The Role of Tissue Geometry in Spinal Cord Regeneration
Abstract
1. Methods
2. The Problem
3. Factors Associated with Glial Scars and Myelin Have Been Implicated in Growth Cone Collapse
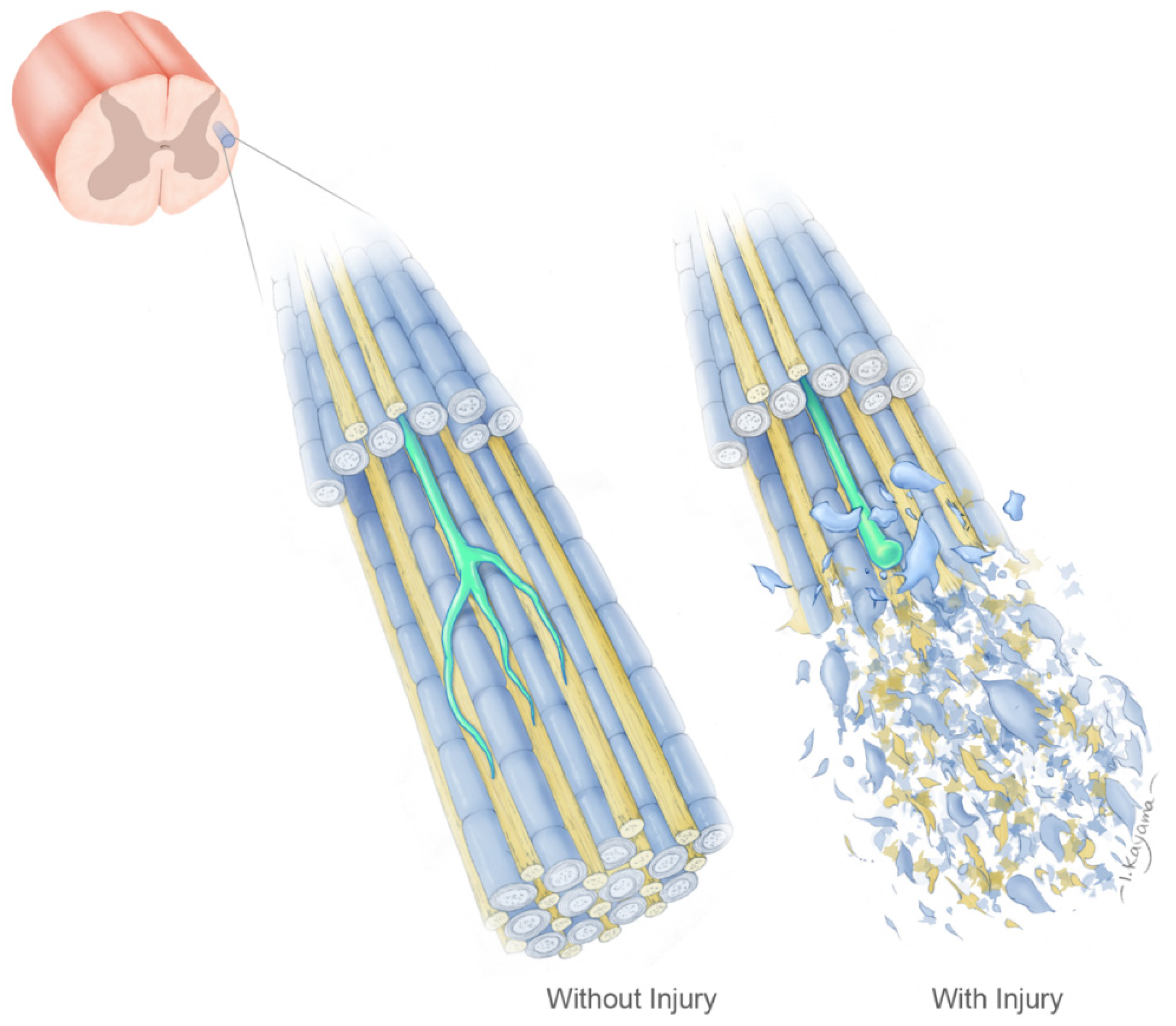
4. Intact White Matter Can Support Axonal Growth
5. The Geometry Hypothesis
6. Reconstruction of Tissue Geometry to Promote Regeneration
7. Guidance Mechanisms
8. Clinical Application of the Tissue Geometry Hypothesis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Quadri, S.A.; Farooqui, M.; Ikram, A.; Zafar, A.; Khan, M.A.; Suriya, S.S.; Claus, C.; Fiani, B.; Rahman, M.; Ramachandran, A.; et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg. Rev. 2018, 43, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Karsy, M.; Hawryluk, G. Modern Medical Management of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Guth, L.; Windle, W.F. The enigma of central nervous regeneration. Summarized transactions of a conference on applications of new technology to the enigma of central nervous regeneration, held in Palm Beach, Florida, February 4–6, 1970 under the auspices of the National Paraplegia Foundation. Exp. Neurol. Suppl. 1970, 5, 28. [Google Scholar]
- Cajal, S.R.Y.; DeFelipe, J.; Jones, E.G. Degeneration and Regeneration of the Nervous System; Oxford University Press: London, UK, 1928. [Google Scholar]
- Anderson, P.; Campbell, G.; Zhang, Y.; Lieberman, A. Chapter 17 Cellular and molecular correlates of the regeneration of adult mammalian CNS axons into peripheral nerve grafts. Prog. Brain Res. 1998, 117, 211–232. [Google Scholar] [CrossRef]
- Benfey, M.; Aguayo, A.J. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature 1982, 296, 150–152. [Google Scholar] [CrossRef]
- David, S.; Aguayo, A.J. Axonal Elongation into Peripheral Nervous System “Bridges” After Central Nervous System Injury in Adult Rats. Science 1981, 214, 931–933. [Google Scholar] [CrossRef]
- Heinicke, E.A.; Kiernan, J.A. Vascular permeability and axonal regeneration in skin autotransplanted into the brain. J. Anat. 1978, 125, 409–420. [Google Scholar]
- Tello, F. La influencia del neurotropismo en la regeneración de los centros nerviosos. Trab. Del. Lab. Invest Biol. 1911, 9, 23. [Google Scholar]
- Nathaniel, E.; Clemente, C. Growth of nerve fibers into skin and muscle graffs in rat brains. Exp. Neurol. 1959, 1, 65–81. [Google Scholar] [CrossRef]
- Björklund, A.; Stenevi, U. Growth of central catecholamine neurones into smooth muscle grafts in the rat mesencephalon. Brain Res. 1971, 31, 1–20. [Google Scholar] [CrossRef]
- Svendgaard, N.-A.; Björklund, A.; Stenevi, U. Regeneration of central cholinergic neurones in the adult rat brain. Brain Res. 1976, 102, 1–22. [Google Scholar] [CrossRef]
- Clark, W.E.l.G. The problem of neuronal regeneration in the central nervous system: I. The influence of spinal ganglia and nerve fragments grafted in the brain. J. Anat. 1942, 77, 20–48. [Google Scholar]
- Clark, W.E.l.G. The problem of neuronal regeneration in the central nervous system: II. The insertion of peripheral nerve stumps into the brain. J. Anat. 1943, 7, 251–259. [Google Scholar]
- Waller, A. Experiments on the Section of the Glosso-Pharyngeal and Hypoglossal Nerves of the Frog, and Observations of the Alterations Produced Thereby in the Structure of Their Primitive Fibres. Edinb. Med. Surg. J. 1851, 76, 369–376. [Google Scholar]
- Büngner, O.V. Über die Degenerations-und Regenerationsvorgänge am Nerven nach Verletzungen. Beiträge Zur. Pathol. Anat. 1891, 10, 321. [Google Scholar]
- Brown, M.C.; Perry, V.H.; Lunn, E.R.; Gordon, S.; Heumann, R. Macrophage dependence of peripheral sensory nerve regeneration: Possible involvement of nerve growth factor. Neuron 1991, 6, 359–370. [Google Scholar] [CrossRef]
- Brown, M.C.; Lunn, E.R.; Perry, V.H. Consequences of slow wallerian degeneration for regenerating motor and sensory axons. J. Neurobiol. 1992, 23, 521–536. [Google Scholar] [CrossRef]
- Perry, V.H.; Brown, M.C. Role of macrophages in peripheral nerve degeneration and repair. BioEssays 1992, 14, 401–406. [Google Scholar] [CrossRef]
- Wagner, F.C.; Dohrmann, G.J. Alterations in nerve cells and myelinated fibers in spinal cord injury. Surg. Neurol. 1975, 3, 125–131. [Google Scholar]
- Dohrmann, G.J.; Wagner, F.C.; Bucy, P.C. Transitory traumatic paraplegia: Electron microscopy of early alterations in myelinated nerve fibers. J. Neurosurg. 1972, 36, 407–415. [Google Scholar] [CrossRef]
- Wagner, F.C.; Dohrmann, G.J.; Bucy, P.C. Histopathology of transistory traumatic paraplegia in the monkey. J. Neurosurg. 1971, 35, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Buss, A.; Brook, G.A.; Kakulas, B.; Martin, D.; Franzen, R.; Schoenen, J.; Noth, J.; Schmitt, A.B. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 2004, 127, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, C.; Eidelberg, E. Electron microscopic observations of the delayed effects of spinal cord compression. Exp. Neurol. 1975, 48, 637–646. [Google Scholar] [CrossRef]
- Lampert, P.; Cressman, M. Axonal regeneration in the dorsal columns of the spinal cord of adult rats. An electron microscopic study. Lab. Investig. 1964, 13, 825–839. [Google Scholar]
- Lampert, P.W.; Cressman, M.R. Fine-structural changes of myelin sheaths after axonal degeneration in the spinal cord of rats. Am. J. Pathol. 1966, 49, 1139–1155. [Google Scholar]
