Spreading Allergic Contact Dermatitis to Tea Tree Oil in an Over-the-Counter Product Applied on a Wart
Abstract
:1. Introduction
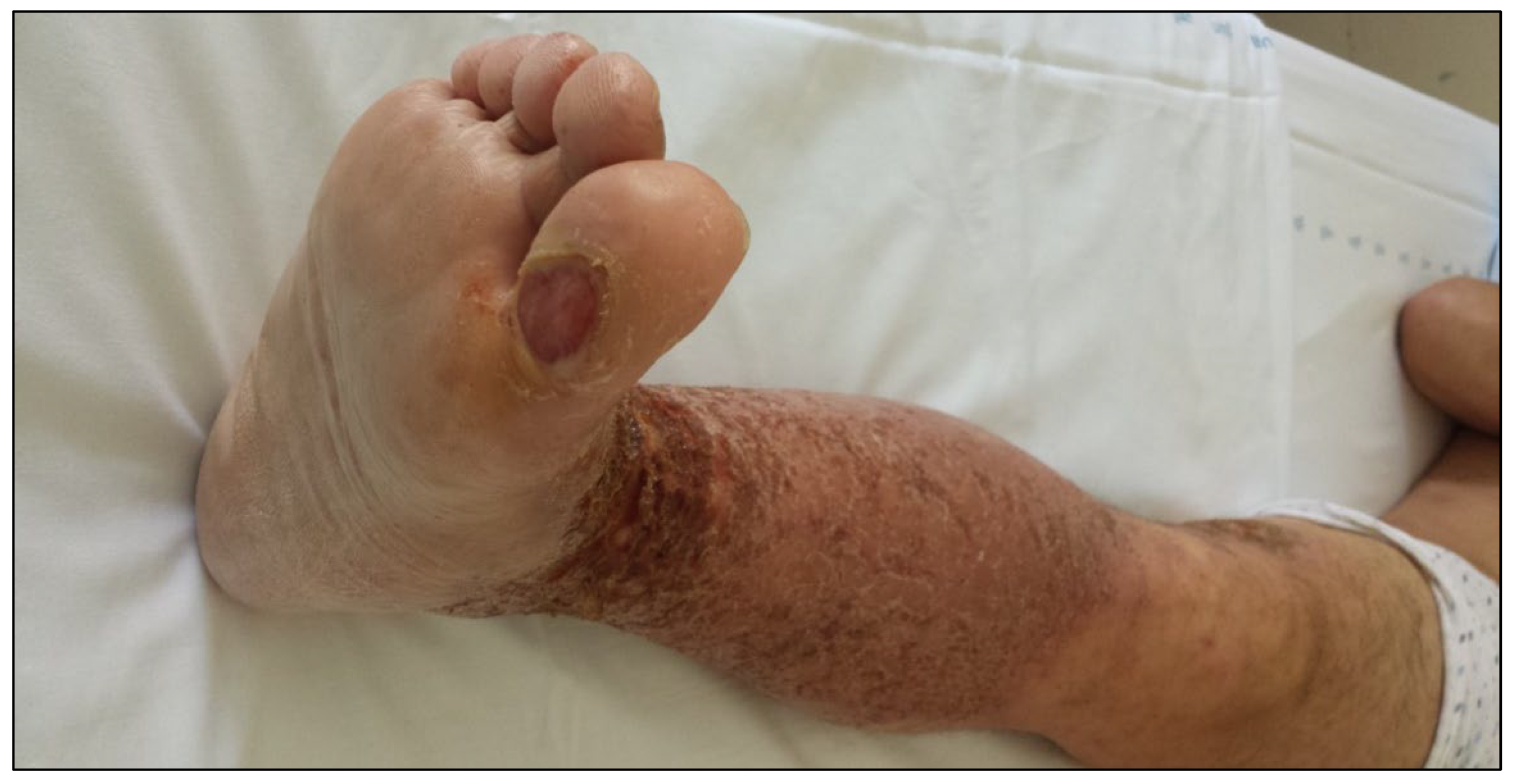
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magalhães, G.M.; Vieira, É.C.; Garcia, L.C.; de Lourdes Ribeiro De Carvalho-Leite, M.; Guedes, A.C.M.; Araújo, M.G. Update on human papilloma virus—part I: Epidemiology, pathogenesis, and clinical spectrum. An. Bras. Dermatol. 2021, 96, 1–16. [Google Scholar] [CrossRef]
- Kwok, C.S.; Holland, R.; Gibbs, S. Efficacy of topical treatments for cutaneous warts: A meta-analysis and pooled analysis of randomized controlled trials. Br. J. Dermatol. 2011, 165, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Gibbs, S.; Bennett, C.; Holland, R.; Abbott, R. Topical treatments for cutaneous warts. Cochrane Database Syst. Rev. 2012, 9, CD001781. [Google Scholar]
- García-Oreja, S.; Álvaro-Afonso, F.J.; García-Álvarez, Y.; García-Morales, E.; Sanz-Corbalán, I.; Lázaro Martínez, J.L. Topical treatment for plantar warts: A systematic review. Dermatol. Ther. 2021, 34, e14621. [Google Scholar] [CrossRef]
- Hekmatjah, J.; Farshchian, M.; Grant-Kels, J.M.; Mehregan, D. The status of treatment for plantar warts in 2021: No definitive advancements in decades for a common dermatology disease. Clin. Dermatol. 2021, 39, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.A.; Schmid, M.M. Use of OTC Essential Oils to Clear Plantar Warts. Nurse Pract. 2006, 31, 53–55. [Google Scholar] [CrossRef]
- Aberer, W. Contact allergy and medicinal herbs. J. Dtsch. Dermatol. Ges. 2008, 6, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, S.; Ozkol, H.; Karadag, A.; Calka, O. The use of complementary and alternative medicine among dermatology outpatients in Eastern Turkey. Hum. Exp. Toxicol. 2014, 33, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- de Groot, A.C.; Schmidt, E. Tea tree oil: Contact allergy and chemical composition. Contact Derm. 2016, 75, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid. Based Complement. Alternat. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, G.; Sciacca, J.; James, W. Tea Tree Oil: Cutaneous Effects of the Extracted Oil of Melaleuca alternifolia. Dermatitis 2004, 15, 59–66. [Google Scholar] [CrossRef]
- Rutherford, T.; Nixon, R.; Tam, M.; Tate, B. Allergy to tea tree oil: Retrospective review of 41 cases with positive patch tests over 4.5 years. Australas. J. Dermatol. 2007, 48, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Stingeni, L.; Bianchi, L.; Hansel, K.; Corazza, M.; Gallo, R.; Guarneri, F.; Patruno, C.; Rigano, L.; Romita, P.; Pigatto, P.D.; et al. “Skin Allergy group of SIDeMAST” and “SIDAPA” (Società Italiana di Dermatologia Allergologica, Professionale e Ambientale). Italian Guidelines in Patch Testing—Adapted from the European Society of Contact Dermatitis (ESCD). G. Ital. Dermatol. Venereol. 2019, 154, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Mozelsio, N.B.; Harris, K.E.; McGrath, K.G.; Grammer, L.C. Immediate systemic hypersensitivity reaction associated with topical application of Australian tea tree oil. Allergy Asthma Proc. 2003, 24, 73–75. [Google Scholar] [PubMed]
