The Role of Power Doppler Ultrasonography in Caudal Epidural Injection
Abstract
1. Introduction
2. Materials and Methods
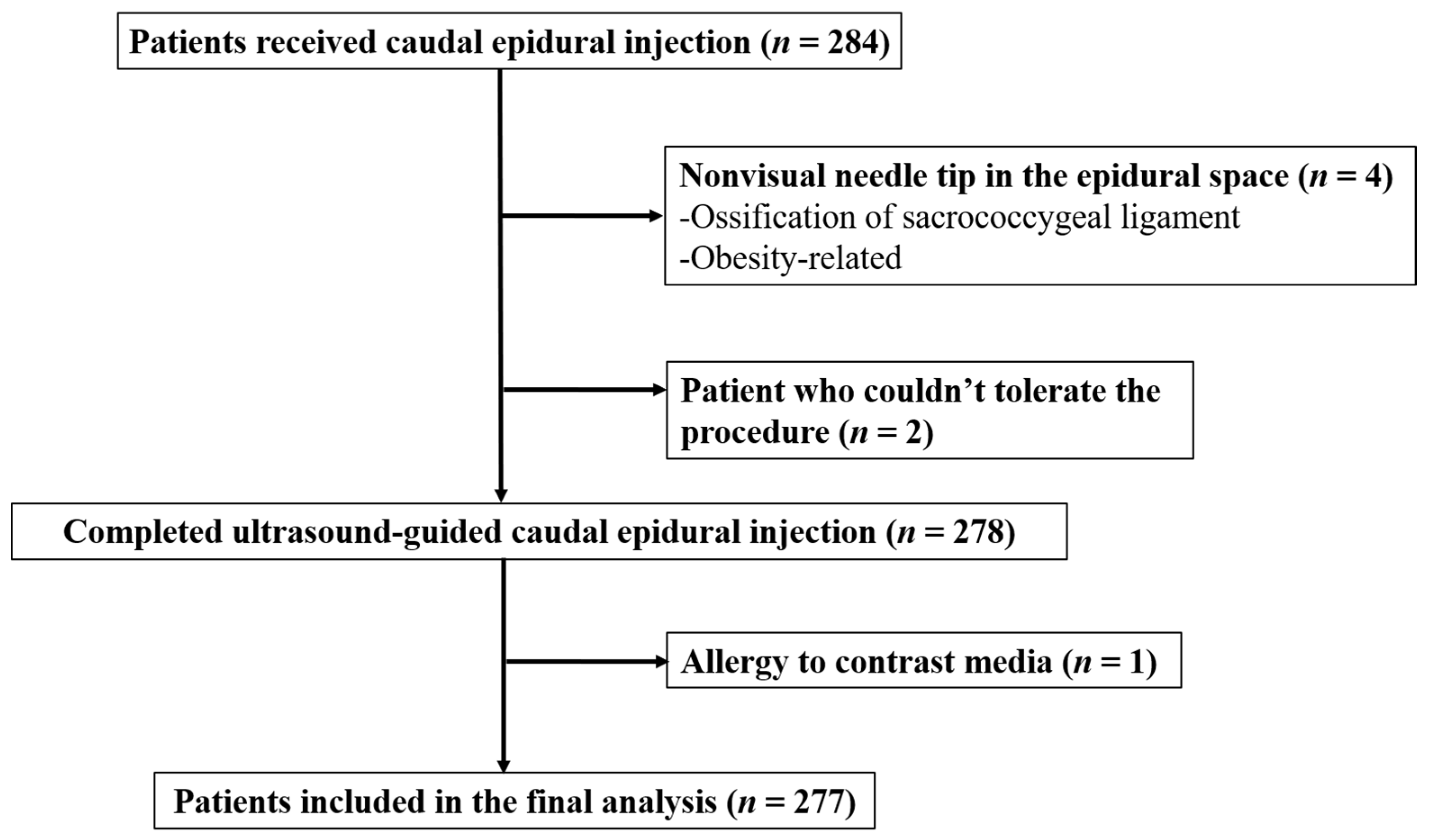
2.1. Patients
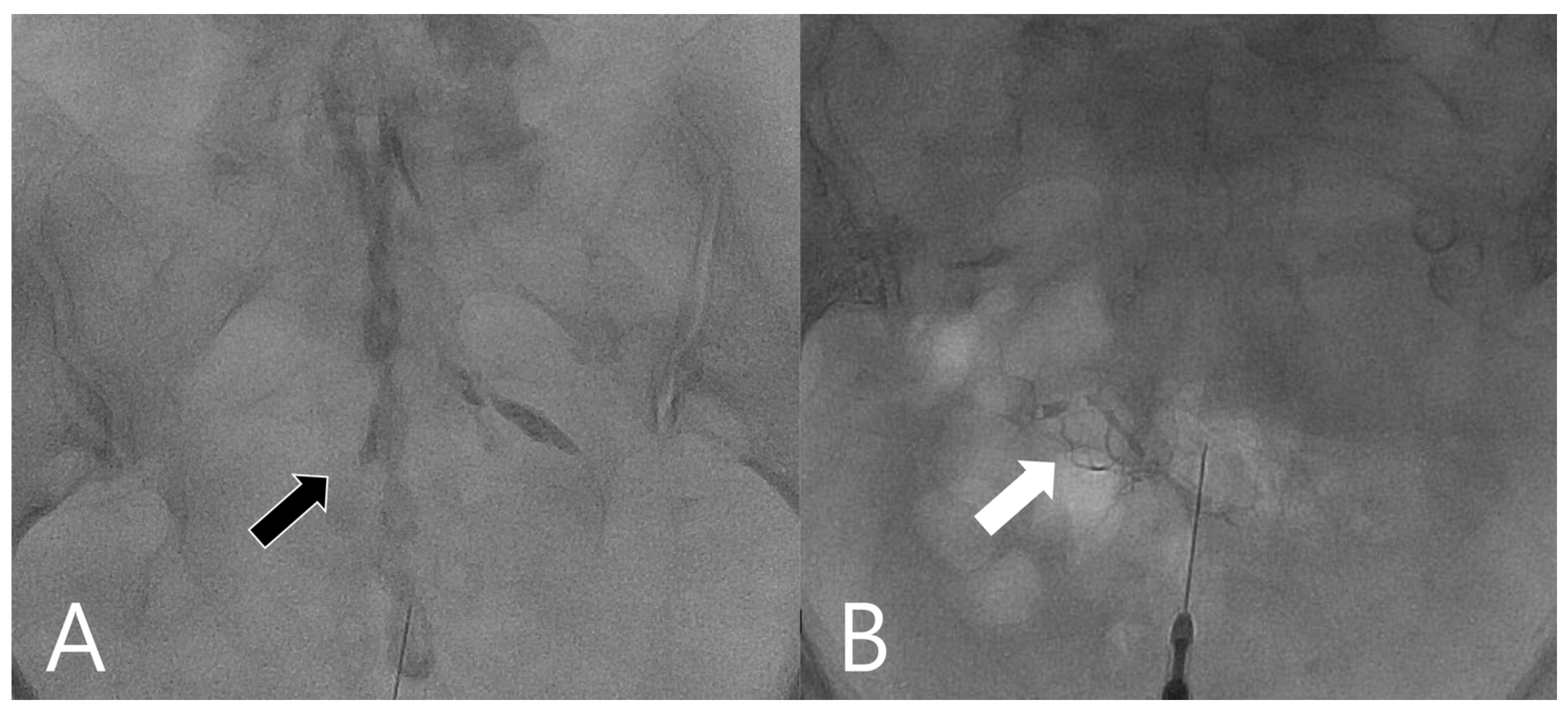
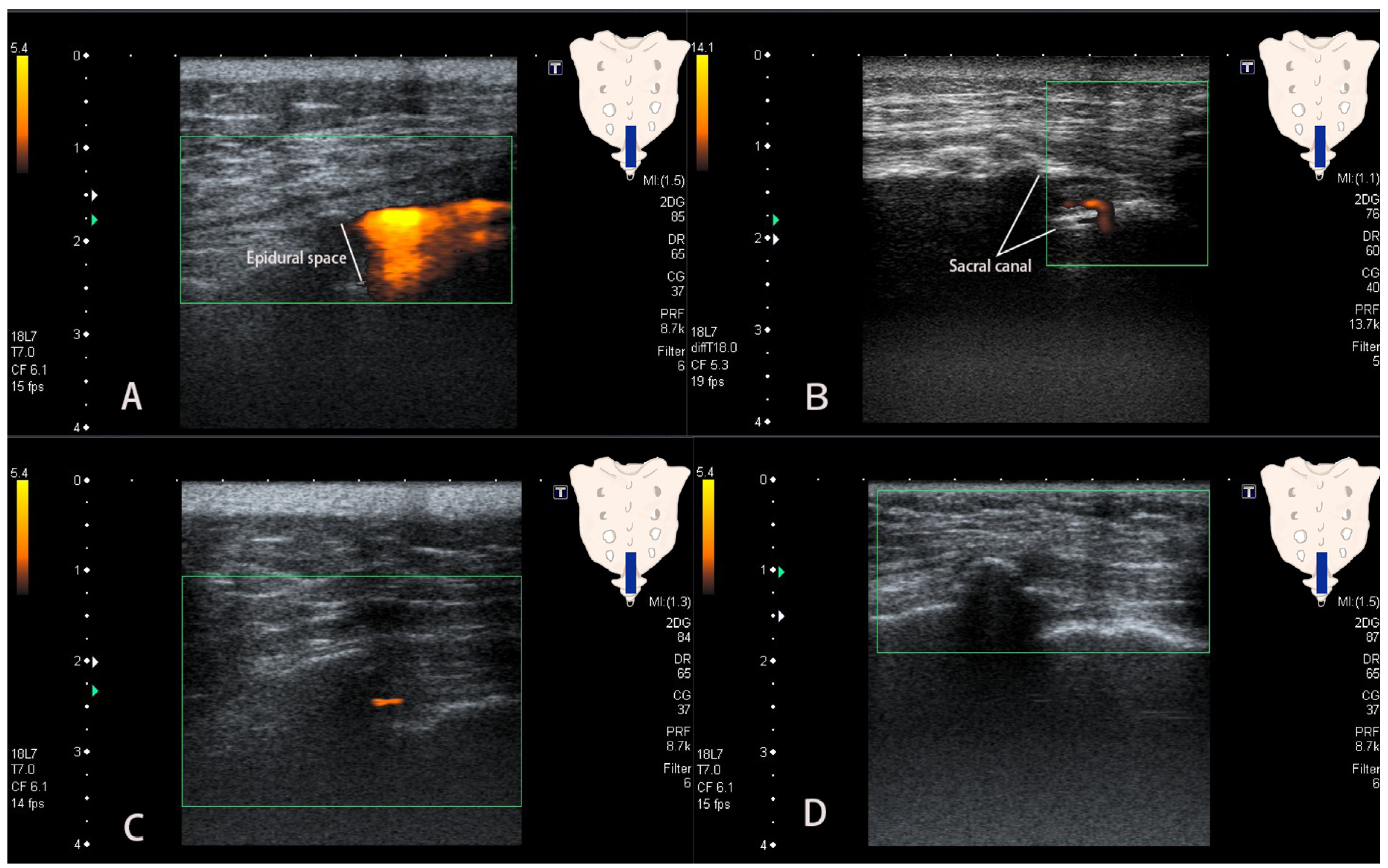
2.2. Procedures
2.3. Data Collection and Analysis
2.4. Statistics Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buttermann, G.R. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004, 4, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L. Role of neuraxial steroids in interventional pain management. Pain Physician 2002, 5, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, H.; Lu, L.; Li, X.; Jia, J.; Shi, Z.; Yao, X.; Wu, Q.; Feng, S. The Effectiveness of Transforaminal Versus Caudal Routes for Epidural Steroid Injections in Managing Lumbosacral Radicular Pain: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e3373. [Google Scholar] [CrossRef] [PubMed]
- Blanchais, A.; Le Goff, B.; Guillot, P.; Berthelot, J.M.; Glemarec, J.; Maugars, Y. Feasibility and safety of ultrasound-guided caudal epidural glucocorticoid injections. Jt. Bone Spine 2010, 77, 440–444. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, K.S.; Kim, Y.H.; Jung, H.D.; Lim, S.J.; Moon, D.E. Accuracy of live fluoroscopy to detect intravascular injection during lumbar transforaminal epidural injections. Korean J. Pain 2010, 23, 18–23. [Google Scholar] [CrossRef]
- Wang, G.; Liang, J.; Jia, Z.; Wan, L.; Yang, M. Spinal cord infarction caused by sacral canal epidural steroid injection: A case report. Medicine 2018, 97, e0111. [Google Scholar] [CrossRef] [PubMed]