- Davies, S.J.A.; Goucher, D.R.; Doller, C.; Silver, J. Robust Regeneration of Adult Sensory Axons in Degenerating White Matter of the Adult Rat Spinal Cord. J. Neurosci. 1999, 19, 5810–5822. [Google Scholar] [CrossRef]
- McKeon, R.; Schreiber, R.; Rudge, J.; Silver, J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991, 11, 3398–3411. [Google Scholar] [CrossRef]
- Bignami, A.; Ralston, H.J. The cellular reaction to wallerian degeneration in the central nervous system of the cat. Brain Res. 1969, 13, 444–461. [Google Scholar] [CrossRef]
- Daniel, P.M.; Strich, S.J. Histological observations on Wallerian degeneration in the spinal cord of the baboon, Papio papio. Acta Neuropathol. 1969, 12, 314–328. [Google Scholar] [CrossRef]
- Perry, V.H.; Brown, M.C.; Gordon, S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J. Exp. Med. 1987, 165, 1218–1223. [Google Scholar] [CrossRef]
- Petrova, V.; Eva, R. The Virtuous Cycle of Axon Growth: Axonal Transport of Growth-Promoting Machinery as an Intrinsic Determinant of Axon Regeneration. Dev. Neurobiol. 2018, 78, 898–925. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, D.-H.; Levchenko, A. Topotaxis: A New Mechanism of Directed Cell Migration in Topographic ECM Gradients. Biophys. J. 2018, 114, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; Liu, K.; Hu, Y.; Kanter, J.L.; He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010, 223, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Luo, L.; Chen, J. Roles of mTOR Signaling in Tissue Regeneration. Cells 2019, 8, 1075. [Google Scholar] [CrossRef]
- Berry, M.; Ahmed, Z.; Morgan-Warren, P.; Fulton, D.; Logan, A. Prospects for mTOR-mediated functional repair after central nervous system trauma. Neurobiol. Dis. 2016, 85, 99–110. [Google Scholar] [CrossRef]
- Sun, T.; Duan, L.; Li, J.; Guo, H.; Xiong, M. Gypenoside XVII protects against spinal cord injury in mice by regulating the microRNA-21-mediated PTEN/AKT/mTOR pathway. Int. J. Mol. Med. 2021, 48, 146. [Google Scholar] [CrossRef]
- Guan, C.; Luan, L.; Li, J.; Yang, L. MiR-212-3p improves rat functional recovery and inhibits neurocyte apoptosis in spinal cord injury models via PTEN downregulation-mediated activation of AKT/mTOR pathway. Brain Res. 2021, 1768, 147576. [Google Scholar] [CrossRef]
- Chung, D.; Shum, A.; Caraveo, G. GAP-43 and BASP1 in Axon Regeneration: Implications for the Treatment of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 890. [Google Scholar] [CrossRef]
- Pan, L.; Tan, B.; Tang, W.; Luo, M.; Liu, Y.; Yu, L.; Yin, Y. Combining task-based rehabilitative training with PTEN inhibition promotes axon regeneration and upper extremity skilled motor function recovery after cervical spinal cord injury in adult mice. Behav. Brain Res. 2021, 405, 113197. [Google Scholar] [CrossRef]
- Harvey, A.R.; Lovett, S.J.; Majda, B.T.; Yoon, J.H.; Wheeler, L.P.; Hodgetts, S. Neurotrophic factors for spinal cord repair: Which, where, how and when to apply, and for what period of time? Brain Res. 2015, 1619, 36–71. [Google Scholar] [CrossRef]
- Muheremu, A.; Shu, L.; Liang, J.; Aili, A.; Jiang, K. Sustained delivery of neurotrophic factors to treat spinal cord injury. Transl. Neurosci. 2021, 12, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Madigan, N.N.; Hakim, J.S.; Dadsetan, M.; McMahon, S.S.; Yaszemski, M.J.; Windebank, A.J. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J. Tissue Eng. Regen. Med. 2017, 12, e398–e407. [Google Scholar] [CrossRef] [PubMed]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Berry, M.; Maxwell, W.L.; Logan, A.; Mathewson, A.; Mcconnell, P.; Ashhurst, D.E.; Thomas, G.H. Deposition of Scar Tissue in the Central Nervous System. Trauma Regen. 1983, 32, 31–53. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Carter, L.M. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 2011, 84, 306–316. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Asher, R. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Kawano, H.; Kimura-Kuroda, J.; Komuta, Y.; Yoshioka, N.; Li, H.P.; Kawamura, K.; Li, Y.; Raisman, G. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 2012, 349, 169–180. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Davies, S.J.A.; Fitch, M.T.; Memberg, S.P.; Hall, A.K.; Raisman, G.; Silver, J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 1997, 390, 680–683. [Google Scholar] [CrossRef]
- McKeon, R.J.; Höke, A.; Silver, J. Injury-Induced Proteoglycans Inhibit the Potential for Laminin-Mediated Axon Growth on Astrocytic Scars. Exp. Neurol. 1995, 136, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.M.; Lemmon, V.; Carrino, D.A.; Caplan, A.; Silver, J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp. Neurol. 1990, 109, 111–130. [Google Scholar] [CrossRef]
- Lemonsab, M.L.; Howlandab, D.R.; Anderson, D.K. Chondroitin Sulfate Proteoglycan Immunoreactivity Increases Following Spinal Cord Injury and Transplantation. Exp. Neurol. 1999, 160, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Niederöst, B.P.; Zimmermann, D.R.; Schwab, M.E.; Bandtlow, C.E. Bovine CNS Myelin Contains Neurite Growth-Inhibitory Activity Associated with Chondroitin Sulfate Proteoglycans. J. Neurosci. 1999, 19, 8979–8989. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, X.; He, X. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen. Res. 2014, 9, 1787–1795. [Google Scholar] [CrossRef]
