Therapeutic Strategies for Ovarian Cancer in Point of HGF/c-MET Targeting
Abstract
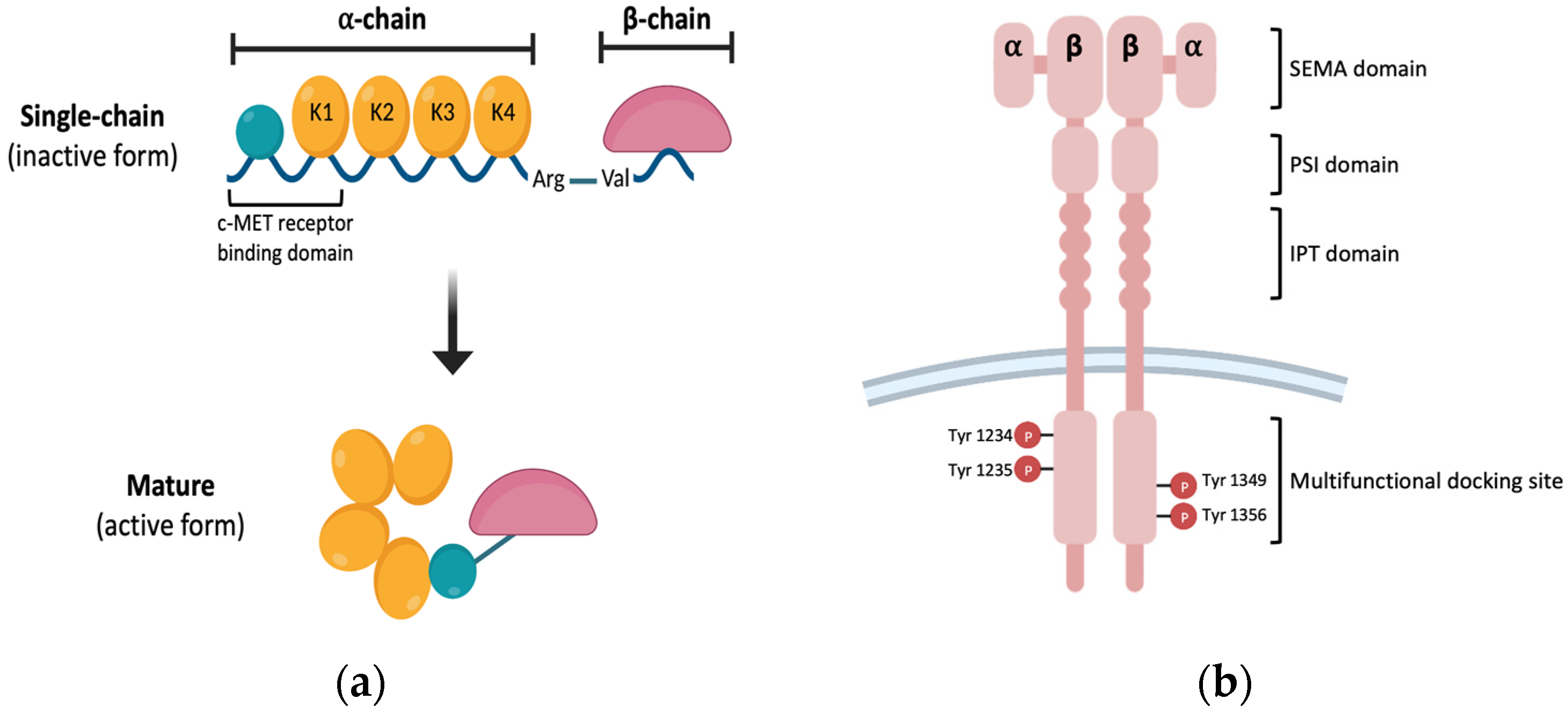
:1. Introduction
2. HGF Biology in Cancer
2.1. The Roles of the HGF/c-MET Axis Signaling Pathway in Cancer
2.2. Relationship between HGF/c-MET and Cancer Metastasis
3. HGF/c-MET Axis Signaling Pathway in Ovarian Cancer
3.1. Ovarian Cancer Incidence and Standard Treatment Strategies
3.2. Limitation of Chemotherapy and Current Status of Other Therapeutic Strategies in Ovarian Cancer
3.3. Function of HGF/c-MET Axis in Ovarian Cancer
4. Targeting HGF/c-MET Axis in Cancer
4.1. Efficacy of Therapeutics with HGF and c-MET Inhibitors in Cancer
4.2. Preclinical and Clinical Trials of HGF/c-MET Inhibitors in Ovarian Cancer
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gherardi, E.; Gray, J.; Stoker, M.; Perryman, M.; Furlong, R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc. Natl. Acad. Sci. USA 1989, 86, 5844–5848. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Nawa, K.; Ichihara, A.; Kaise, N.; Nishino, T. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987, 224, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Stoker, M.; Gherardi, E.; Perryman, M.; Gray, J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 1987, 327, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Tamagnone, L.; Vigna, E.; Sachs, M.; Hartmann, G.; Birchmeier, W.; Daikuhara, Y.; Tsubouchi, H.; Blasi, F.; Comoglio, P.M. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992, 11, 4825–4833. [Google Scholar] [CrossRef]
- Naldini, L.; Vigna, E.; Bardelli, A.; Follenzi, A.; Galimi, F.; Comoglio, P.M. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J. Biol. Chem. 1995, 270, 603–611. [Google Scholar] [CrossRef] [Green Version]
- Lokker, N.A.; Mark, M.R.; Luis, E.A.; Bennett, G.L.; Robbins, K.A.; Baker, J.B.; Godowski, P.J. Structure-function analysis of hepatocyte growth factor: Identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992, 11, 2503–2510. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kataoka, H.; Date, K.; Nakamura, T. Cooperative interaction between alpha- and beta-chains of hepatocyte growth factor on c-Met receptor confers ligand-induced receptor tyrosine phosphorylation and multiple biological responses. J. Biol. Chem. 1998, 273, 22913–22920. [Google Scholar] [CrossRef] [Green Version]
- Basilico, C.; Arnesano, A.; Galluzzo, M.; Comoglio, P.M.; Michieli, P. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J. Biol. Chem. 2008, 283, 21267–21277. [Google Scholar] [CrossRef] [Green Version]