- Boswell, M.V.; Trescot, A.M.; Datta, S.; Schultz, D.M.; Hansen, H.C.; Abdi, S.; Sehgal, N.; Shah, R.V.; Singh, V.; Benyamin, R.M.; et al. Interventional techniques: Evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician 2007, 10, 7–111. [Google Scholar]
- Park, K.D.; Kim, T.K.; Lee, W.Y.; Ahn, J.; Koh, S.H.; Park, Y. Ultrasound-Guided Versus Fluoroscopy-Guided Caudal Epidural Steroid Injection for the Treatment of Unilateral Lower Lumbar Radicular Pain: Case-Controlled, Retrospective, Comparative Study. Medicine 2015, 94, e2261. [Google Scholar] [CrossRef]
- Yoon, J.S.; Sim, K.H.; Kim, S.J.; Kim, W.S.; Koh, S.B.; Kim, B.J. The feasibility of color Doppler ultrasonography for caudal epidural steroid injection. Pain 2005, 118, 210–214. [Google Scholar] [CrossRef]
- Furman, M.B.; O’Brien, E.M.; Zgleszewski, T.M. Incidence of intravascular penetration in transforaminal lumbosacral epidural steroid injections. Spine 2000, 25, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.J.; Willick, S.E.; Chira-Adisai, W.; Zuhosky, J.; Tyburski, M.; Dreyfuss, P.; Prather, H.; Press, J.M. Incidence of intravascular uptake in lumbar spinal injection procedures. Spine 2000, 25, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, D.L.; Moore, T.E.; Kathol, M.H.; el-Khoury, G.Y.; Lemke, J.H.; Walker, C.W. Correct placement of epidural steroid injections: Fluoroscopic guidance and contrast administration. AJNR Am. J. Neuroradiol. 1991, 12, 1003–1007. [Google Scholar] [PubMed]