- Zhu, Y.; Soderblom, C.; Trojanowsky, M.; Lee, D.-H.; Lee, J.K. Fibronectin Matrix Assembly after Spinal Cord Injury. J. Neurotrauma 2015, 32, 1158–1167. [Google Scholar] [CrossRef]
- Boghdadi, A.G.; Teo, L.; Bourne, J.A. The Neuroprotective Role of Reactive Astrocytes after Central Nervous System Injury. J. Neurotrauma 2020, 37, 681–691. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Yu, B.; Gu, X. Combination of biomaterial transplantation and genetic enhancement of intrinsic growth capacities to promote CNS axon regeneration after spinal cord injury. Front. Med. 2018, 13, 131–137. [Google Scholar] [CrossRef]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Corps, K.N.; Roth, T.; McGavern, D.B. Inflammation and Neuroprotection in Traumatic Brain Injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Fonken, L.K. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef] [PubMed]
- Bandtlow, C.; Zachleder, T.; Schwab, M. Oligodendrocytes arrest neurite growth by contact inhibition. J. Neurosci. 1990, 10, 3837–3848. [Google Scholar] [CrossRef] [PubMed]
- Caroni, P.; Schwab, M.E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 1988, 106, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kottis, V.; Thibault, P.; Mikol, D.; Xiao, Z.-C.; Zhang, R.; Dergham, P.; Braun, P.E. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J. Neurochem. 2002, 82, 1566–1569. [Google Scholar] [CrossRef]
- McKerracher, L.; David, S.; Jackson, D.L.; Kottis, V.; Dunn, R.J.; Braun, P.E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 1994, 13, 805–811. [Google Scholar] [CrossRef]
- Li, M.; Shibata, A.; Li, C.; Braun, P.E.; McKerracher, L.; Roder, J.; Kater, S.B.; David, S. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J. Neurosci. Res. 1996, 46, 404–414. [Google Scholar] [CrossRef]
- Schwab, M.E. Myelin-associated inhibitors of neurite growth. Exp. Neurol. 1990, 109, 2–5. [Google Scholar] [CrossRef]
- Shen, Y.J.; DeBellard, M.E.; Salzer, J.L.; Roderc, J.; Filbin, M.T. Myelin-Associated Glycoprotein in Myelin and Expressed by Schwann Cells Inhibits Axonal Regeneration and Branching. Mol. Cell. Neurosci. 1998, 12, 79–91. [Google Scholar] [CrossRef]
- Spillmann, A.A.; Amberger, V.R.; Schwab, M.E. High Molecular Weight Protein of Human Central Nervous System Myelin Inhibits Neurite Outgrowth: An Effect which can be Neutralized by the Monoclonal Antibody IN-1. Eur. J. Neurosci. 1997, 9, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Tanga, S.; Woodhall, R.W.; Shen, Y.J.; Debellard, M.E.; Saffell, J.L.; Dohertyb, P.; Walsh, F.S.; Filbin, M.T. Soluble Myelin-Associated Glycoprotein (MAG) Foundin VivoInhibits Axonal Regeneration. Mol. Cell. Neurosci. 1997, 9, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Mar, F.M.; da Silva, T.F.; Morgado, M.M.; Rodrigues, L.G.; Rodrigues, D.; Pereira, M.I.L.; Marques, A.; Sousa, V.F.; Coentro, J.; Sá-Miranda, C.; et al. Myelin Lipids Inhibit Axon Regeneration Following Spinal Cord Injury: A Novel Perspective for Therapy. Mol. Neurobiol. 2015, 53, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Winzeler, A.M.; Mandemakers, W.J.; Sun, M.Z.; Stafford, M.; Phillips, C.T.; Barres, B.A. The Lipid Sulfatide Is a Novel Myelin-Associated Inhibitor of CNS Axon Outgrowth. J. Neurosci. 2011, 31, 6481–6492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boghdadi, A.G.; Teo, L.; Bourne, J.A. The Involvement of the Myelin-Associated Inhibitors and Their Receptors in CNS Plasticity and Injury. Mol. Neurobiol. 2017, 55, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Shen, Y.; De Bellard, M.; Tang, S.; Filbin, M.T. Prior Exposure to Neurotrophins Blocks Inhibition of Axonal Regeneration by MAG and Myelin via a cAMP-Dependent Mechanism. Neuron 1999, 22, 89–101. [Google Scholar] [CrossRef]
- Schwab, M.E.; Caroni, P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 1988, 8, 2381–2393. [Google Scholar] [CrossRef]
- Caroni, P.; Schwab, M.E. Antibody against myelin associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1988, 1, 85–96. [Google Scholar] [CrossRef]
- Rubin, B.P.; Spillmann, A.A.; Bandtlow, C.E.; Hillenbrand, R.; Keller, F.; Schwab, M.E. Inhibition of PC12 Cell Attachment and Neurite Outgrowth by Detergent Solubilized CNS Myelin Proteins. Eur. J. Neurosci. 1995, 7, 2524–2529. [Google Scholar] [CrossRef]
- Mukhopadhyay, G.; Doherty, P.; Walsh, F.S.; Crocker, P.; Filbin, M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 1994, 13, 757–767. [Google Scholar] [CrossRef]
- Tang, S.; Qiu, J.; Nikulina, E.; Filbin, M.T. Soluble Myelin-Associated Glycoprotein Released from Damaged White Matter Inhibits Axonal Regeneration. Mol. Cell. Neurosci. 2001, 18, 259–269. [Google Scholar] [CrossRef]
- Bregman, B.S.; Kunkel-Bagden, E.; Schnell, L.; Dai, H.N.; Gao, D.; Schwab, M.E. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 1995, 378, 498–501. [Google Scholar] [CrossRef]
- Liebscher, T.; Schnell, L.; Schnell, D.; Scholl, J.; Schneider, R.; Gullo, M.; Fouad, K.; Mir, A.; Rausch, M.; Kindler, D.; et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 2005, 58, 706–719. [Google Scholar] [CrossRef]
