 89% (54/61) | ||
| New bone fusion | 42% (81/191) | ||||
| Existing bone fusion | 12% (23/191) | ||||
| Fusion morphology | Type B | 54% (56/104, 18 cases) | 72% (168/231)B + I 37%, B + P 30% | 74% (40/54) | |
| Type I | 10% (10/104, 3 cases) | 27% (63/231) | 19% (10/54) | ||
| Type P | 36% (38/104, 12 cases) | 0% (0/231) | 6% (3/54) | ||
| Type N (n = 40 Cases 124 Levels) | Type D (n = 11 Cases 48 Levels) | Type O (n = 10 Cases 31 Levels) | p-Value | |||
|---|---|---|---|---|---|---|
| Type N vs. Type D | Type N vs. Type O | |||||
| Fusion rate | (%) | 35% | 100% | 80% | <0.001 * | 0.013 * |
| cases | 14/40 | 11/11 | 8/10 | |||
| levels | 46/124 | 36/36 | 26/31 | |||
| Group U (n = 33) | Group NU (n = 28) | p-Value | |||
|---|---|---|---|---|---|
| Age | 73.5 ± 7.3 | 72.8 ± 7.4 | 0.346 | ||
| Sex (male: female) | 10:23 | 5:23 | 0.207 | ||
| vertebral condition | type D (cases) | 11/33 | 0/28 | <0.001 * | |
| type O (cases) | 8/33 | 2/28 | 0.071 | ||
| type N (cases) | 14/33 | 26/28 | <0.001 * | ||
| complications | screw loosening (cases) | 8/33 | 20/28 | <0.001 * | |
| PJK (cases) | 5/33 | 7/28 | 0.466 | ||
| RF (case) | thoracic | 0/33 | 0/28 | 1.000 | |
| lumbar | 3/33 | 5/28 | 0.260 | ||
| lumbosacral | 2/33 | 6/28 | 0.264 | ||
| total | 5/33 | 11/33 | 0.032 * | ||
| revision rate | RF lumbosacral (cases) | 5/33 | 9/28 | 0.102 | |
| PJK (cases) | 3/33 | 5/28 | 0.264 | ||
| Total (cases) | 7/33 | 10/28 | 0.165 | ||
| HU | 125.1 ± 43.1 | 134.7 ± 50.1 | 0.212 | ||
| Group U (n = 33) | Group NU (n = 28) | p-Value | |
|---|---|---|---|
| Pre-PI (°) | 46.1 ± 12.9 | 48.0 ± 12.3 | 0.310 |
| Post-PI (°) | 48.2 ± 12.5 | 49.2 ± 11.9 | 0.351 |
| Pre-LL (°) | 13.5 ± 13.8 | 6.8 ± 18.7 | 0.081 |
| Post-LL (°) | 47.4 ± 12.2 | 47.2 ± 10.0 | 0.473 |
| Pre-PI-LL (°) | 32.6 ± 15.7 | 41.1 ± 20.1 | 0.054 |
| post-PI-LL (°) | −1.2 ± 11.6 | 0.7 ± 11.1 | 0.269 |
| Pre-PT (°) | 28.7 ± 10.6 | 32.2 ± 21.3 | 0.149 |
| Post-PT (°) | 16.6 ± 8.0 | 15.9 ± 11.6 | 0.399 |
| Pre-TK (°) | 22.8 ± 14.4 | 13.6 ± 13.4 | 0.006 * |
| Post-TK (°) | 38.9 ± 10.0 | 36.1 ± 8.7 | 0.140 |
| Pre-SVA (mm) | 82.3 ± 45.1 | 107.9 ± 40.1 | 0.076 |
| Post-SVA (mm) | 24.4 ± 28.5 | 37.8 ± 28.8 | 0.037 * |
| PSA (°) | 10.9 ± 8.2 | 6.6 ± 8.8 | 0.025 * |
| RKA (°) | 20.4 ± 6.9 | 20.4 ± 7.9 | 0.493 |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Pre-TK | 0.979 | 0.927–1.029 | 0.409 |
| Post-SVA | 1.028 | 1.003–1.059 | 0.022 * |
| PSA | 0.989 | 0.899–1.087 | 0.830 |
| Vertebral condition type N | 23.812 | 3.443–329.921 | <0.001 * |
| screw loosening | 6.177 | 1.356–35.964 | 0.017 * |
| Group NSL (n = 33) | Group SL (n = 28) | p-Value | |||
|---|---|---|---|---|---|
| Age | 72.5 ± 8.5 | 74.1 ± 5.7 | 0.207 | ||
| Gender (male: female) | 9:24 | 5:23 | 0.207 | ||
| Vertebral condition | type D (cases) | 9/33 | 2/28 | 0.028 * | |
| type O (cases) | 7/33 | 3/28 | 0.568 | ||
| type N (cases) | 17/33 | 23/28 | 0.001 * | ||
| Complications | PJK (cases) | 12% (4/33) | 29% (8/28) | 0.118 | |
| RF (case) | thoracic | 0/33 | 0/28 | 1.000 | |
| lumbar | 2/33 | 6/28 | 0.082 | ||
| lumbosacral | 4/33 | 4/28 | 0.549 | ||
| Total | 6/33 | 10/28 | 0.104 | ||