- Tsui, B.; Leipoldt, C.; Desai, S. Color flow Doppler ultrasonography can distinguish caudal epidural injection from intrathecal injection. Anesth. Analg. 2013, 116, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Dsc, N.; Md, P.; Baker, R.; Yin, W.; Landers, M.; Md, M.; Aprill, C. Complications of Spinal Diagnostic and Treatment Procedures. Pain Med. 2008, 9, S11–S34. [Google Scholar] [CrossRef]
- Reitman, C.A.; Watters, W., III. Subdural hematoma after cervical epidural steroid injection. Spine 2002, 27, E174–E176. [Google Scholar] [CrossRef] [PubMed]
- Martinoli, C.; Derchi, L.E.; Rizzatto, G.; Solbiati, L. Power Doppler sonography: General principles, clinical applications, and future prospects. Eur. Radiol. 1998, 8, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, A.; Ando, G.; Salvo, A.; Consolo, A.; Coppolino, F.; Giannino, D. A comparison of Power Doppler with conventional sonographic imaging for the evaluation of renal artery stenosis. Cardiovasc. Ultrasound 2004, 2, 1–4. [Google Scholar] [CrossRef][Green Version]
- Rubens, D.J.; Bhatt, S.; Nedelka, S.; Cullinan, J. Doppler artifacts and pitfalls. Radiol. Clin. N. Am. 2006, 44, 805–835. [Google Scholar] [CrossRef]
- Somanchi, B.V.; Mohammad, S.; Ross, R. An unusual complication following caudal epidural steroid injection: A case report. Acta Orthop. Belg. 2008, 74, 720–722. [Google Scholar] [PubMed]
- Maniquis-Smigel, L.; Reeves, K.D.; Rosen, H.J.; Lyftogt, J.; Graham-Coleman, C.; Cheng, A.-L.; Rabago, D. Analgesic Effect and Potential Cumulative Benefit from Caudal Epidural D5W in Consecutive Participants with Chronic Low-Back and Buttock/Leg Pain. J. Altern. Complement. Med. 2018, 24, 1189–1196. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.H.; Park, K.D.; Ahn, J.K.; Park, J.; Jee, H. Ultrasound-guided vs. fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: A prospective, randomized, single-blind clinical study. Am. J. Phys. Med. Rehabil. 2013, 92, 575–586. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef] [PubMed]
- McGee, S. Simplifying likelihood ratios. J. Gen. Intern. Med. 2002, 17, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Ogoke, B.A. Caudal epidural steroid injections. Pain Physician 2000, 3, 305–312. [Google Scholar] [CrossRef]
- Goeller, J.K.; Joselyn, A.; Martin, D.P.; Bhalla, T.; Dairo, O.; Herz, D.B.; Alpert, S.A.; Tobias, J.D. Epidural pressure changes following caudal blockade: A prospective, observational study. J. Anesth. 2016, 30, 578–582. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.H.; Sim, W.S.; Kim, D.K.; Lee, S.H.; Yun, H.M.; Park, H.J. The influence of epidural catheter on the incidence of intravascular injection during caudal block. Skelet. Radiol. 2017, 46, 1707–1713. [Google Scholar] [CrossRef]
- Fukazawa, K.; Matsuki, Y.; Ueno, H.; Hosokawa, T.; Hirose, M. Risk factors related to accidental intravascular injection during caudal anesthesia. J. Anesth. 2014, 28, 940–943. [Google Scholar] [CrossRef]
- Nikooseresht, M.; Hashemi, M.; Mohajerani, S.A.; Shahandeh, F.; Agah, M. Ultrasound as a screening tool for performing caudal epidural injections. Iran J. Radiol. 2014, 11, e13262. [Google Scholar] [CrossRef]
- Chen, C.P.; Tang, S.F.; Hsu, T.C.; Tsai, W.C.; Liu, H.P.; Chen, M.J.; Date, E.; Lew, H.L. Ultrasound guidance in caudal epidural needle placement. Anesthesiology 2004, 101, 181–184. [Google Scholar] [CrossRef]