- Von Meyenburg, J.; Brösamle, C.; Metz, G.A.S.; Schwab, M.E. Regeneration and Sprouting of Chronically Injured Corticospinal Tract Fibers in Adult Rats Promoted by NT-3 and the mAb IN-1, Which Neutralizes Myelin-Associated Neurite Growth Inhibitors. Exp. Neurol. 1998, 154, 583–594. [Google Scholar] [CrossRef]
- Raineteau, O.; Z’Graggen, W.J.; Thallmair, M.; Schwab, M.E. Sprouting and regeneration after pyramidotomy and blockade of the myelin-associated neurite growth inhibitors NI 35/250 in adult rats. Eur. J. Neurosci. 1999, 11, 1486–1490. [Google Scholar] [CrossRef]
- Schnell, L.; Schwab, M.E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 1990, 343, 269–272. [Google Scholar] [CrossRef]
- Schnell, L.; Schwab, M.E. Sprouting and Regeneration of Lesioned Corticospinal Tract Fibres in the Adult Rat Spinal Cord. Eur. J. Neurosci. 1993, 5, 1156–1171. [Google Scholar] [CrossRef]
- Weibel, D.; Cadelli, D.; Schwab, M.E. Regeneration of lesioned rat optic nerve fibers is improved after neutralization of myelin-associated neurite growth inhibitors. Brain Res. 1994, 642, 259–266. [Google Scholar] [CrossRef]
- Z’Graggen, W.J.; Metz, G.; Kartje, G.L.; Thallmair, M.; Schwab, M.E. Functional Recovery and Enhanced Corticofugal Plasticity after Unilateral Pyramidal Tract Lesion and Blockade of Myelin-Associated Neurite Growth Inhibitors in Adult Rats. J. Neurosci. 1998, 18, 4744–4757. [Google Scholar] [CrossRef]
- Schwab, M.E.; Thoenen, H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J. Neurosci. 1985, 5, 2415–2423. [Google Scholar] [CrossRef]
- Varga, Z.M.; Schwab, M.E.; Nicholls, J.G. Myelin-associated neurite growth-inhibitory proteins and suppression of regeneration of immature mammalian spinal cord in culture. Proc. Natl. Acad. Sci. USA 1995, 92, 10959–10963. [Google Scholar] [CrossRef]
- Sicotte, M.; Tsatas, O.; Jeong, S.Y.; Cai, C.-Q.; He, Z.; David, S. Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol. Cell. Neurosci. 2003, 23, 251–263. [Google Scholar] [CrossRef]
- Wong, E.V.; David, S.; Jacob, M.H.; Jay, D.G. Inactivation of Myelin-Associated Glycoprotein Enhances Optic Nerve Regeneration. J. Neurosci. 2003, 23, 3112–3117. [Google Scholar] [CrossRef]
- Kim, J.-E.; Li, S.; GrandPré, T.; Qiu, D.; Strittmatter, S. Axon Regeneration in Young Adult Mice Lacking Nogo-A/B. Neuron 2003, 38, 187–199. [Google Scholar] [CrossRef]
- Simonen, M.; Pedersen, V.; Weinmann, O.; Schnell, L.; Buss, A.; Ledermann, B.; Christ, F.; Sansig, G.; van der Putten, H.; Schwab, M.E. Systemic Deletion of the Myelin-Associated Outgrowth Inhibitor Nogo-A Improves Regenerative and Plastic Responses after Spinal Cord Injury. Neuron 2003, 38, 201–211. [Google Scholar] [CrossRef]
- Dimou, L.; Schnell, L.; Montani, L.; Duncan, C.; Simonen, M.; Schneider, R.; Liebscher, T.; Gullo, M.; Schwab, M.E. Nogo-A-Deficient Mice Reveal Strain-Dependent Differences in Axonal Regeneration. J. Neurosci. 2006, 26, 5591–5603. [Google Scholar] [CrossRef]
- Lee, J.K.; Chan, A.F.; Luu, S.M.; Zhu, Y.; Ho, C.; Tessier-Lavigne, M.; Zheng, B. Reassessment of Corticospinal Tract Regeneration in Nogo-Deficient Mice. J. Neurosci. 2009, 29, 8649–8654. [Google Scholar] [CrossRef]
- Zheng, B.; Ho, C.; Li, S.; Keirstead, H.; Steward, O.; Tessier-Lavigne, M. Lack of Enhanced Spinal Regeneration in Nogo-Deficient Mice. Neuron 2003, 38, 213–224. [Google Scholar] [CrossRef]
- Domeniconi, M.; Cao, Z.; Spencer, T.; Sivasankaran, R.; Wang, K.C.; Nikulina, E.; Kimura, N.; Cai, H.; Deng, K.; Gao, Y.; et al. Myelin-Associated Glycoprotein Interacts with the Nogo66 Receptor to Inhibit Neurite Outgrowth. Neuron 2002, 35, 283–290. [Google Scholar] [CrossRef]
- Fournier, A.E.; Grandpre, T.; Strittmatter, S. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001, 409, 341–346. [Google Scholar] [CrossRef]
- Wang, X.; Ba, K.W.B.; Basso, D.M.; Strittmatter, S.M. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann. Neurol. 2006, 60, 540–549. [Google Scholar] [CrossRef]
- Fischer, D.; He, Z.; Benowitz, L.I. Counteracting the Nogo Receptor Enhances Optic Nerve Regeneration If Retinal Ganglion Cells Are in an Active Growth State. J. Neurosci. 2004, 24, 1646–1651. [Google Scholar] [CrossRef]
- Li, S.; Strittmatter, S.M. Delayed Systemic Nogo-66 Receptor Antagonist Promotes Recovery from Spinal Cord Injury. J. Neurosci. 2003, 23, 4219–4227. [Google Scholar] [CrossRef]
- Li, S. Blockade of Nogo-66, Myelin-Associated Glycoprotein, and Oligodendrocyte Myelin Glycoprotein by Soluble Nogo-66 Receptor Promotes Axonal Sprouting and Recovery after Spinal Injury. J. Neurosci. 2004, 105, 11–20. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, T.; Maynard, G.D.; Terse, P.S.; Cafferty, W.B.; Kocsis, J.D.; Strittmatter, S.M. Nogo receptor decoy promotes recovery and cortico-spinal growth in non-human primate spinal cord injury. Brain 2020, 143, 1697–1713. [Google Scholar] [CrossRef]
- Cao, Y.; Shumsky, J.S.; Sabol, M.A.; Kushner, R.A.; Strittmatter, S.; Hamers, F.P.; Lee, D.H.; Rabacchi, S.A.; Murray, M. Nogo-66 Receptor Antagonist Peptide (NEP1-40) Administration Promotes Functional Recovery and Axonal Growth After Lateral Funiculus Injury in the Adult Rat. Neurorehabil. Neural Repair 2008, 22, 262–278. [Google Scholar] [CrossRef]