- Bottaro, D.P.; Rubin, J.S.; Faletto, D.L.; Chan, A.M.; Kmiecik, T.E.; Vande Woude, G.F.; Aaronson, S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991, 251, 802–804. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kataoka, H. Mechanisms of hepatocyte growth factor activation in cancer tissues. Cancers 2014, 6, 1890–1904. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, G.A.; Naujokas, M.A.; Park, M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol. Cell. Biol. 1991, 11, 2962–2970. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, E.; Sandin, S.; Petoukhov, M.V.; Finch, J.; Youles, M.E.; Ofverstedt, L.G.; Miguel, R.N.; Blundell, T.L.; Vande Woude, G.F.; Skoglund, U.; et al. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc. Natl. Acad. Sci. USA 2006, 103, 4046–4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longati, P.; Bardelli, A.; Ponzetto, C.; Naldini, L.; Comoglio, P.M. Tyrosines1234-1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor). Oncogene 1994, 9, 49–57. [Google Scholar]
- Sachs, M.; Brohmann, H.; Zechner, D.; Muller, T.; Hulsken, J.; Walther, I.; Schaeper, U.; Birchmeier, C.; Birchmeier, W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 2000, 150, 1375–1384. [Google Scholar] [CrossRef] [Green Version]
- Montesano, R.; Matsumoto, K.; Nakamura, T.; Orci, L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991, 67, 901–908. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Vande Woude, G.F. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J. Cell. Biochem. 2003, 88, 408–417. [Google Scholar] [CrossRef]
- Petrini, I. Biology of MET: A double life between normal tissue repair and tumor progression. Ann. Transl. Med. 2015, 3, 82. [Google Scholar] [CrossRef]
- Gherardi, E.; Birchmeier, W.; Birchmeier, C.; Vande Woude, G. Targeting MET in cancer: Rationale and progress. Nat. Rev. Cancer 2012, 12, 89–103. [Google Scholar] [CrossRef]
- Canadas, I.; Taus, A.; Gonzalez, I.; Villanueva, X.; Gimeno, J.; Pijuan, L.; Domine, M.; Sanchez-Font, A.; Vollmer, I.; Menendez, S.; et al. High circulating hepatocyte growth factor levels associate with epithelial to mesenchymal transition and poor outcome in small cell lung cancer patients. Oncotarget 2014, 5, 5246–5256. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Horikoshi, M.; Rikimaru, K.; Enomoto, S. A study of an in vitro model for invasion of oral squamous cell carcinoma. J. Oral Pathol. Med. 1989, 18, 498–501. [Google Scholar] [CrossRef]
- Weidner, K.M.; Behrens, J.; Vandekerckhove, J.; Birchmeier, W. Scatter factor: Molecular characteristics and effect on the invasiveness of epithelial cells. J. Cell Biol. 1990, 111, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Desiderio, M.A. Epigenetic regulation of HGF/Met receptor axis is critical for the outgrowth of bone metastasis from breast carcinoma. Cell Death Dis. 2017, 8, e2578. [Google Scholar] [CrossRef] [Green Version]
- Baykal, C.; Ayhan, A.; Al, A.; Yuce, K.; Ayhan, A. Overexpression of the c-Met/HGF receptor and its prognostic significance in uterine cervix carcinomas. Gynecol. Oncol. 2003, 88, 123–129. [Google Scholar] [CrossRef]
- Kataoka, H.; Hamasuna, R.; Itoh, H.; Kitamura, N.; Koono, M. Activation of hepatocyte growth factor/scatter factor in colorectal carcinoma. Cancer Res. 2000, 60, 6148–6159. [Google Scholar]