- Lee, B.J.; Han, J.; Park, D. A Video of Ultrasound-Guided S1 Transforaminal Epidural Injection Using Color Doppler: Technical Reports. Pain Pract. 2020, 20, 396–398. [Google Scholar] [CrossRef]
- Soni, P.; Punj, J. Ultrasound-Guided Lumbar Transforaminal Epidural Injection: A Narrative Review. Asian Spine J. 2020, 15, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Lee, W.Y.; Kim, J.W.; Cho, K.R.; Nam, S.H.; Park, Y. Ultrasound-Guided Selective Nerve Root Block versus Fluoroscopy-Guided Interlaminar Epidural Block versus Fluoroscopy-Guided Transforaminal Epidural Block for the Treatment of Radicular Pain in the Lower Cervical Spine: A Retrospective Comparative Study. Pain Res. Manag. 2020, 2020, 9103421. [Google Scholar] [CrossRef] [PubMed]
| Variable | Intravascular Injection | Intraepidural Injection | p-Value |
|---|---|---|---|
| Sex | 0.327 | ||
| male * | 2 | 104 | |
| female * | 8 | 163 | |
| Age (y) | 0.155 | ||
| range | 56~86 | 23~97 | |
| mean ± SD | 74.4 ± 9.3 | 68.4 ± 13.3 | |
| BMI (kg/cm2) | 0.347 | ||
| range | 16.9~28.0 | 16.9~44.7 | |
| mean ± SD | 23.5 ± 4.0 | 24.7 ± 3.9 | |
| History of laminectomy | 0.138 | ||
| yes * | 5 | 69 | |
| no * | 5 | 198 |
| Variable | Intravascular Injection | Intraepidural Injection | p-Value |
|---|---|---|---|
| Flow pattern | <0.001 | ||
| Patch sign | 2 | 237 | |
| Earthworm sign | 7 | 0 | |
| Tubular sign | 0 | 25 | |
| Absent flow | 1 | 5 |
| Sensitivity% [95% CI] | Specificity% [95% CI] | PLR [95% CI] | NLR [95% CI] | |
|---|---|---|---|---|
| Flow pattern | ||||
| Patch sign | 88.8 [84.35–92.29] | 80.0 [44.39–97.48] | 4.44 [1.28–15.34] | 0.14 [1.28–15.34] |