- Davies, S.J.A.; Field, P.M.; Raisman, G. Long Fibre Growth by Axons of Embryonic Mouse Hippocampal Neurons Microtransplanted into the Adult Rat Fimbria. Eur. J. Neurosci. 1993, 5, 95–106. [Google Scholar] [CrossRef]
- Humpel, C.; Bygdeman, M.; Olson, L.; Strömberg, I. Human fetal neocortical tissue grafted to rat brain cavities survives, leads to reciprocal nerve fiber growth, and accumulates host IgG. J. Comp. Neurol. 1994, 340, 337–348. [Google Scholar] [CrossRef]
- Lehman, M.N.; Lesauter, J.; Silver, R. Fiber outgrowth from anterior hypothalamic and cortical xenografts in the third ventricle. J. Comp. Neurol. 1998, 391, 133–145. [Google Scholar] [CrossRef]
- Tønder, N.; Sørensen, T.; Zimmer, J. Chapter 28 Grafting of fetal CA3 neurons to excitotoxic, axon-sparing lesions of the hippocampal CA3 area in adult rats. Prog. Brain Res. 1990, 83, 391–409. [Google Scholar] [CrossRef]
- Wictorin, K.; Brundin, P.; Sauer, H.; Lindvall, O.; Björklund, A. Long distance directed axonal growth from human dopaminergic mesencephalic neuroblasts implanted along the nigrostriatal pathway in 6-hydroxydopamine lesioned adult rats. J. Comp. Neurol. 1992, 323, 475–494. [Google Scholar] [CrossRef]
- Wictorin, K.; Simerly, R.B.; Isacson, O.; Swanson, L.W.; Björklund, A. Connectivity of striatal grafts implanted into the ibotenic acid-lesioned striatum—III. Efferent projecting graft neurons and their relation to host afferents within the grafts. Neuroscience 1989, 30, 313–330. [Google Scholar] [CrossRef]
- Wictorin, K.; Brundin, P.; Gustavii, B.; Lindvall, O.; Björklund, A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature 1990, 347, 556–558. [Google Scholar] [CrossRef]
- Wictorin, K.; Lagenaur, C.F.; Lund, R.D.; Bjorklund, A. Efferent Projections to the Host Brain from Intrastriatal Striatal Mouse-to-rat Grafts: Time Course and Tissue-type Specificity as Revealed by a Mouse Specific Neuronal Marker. Eur. J. Neurosci. 1991, 3, 86–101. [Google Scholar] [CrossRef]
- Wictorin, K.; Clarke, D.J.; Bolam, J.P.; Björklund, A. Fetal striatal neurons grafted into the ibotenate lesioned adult striatum: Efferent projections and synaptic contacts in the host globus pallidus. Neuroscience 1990, 37, 301–315. [Google Scholar] [CrossRef]
- Pettigrew, D.B.; Li, Y.-Q.; Kuntz, C.; Crutcher, K.A. Global expression of NGF promotes sympathetic axonal growth in CNS white matter but does not alter its parallel orientation. Exp. Neurol. 2007, 203, 95–109. [Google Scholar] [CrossRef][Green Version]
- Carbonetto, S.; Evans, D.; Cochard, P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J. Neurosci. 1987, 7, 610–620. [Google Scholar] [CrossRef]
- Carpenter, M.K.; Hassinge, T.D.; Whalen, L.R.; Kater, S.B. CNS white matter can be altered to support neuronal outgrowth. J. Neurosci. Res. 1994, 37, 1–14. [Google Scholar] [CrossRef]
- Crutcher, K.A. Tissue sections as culture substrates: Overview and critique. Hippocampus 1993, 3, 157–163. [Google Scholar] [CrossRef]
- Crutcher, K. Tissue sections from the mature rat brain and spinal cord as substrates for neurite outgrowth in vitro: Extensive growth on gray matter but little growth on white matter. Exp. Neurol. 1989, 104, 39–54. [Google Scholar] [CrossRef]
- Sagot, Y.; Swerts, J.-P.; Cochard, P. Changes in permissivity for neuronal attachment and neurite outgrowth of spinal cord grey and white matters during development: A study with the ‘ryoculture’ bioassay. Brain Res. 1991, 543, 25–35. [Google Scholar] [CrossRef]
- Savio, T.; Schwab, M. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J. Neurosci. 1989, 9, 1126–1133. [Google Scholar] [CrossRef]
- Watanabe, E.; Murakami, F. Preferential adhesion of chick central neurons to the gray matter of the central nervous system. Neurosci. Lett. 1989, 97, 69–74. [Google Scholar] [CrossRef]
- Watanabe, E.; Murakami, F. Cell attachment to and neurite outgrowth on tissue sections of developing, mature and lesioned brain: The role of inhibitory factor(s) in the CNS white matter. Neurosci. Res. 1990, 8, 83–99. [Google Scholar] [CrossRef]
- Pettigrew, D.B.; Shockley, K.P.; Crutcher, K.A. Disruption of spinal cord white matter and sciatic nerve geometry inhibits axonal growth in vitro in the absence of glial scarring. BMC Neurosci. 2001, 2, 8. [Google Scholar] [CrossRef]
- Pettigrew, D.B.; Crutcher, K.A. Myelin contributes to the parallel orientation of axonal growth on white matter in vitro. BMC Neurosci. 2001, 2, 9. [Google Scholar] [CrossRef]
- Bähr, M.; Przyrembel, C. Myelin from Peripheral and Central Nervous System Is a Nonpermissive Substrate for Retinal Ganglion Cell Axons. Exp. Neurol. 1995, 5, 87–93. [Google Scholar] [CrossRef]
- Cadelli, D.; Bandtlow, C.; Schwab, M. Oligodendrocyte- and myelin-associated inhibitors of neurite outgrowth: Their involvement in the lack of CNS regeneration. Exp. Neurol. 1992, 115, 189–192. [Google Scholar] [CrossRef]
- Crutcher, K.; Jayasinghe, C.; Yun, Y.; Shanov, V. Progress in the Use of Aligned Carbon Nanotubes to Support Neuronal At-tachment and Directional Neurite Growth. In Nanomedicine Materials, Devices and Systems; Schulz, M., Shanov, V., Yun, Y., Eds.; Artech House: Boston, CA, USA, 2009; pp. 189–209. [Google Scholar]
- Raisman, G. Myelin inhibitors: Does NO mean GO? Nat. Rev. Neurosci. 2004, 5, 157–161. [Google Scholar] [CrossRef]