- De Groot, A.C.; Willem Weyland, J. Systemic contact dermatitis from tea tree oil. Contact Derm. 1992, 27, 279–280. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.C. Airborne allergic contact dermatitis from tea tree oil. Contact Derm. 1996, 35, 304–305. [Google Scholar] [CrossRef]
- Dharmagunawardena, B.; Takwale, A.; Sanders, K.J.; Cannan, S.; Rodger, A.; Ilchyshyn, A. Gas chromatography: An investigative tool in multiple allergies to essential oils. Contact Derm. 2002, 47, 288–292. [Google Scholar] [CrossRef]
- Gilissen, L.; Huygens, S.; Goossens, A. Allergic contact dermatitis caused by topical herbal remedies: Importance of patch testing with the patients’ own products. Contact Dermat. 2018, 78, 177–184. [Google Scholar] [CrossRef]
- Khanna, M.; Qasem, K.; Sasseville, D. Allergic contact dermatitis to tea tree oil with erythema multiforme-like id reaction. Am. J. Contact. Dermat. 2000, 11, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Perrett, C.M.; Evans, A.V.; Russell-Jones, R. Tea tree oil dermatitis associated with linear IgA disease. Clin. Exp. Dermatol. 2003, 28, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Hausen, B.M.; Reichling, J.; Harkenthal, M. Degradation products of monoterpenes are the sensitizing agents in tea tree oil. Am. J. Contact. Dermat. 1999, 10, 68–77. [Google Scholar] [CrossRef]
- COLIPA Recommendation N° 12. Use of Tea-Tree Oil in Cosmetic Products. Available online: https://www.cosmeticseurope.eu/files/5514/6408/3534/CR-12-Tea-TreeOil.PDF (accessed on 25 November 2021).
- Selvaag, E.; Eriksen, B.; Thune, P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Derm. 1994, 31, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Storan, E.R.; Nolan, U.; Kirby, B. Allergic contact dermatitis caused by the tea tree oil-containing hydrogel Burnshield®. Contact Derm. 2016, 74, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Zambello, A.; Caroppo, F.; Belloni Fortina, A. Tea tree oil contact sensitization in children. Contact Derm. 2021, 84, 139–140. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrogio, F.; Foti, C.; Cazzato, G.; Mortato, E.; Mazzoccoli, S.; De Caro, A.P.; Cassano, N.; Vena, G.A.; Calogiuri, G.; Romita, P. Spreading Allergic Contact Dermatitis to Tea Tree Oil in an Over-the-Counter Product Applied on a Wart. Medicina 2022, 58, 561. https://doi.org/10.3390/medicina58050561
Ambrogio F, Foti C, Cazzato G, Mortato E, Mazzoccoli S, De Caro AP, Cassano N, Vena GA, Calogiuri G, Romita P. Spreading Allergic Contact Dermatitis to Tea Tree Oil in an Over-the-Counter Product Applied on a Wart. Medicina. 2022; 58(5):561. https://doi.org/10.3390/medicina58050561
Chicago/Turabian StyleAmbrogio, Francesca, Caterina Foti, Gerardo Cazzato, Edoardo Mortato, Stella Mazzoccoli, Anna Paola De Caro, Nicoletta Cassano, Gino Antonio Vena, Gianfranco Calogiuri, and Paolo Romita. 2022. "Spreading Allergic Contact Dermatitis to Tea Tree Oil in an Over-the-Counter Product Applied on a Wart" Medicina 58, no. 5: 561. https://doi.org/10.3390/medicina58050561
APA StyleAmbrogio, F., Foti, C., Cazzato, G., Mortato, E., Mazzoccoli, S., De Caro, A. P., Cassano, N., Vena, G. A., Calogiuri, G., & Romita, P. (2022). Spreading Allergic Contact Dermatitis to Tea Tree Oil in an Over-the-Counter Product Applied on a Wart. Medicina, 58(5), 561. https://doi.org/10.3390/medicina58050561