| Earthworm sign | 0 [0–1.37] | 30 [6.67–65.25] | 0 | 3.3 [1.29–8.59] |
| Tubular sign | 9.4 [6.15–13.51] | 100 [69.15–100] | ∞ | 0.91 [0.87–0.94] |
| Absent flow | 1.87 [0.61–4.32] | 90.0 [55.50–99.75] | 0.19 [0.02–1.46] | 1.09 [0.89–1.34] |
| Sensitivity% [95% CI] | Specificity% [95% CI] | PLR [95% CI] | NLR [95% CI] | |
|---|---|---|---|---|
| Flow pattern | ||||
| Patch sign | 20 [2.52–55.61] | 11.2 [7.71–15.65] | 0.22 [0.07–0.78] | 7.12 [4.50–11.26] |
| Earthworm sign | 70 [34.75–93.33] | 100 [98.63–100] | ∞ | 0.3 [0.12–0.77] |
| Tubular sign | 0 [0–30.85] | 90.6 [86.49–93.85] | 0 | 1.1 [1.06–1.15] |
| Absent flow | 10 [0.25–44.5] | 98.1 [95.68–99.39] | 5.34 [0.69–41.57] | 0.92 [0.75–1.13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-H.; Huang, G.-S.; Tang, C.-T.; Yang, F.-C.; Hsu, Y.-C. The Role of Power Doppler Ultrasonography in Caudal Epidural Injection. Medicina 2022, 58, 575. https://doi.org/10.3390/medicina58050575
Tsai Y-H, Huang G-S, Tang C-T, Yang F-C, Hsu Y-C. The Role of Power Doppler Ultrasonography in Caudal Epidural Injection. Medicina. 2022; 58(5):575. https://doi.org/10.3390/medicina58050575
Chicago/Turabian StyleTsai, Yueh-Hsun, Guo-Shu Huang, Chi-Tun Tang, Fu-Chi Yang, and Yi-Chih Hsu. 2022. "The Role of Power Doppler Ultrasonography in Caudal Epidural Injection" Medicina 58, no. 5: 575. https://doi.org/10.3390/medicina58050575
APA StyleTsai, Y.-H., Huang, G.-S., Tang, C.-T., Yang, F.-C., & Hsu, Y.-C. (2022). The Role of Power Doppler Ultrasonography in Caudal Epidural Injection. Medicina, 58(5), 575. https://doi.org/10.3390/medicina58050575