- Schnell, L.; Hunanyan, A.S.; Bowers, W.J.; Horner, P.J.; Federoff, H.J.; Gullo, M.; Schwab, M.E.; Mendell, L.M.; Arvanian, V.L. Combined delivery of Nogo-A antibody, neurotrophin-3 and the NMDA-NR2d subunit establishes a functional ‘detour’ in the hemisected spinal cord. Eur. J. Neurosci. 2011, 34, 1256–1267. [Google Scholar] [CrossRef]
- Freund, P.; Wannier, T.; Al, E. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J. Comp. Neurol. 2007, 502, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Lindau, N.T.; Bänninger, B.J.; Gullo, M.; Good, N.A.; Al, E. Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy. Brain 2014, 10, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Raineteau, O.; Fouad, K.; Bareyre, F.M.; Schwab, M.E. Reorganization of descending motor tracts in the rat spinal cord. Eur. J. Neurosci. 2002, 16, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Schwegler, G.; Schwab, M.; Kapfhammer, J. Increased collateral sprouting of primary afferents in the myelin-free spinal cord. J. Neurosci. 1995, 15, 2756–2767. [Google Scholar] [CrossRef] [PubMed]
- Vanek, P.; Thallmair, M.; Schwab Martin, E.; Kapfhammer, J.P. Increased lesion-induced sprouting of corticospinal fibres in the myelin-free rat spinal cord. Eur. J. Neurosci. 1998, 10, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wenk, C.A.; Thallmair, M.; Kartje, G.L.; Schwab, M.E. Increased corticofugal plasticity after unilateral cortical lesions combined with neutralization of the IN-1 antigen in adult rats. J. Comp. Neurol. 1999, 410, 143–157. [Google Scholar] [CrossRef]
- Buffo, A.; Zagrebelsky, M.; Huber, A.B.; Skerra, A.; Schwab, M.E.; Strata, P.; Rossi, F. Application of Neutralizing Antibodies against NI-35/250 Myelin-Associated Neurite Growth Inhibitory Proteins to the Adult Rat Cerebellum Induces Sprouting of Uninjured Purkinje Cell Axons. J. Neurosci. 2000, 20, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Meves, J.M.; Geoffroy, C.G.; Kim, N.D.; Kim, J.J.; Zheng, B. Oligodendrocytic but not neuronal Nogo restricts corticospinal axon sprouting after CNS injury. Exp. Neurol. 2018, 309, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kubo, T.; Matsuda, K.; Yano, K.; Tohyama, M.; Hosokawa, K. Myelin-associated glycoprotein reduces axonal branching and enhances functional recovery after sciatic nerve transection in rats. Glia 2007, 309, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Bastmeyer, M.; Beckmann, M.; Schwab, M.; Stuermer, C. Growth of regenerating goldfish axons is inhibited by rat oligodendrocytes and CNS myelin but not but not by goldfish optic nerve tract oligodendrocytelike cells and fish CNS myelin. J. Neurosci. 1991, 11, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, D.B.; Crutcher, K.A. White Matter of the CNS Supports or Inhibits Neurite Outgrowth In Vitro Depending on Geometry. J. Neurosci. 1999, 19, 8358–8366. [Google Scholar] [CrossRef] [PubMed]
- Raisman, G.A. promising therapeutic approach to spinal cord repair. J. R. Soc. Med. 2003, 96, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.G.; Ward, M.B. Reconstitution of the spinal cord following ablation in urodele larvae. J. Exp. Zoöl. 1965, 160, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.G.; Ward, M.B. Reconstitution of the spinal cord after ablation in adult Triturus. Dev. Biol. 1967, 15, 464–486. [Google Scholar] [CrossRef]
- Albors, A.R.; Tazaki, A.; Rost, F.; Nowoshilow, S.; Chara, O.; Tanaka, E.M. Planar cell polarity-mediated induction of neural stem cell expansion during axolotl spinal cord regeneration. eLife 2015, 4, e10230. [Google Scholar] [CrossRef]
- Tazaki, A.; Tanaka, E.M.; Fei, J.-F. Salamander spinal cord regeneration: The ultimate positive control in vertebrate spinal cord regeneration. Dev. Biol. 2017, 432, 63–71. [Google Scholar] [CrossRef]
- Silvio, V.; Manthorpe, M. Trophic and neurite-promoting factors for cholinergic neurons. Cell Mol. Biol. Neuronal. Dev. 1984, 17, 251–275. [Google Scholar]
- Qian, Y.; Lin, H.; Yan, Z.; Shi, J.; Fan, C. Functional nanomaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater. Today 2021, 51, 165–187. [Google Scholar] [CrossRef]
- Sarker, M.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, 171, 125–150. [Google Scholar] [CrossRef]
- Higuchi, A.; Kumar, S.S.; Benelli, G.; Ling, Q.-D.; Li, H.-F.; Alarfaj, A.A.; Munusamy, M.A.; Sung, T.-C.; Chang, Y.; Murugan, K. Biomaterials used in stem cell therapy for spinal cord injury. Prog. Mater. Sci. 2019, 103, 374–424. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Yun, Y.; Lee, S.-J.; Ha, Y.; Gwak, S.-J. Biomaterials and strategies for repairing spinal cord lesions. Neurochem. Int. 2021, 144, 104973. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xue, F.; Liu, K.; Li, B.; Fu, C.; Ding, J. Physical and biological engineering of polymer scaffolds to potentiate repair of spinal cord injury. Mater. Des. 2021, 201, 109484. [Google Scholar] [CrossRef]