| Revision rate | RF (lumbosacral) (cases) | 5/33 | 10/28 | 0.059 | |
| PJK (cases) | 3/33 | 5/28 | 0.264 | ||
| Total (cases) | 7/33 | 10/28 | 0.165 | ||
| Bone fusion rate in thoracic spine | 76% (25/33) (78/102) segments) | 29% (8/28) (26/89 segments) | <0.001 * | ||
| HU | 138.1 ± 40.7 | 121.1 ± 51.3 | 0.078 | ||
| Group NSL (n = 33) | Group SL (n = 28) | p-Value | |
|---|---|---|---|
| Pre-PI (°) | 44.7 ± 12.8 | 51.0 ± 11.1 | 0.023 * |
| Post-PI (°) | 45.3 ± 11.9 | 51.2 ± 10.7 | 0.038 * |
| Pre-LL (°) | 5.2 ± 17.5 | 12.9 ± 16.9 | 0.04 |
| Post-LL (°) | 47.4 ± 11.1 | 47.2 ± 11.1 | 0.476 |
| Pre-PI-LL (°) | 35.9 ± 19.1 | 38.0 ± 18.0 | 0.344 |
| post-PI-LL (°) | −1.0 ± 11.1 | 3.0 ± 11.0 | 0.078 |
| Pre-PT (°) | 30.3 ± 12.8 | 30.7 ± 10.3 | 0.456 |
| Post-PT (°) | 14.2 ± 10.4 | 18.6 ± 8.9 | 0.064 |
| Pre-TK (°) | 22.3 ± 15.4 | 14.2 ± 12.6 | 0.014 * |
| Post-TK (°) | 38.8 ± 9.6 | 36.1 ± 9.2 | 0.165 |
| Pre-SVA (mm) | 93.7 ± 61.3 | 106.1 ± 51.7 | 0.201 |
| Post-SVA (mm) | 27.5 ± 29.4 | 31.9 ± 30.7 | 0.311 |
| PSA (°) | 12.1 ± 7.9 | 5.1 ± 7.8 | <0.001 * |
| RKA (°) | 20.1 ± 7.1 | 20.7 ± 7.7 | 0.055 |
| Odds Ratio | 95%CI | p-Value | |
|---|---|---|---|
| PI | 1.041 | 0.985–1.108 | 0.147 |
| Pre-LL | 1.040 | 0.995–1.093 | 0.079 |
| Pre-TK | 0.978 | 0.927–1.026 | 0.381 |
| PSA | 1.115 | 1.025–1.231 | 0.009 * |
| Vertebral condition type N | 4.107 | 0.967–20.666 | 0.055 |
| Cutoff Value | Sensitivity | Specificity | AUC | |
|---|---|---|---|---|
| PSA | 15.3° | 1.00 | 0.425 | 0.731 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, M.; Taniguchi, S.; Kawashima, K.; Adachi, T.; Paku, M.; Tani, Y.; Ando, M.; Saito, T. Bone Fusion Morphology after Circumferential Minimally Invasive Spine Surgery Using Lateral Lumbar Interbody Fusion and Percutaneous Pedicle Screws without Bone Grafting in the Thoracic Spine: A Retrospective Study. Medicina 2022, 58, 496. https://doi.org/10.3390/medicina58040496
Ishihara M, Taniguchi S, Kawashima K, Adachi T, Paku M, Tani Y, Ando M, Saito T. Bone Fusion Morphology after Circumferential Minimally Invasive Spine Surgery Using Lateral Lumbar Interbody Fusion and Percutaneous Pedicle Screws without Bone Grafting in the Thoracic Spine: A Retrospective Study. Medicina. 2022; 58(4):496. https://doi.org/10.3390/medicina58040496
Chicago/Turabian StyleIshihara, Masayuki, Shinichirou Taniguchi, Koki Kawashima, Takashi Adachi, Masaaki Paku, Yoichi Tani, Muneharu Ando, and Takanori Saito. 2022. "Bone Fusion Morphology after Circumferential Minimally Invasive Spine Surgery Using Lateral Lumbar Interbody Fusion and Percutaneous Pedicle Screws without Bone Grafting in the Thoracic Spine: A Retrospective Study" Medicina 58, no. 4: 496. https://doi.org/10.3390/medicina58040496
APA StyleIshihara, M., Taniguchi, S., Kawashima, K., Adachi, T., Paku, M., Tani, Y., Ando, M., & Saito, T. (2022). Bone Fusion Morphology after Circumferential Minimally Invasive Spine Surgery Using Lateral Lumbar Interbody Fusion and Percutaneous Pedicle Screws without Bone Grafting in the Thoracic Spine: A Retrospective Study. Medicina, 58(4), 496. https://doi.org/10.3390/medicina58040496