- Mneimneh, A.T.; Mehanna, M.M. Collagen-based scaffolds: An auspicious tool to support repair, recovery, and regeneration post spinal cord injury. Int. J. Pharm. 2021, 601, 120559. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio. 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Schaub, N.J.; Johnson, C.D.; Cooper, B.; Gilbert, R.J. Electrospun Fibers for Spinal Cord Injury Research and Regeneration. J. Neurotrauma 2016, 33, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Agrawal, N.K.; Griffin, J.M.; Schmidt, C.E. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv. Drug Deliv. Rev. 2018, 148, 38–59. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhao, Y.; Chen, B.; Dai, J. Scaffolds for spinal cord injury repair: From proof of concept to first in-human studies and clinical trials. Handb. Innov. Cent. Nerv. Syst. Regen. Med. 2020, 603–619. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, B.; Ding, J.; Yan, L.; Thawani, J.P.; Fu, C.; Chen, X. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019, 88, 57–77. [Google Scholar] [CrossRef]
- Weiss, P. In vitro experiments on the factors determining the course of the outgrowing nerve fiber. J. Exp. Zoöl. 1934, 68, 393–448. [Google Scholar] [CrossRef]
- Weiss, P.; Taylor, A.C. Guides for Nerve Regeneration Across Gaps. J. Neurosurg. 1946, 3, 375–389. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Corey, J.M.; Lin, D.Y.; Mycek, K.B.; Chen, Q.; Samuel, S.; Feldman, E.L.; Martin, D.C. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. Part A 2007, 83, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Cregg, J.; Wang, H.B.; Wendell, D.F.; Oudega, M.; Gilbert, R.J.; McDonald, J.W. Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers. Biomaterials 2011, 32, 6068–6079. [Google Scholar] [CrossRef] [PubMed]
- Puhl, D.L.; Funnell, J.L.; D’Amato, A.R.; Bao, J.; Zagorevski, D.V.; Pressman, Y.; Morone, D.; Haggerty, A.E.; Oudega, M.; Gilbert, R.J. Aligned Fingolimod-Releasing Electrospun Fibers Increase Dorsal Root Ganglia Neurite Extension and Decrease Schwann Cell Expression of Promyelinating Factors. Front. Bioeng. Biotechnol. 2020, 8, 937. [Google Scholar] [CrossRef]
- Xue, W.; Shi, W.; Kong, Y.; Kuss, M.; Duan, B. Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact. Mater. 2021, 6, 4141–4160. [Google Scholar] [CrossRef]
- Ren, Y.-J.; Zhang, S.; Mi, R.; Liu, Q.; Zeng, X.; Rao, M.; Hoke, A.; Mao, H.-Q. Enhanced differentiation of human neural crest stem cells towards the Schwann cell lineage by aligned electrospun fiber matrix. Acta Biomater. 2013, 9, 7727–7736. [Google Scholar] [CrossRef]
- Usmani, S.; Aurand, E.R.; Medelin, M.; Fabbro, A.; Scaini, D.; Laishram, J.; Rosselli, F.B.; Ansuini, A.; Zoccolan, D.; Scarselli, M.; et al. 3D meshes of carbon nanotubes guide functional reconnection of segregated spinal explants. Sci. Adv. 2016, 2, e1600087. [Google Scholar] [CrossRef]
- Maiese, K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen. Res. 2014, 9, 1413–1417. [Google Scholar] [CrossRef]
- Johnson, P.J.; Parker, S.R.; Sakiyama-Elbert, S.E. Fibrin-based tissue engineering scaffolds enhance neural fiber sprouting and delay the accumulation of reactive astrocytes at the lesion in a subacute model of spinal cord injury. J. Biomed. Mater. Res. Part A 2009, 92, 152–163. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J. MicroRNA Dysregulation in Epilepsy: From Pathogenetic Involvement to Diagnostic Biomarker and Therapeutic Agent Development. Front. Mol. Neurosci. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Bakhru, S.; Nain, A.S.; Highley, C.; Wang, J.; Campbell, P.; Amon, C.; Zappe, S. Direct and cell signaling-based, geometry-induced neuronal differentiation of neural stem cells. Integr. Biol. 2011, 3, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Worley, K.; Certo, A.; Wan, L.Q. Geometry–Force Control of Stem Cell Fate. BioNanoScience 2012, 3, 43–51. [Google Scholar] [CrossRef]
- Tessier-Lavigne, M.; Goodman, C.S. The Molecular Biology of Axon Guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Lorenz, S.E.; Wahlsten, D.; Coughlin, J. Axonal guidance during development of the great cerebral commissures: Descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J. Comp. Neurol. 1982, 210, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.A.J.; Bär, D.P.R. Axon guidance of outgrowing corticospinal fibres in the rat. J. Anat. 1999, 194, 15–32. [Google Scholar] [CrossRef]
- Francàs, G.D.R.; Zuñiga, N.R.; Stoeckli, E.T. The spinal cord shows the way—How axons navigate intermediate targets. Dev. Biol. 2017, 432, 43–52. [Google Scholar] [CrossRef]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2020, 33, e2005476. [Google Scholar] [CrossRef]
- Collins, F.; Dawson, A. Conditioned medium increases the rate of neurite elongation: Separation of this activity from the substratum-bound inducer of neurite outgrowth. J. Neurosci. 1982, 2, 1005–1010. [Google Scholar] [CrossRef]
- Collins, F.; Lee, M.R. The spatial control of ganglionic neurite growth by the substrate-associated material from conditioned medium: An experimental model of haptotaxis. J. Neurosci. 1984, 4, 2823–2829. [Google Scholar] [CrossRef]
- Leclech, C.; Villard, C. Cellular and Subcellular Contact Guidance on Microfabricated Substrates. Front. Bioeng. Biotechnol. 2020, 8, 551505. [Google Scholar] [CrossRef]
- Li, N.; Folch, A. Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Exp. Cell Res. 2005, 311, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sunnerberg, J.P.; Descoteaux, M.; Kaplan, D.L.; Staii, C. Axonal growth on surfaces with periodic geometrical patterns. PLoS ONE 2021, 16, e0257659. [Google Scholar] [CrossRef] [PubMed]
- Cnops, V.; Chin, J.S.; Milbreta, U.; Chew, S.Y. Biofunctional scaffolds with high packing density of aligned electrospun fibers support neural regeneration. J. Biomed. Mater. Res. Part A 2020, 108, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.R.; Puhl, D.L.; Ziemba, A.M.; Johnson, C.D.L.; Doedee, J.; Bao, J.; Gilbert, R.J. Exploring the effects of electrospun fiber surface nanotopography on neurite outgrowth and branching in neuron cultures. PLoS ONE 2019, 14, e0211731. [Google Scholar] [CrossRef]
- Johnson, C.D.L.; Zuidema, J.M.; Kearns, K.R.; Maguire, A.B.; Desmond, G.P.; Thompson, D.M.; Gilbert, R.J. The Effect of Electrospun Fiber Diameter on Astrocyte-Mediated Neurite Guidance and Protection. ACS Appl. Bio. Mater. 2018, 2, 104–117. [Google Scholar] [CrossRef]
- López-Fagundo, C.; Mitchel, J.A.; Ramchal, T.D.; Dingle, Y.-T.L.; Hoffman-Kim, D. Navigating neurites utilize cellular topography of Schwann cell somas and processes for optimal guidance. Acta Biomater. 2013, 9, 7158–7168. [Google Scholar] [CrossRef]
- Omidinia-Anarkoli, A.; Ephraim, J.W.; Rimal, R.; De Laporte, L. Hierarchical fibrous guiding cues at different scales influence linear neurite extension. Acta Biomater. 2020, 113, 350–359. [Google Scholar] [CrossRef]
- Wang, H.B.; Mullins, M.E.; Cregg, J.; McCarthy, C.W.; Gilbert, R.J. Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater. 2010, 6, 2970–2978. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, K.S.; Coric, D.; Chang, J.J.; Harrop, J.S.; Theodore, N.; Toselli, R.M. A study of probable benefit of a bioresorbable polymer scaffold for safety and neurological recovery in patients with complete thoracic spinal cord injury: 6-month results from the INSPIRE study. J. Neurosurg. Spine 2021, 34, 808–817. [Google Scholar] [CrossRef]
- Dong, X.; Liu, S.; Yang, Y.; Gao, S.; Li, W.; Cao, J.; Wan, Y.; Huang, Z.; Fan, G.; Chen, Q.; et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021, 272, 120767. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Sitoci-Ficici, K.H.; Matyash, M.; Uckermann, O.; Galli, R.; Leipnitz, E.; Later, R.; Ikonomidou, C.; Gelinsky, M.; Schackert, G.; Kirsch, M. Non-functionalized soft alginate hydrogel promotes locomotor recovery after spinal cord injury in a rat hemimyelonectomy model. Acta Neurochir. 2017, 160, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Coykendall, K.; Li, Y.; Moon, A.; Priyadarshani, P.; Yao, L. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Res. Ther. 2014, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Galindo, M.D.C.; Calderón-Vallejo, D.; Olvera-Sandoval, C.; Quintanar, J.L. Therapeutic approaches of trophic factors in animal models and in patients with spinal cord injury. Growth Factors 2020, 38, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grulova, I.; Slovinska, L.; Blaško, J.; Devaux, S.; Wisztorski, M.; Salzet, M.; Fournier, I.; Kryukov, O.; Cohen, S.; Cizkova, D. Delivery of Alginate Scaffold Releasing Two Trophic Factors for Spinal Cord Injury Repair. Sci. Rep. 2015, 5, 13702. [Google Scholar] [CrossRef]
- Kuihua, Z.; Chunyang, W.; Cunyi, F.; Xiumei, M. Aligned SF/P(LLA-CL)-blended nanofibers encapsulating nerve growth factor for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2013, 102, 2680–2691. [Google Scholar] [CrossRef]
- Robinson, J.; Lu, P. Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp. Neurol. 2017, 291, 87–97. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Kim, H.-J.; Ehsanipour, A.; Bierman, R.D.; Kaarela, O.; Xue, C.; Khademhosseini, A.; Seidlits, S.K. Regenerative Therapies for Spinal Cord Injury. Tissue Eng. Part B Rev. 2019, 25, 471–491. [Google Scholar] [CrossRef]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal Cord Injury. Surg. Clin. N. Am. 2017, 97, 1031–1045. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef]
- Sharif, S.; Ali, M.Y.J. Outcome Prediction in Spinal Cord Injury: Myth or Reality. World Neurosurg. 2020, 140, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.; Verla, T.; Ropper, A.E. Practical Application of Recent Advances in Diagnostic, Prognostic, and Therapeutic Modalities for Spinal Cord Injury. World Neurosurg. 2020, 136, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hutson, T.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; van der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef] [PubMed]
- Badhiwala, J.H.; Ahuja, C.S.; Fehlings, M.G. Time is spine: A review of translational advances in spinal cord injury. J. Neurosurg. Spine 2019, 30, 1–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettigrew, D.B.; Singh, N.; Kirthivasan, S.; Crutcher, K.A. The Role of Tissue Geometry in Spinal Cord Regeneration. Medicina 2022, 58, 542. https://doi.org/10.3390/medicina58040542
Pettigrew DB, Singh N, Kirthivasan S, Crutcher KA. The Role of Tissue Geometry in Spinal Cord Regeneration. Medicina. 2022; 58(4):542. https://doi.org/10.3390/medicina58040542
Chicago/Turabian StylePettigrew, David B., Niharika Singh, Sabarish Kirthivasan, and Keith A. Crutcher. 2022. "The Role of Tissue Geometry in Spinal Cord Regeneration" Medicina 58, no. 4: 542. https://doi.org/10.3390/medicina58040542
APA StylePettigrew, D. B., Singh, N., Kirthivasan, S., & Crutcher, K. A. (2022). The Role of Tissue Geometry in Spinal Cord Regeneration. Medicina, 58(4), 542. https://doi.org/10.3390/medicina58040542