- Noguchi, E.; Saito, N.; Kobayashi, M.; Kameoka, S. Clinical significance of hepatocyte growth factor/c-Met expression in the assessment of gastric cancer progression. Mol. Med. Rep. 2015, 11, 3423–3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirri, P.; Chiarugi, P. Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012, 31, 195–208. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor: Renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001, 59, 2023–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, C.; Paglino, C.; Imarisio, I.; Ganini, C.; Sacchi, L.; Quaglini, S.; Giunta, V.; De Amici, M. Changes in circulating pro-angiogenic cytokines, other than VEGF, before progression to sunitinib therapy in advanced renal cell carcinoma patients. Oncology 2013, 84, 115–122. [Google Scholar] [CrossRef]
- Fukuura, T.; Miki, C.; Inoue, T.; Matsumoto, K.; Suzuki, H. Serum hepatocyte growth factor as an index of disease status of patients with colorectal carcinoma. Br. J. Cancer 1998, 78, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Liu, S. HGF-MET as a breast cancer biomarker. Aging 2015, 7, 150–151. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Bradley, R.; Kang, L.; Koeman, J.; Ascierto, M.L.; Worschech, A.; De Giorgi, V.; Wang, E.; Kefene, L.; Su, Y.; et al. Hepatocyte growth factor (HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proc. Natl. Acad. Sci. USA 2012, 109, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, C.; Borset, M.; Turesson, I.; Abildgaard, N.; Sundan, A.; Waage, A. Elevated serum concentrations of hepatocyte growth factor in patients with multiple myeloma. The Nordic Myeloma Study Group. Blood 1998, 91, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int. J. Cancer 2006, 119, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Okazaki, H.; Nakamura, T. Novel function of prostaglandins as inducers of gene expression of HGF and putative mediators of tissue regeneration. J. Biochem. 1995, 117, 458–464. [Google Scholar] [CrossRef]
- Lam, B.Q.; Dai, L.; Qin, Z. The role of HGF/c-MET signaling pathway in lymphoma. J. Hematol. Oncol. 2016, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.H.; Cheng, C.Y.; Su, T.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.; Kwan, H.Y.; Yu, H.; Yu, Z.L. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol. Cancer 2015, 14, 103. [Google Scholar] [CrossRef] [Green Version]
- Koochekpour, S.; Jeffers, M.; Rulong, S.; Taylor, G.; Klineberg, E.; Hudson, E.A.; Resau, J.H.; Vande Woude, G.F. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997, 57, 5391–5398. [Google Scholar]
- Lengyel, E.; Prechtel, D.; Resau, J.H.; Gauger, K.; Welk, A.; Lindemann, K.; Salanti, G.; Richter, T.; Knudsen, B.; Vande Woude, G.F.; et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int. J. Cancer 2005, 113, 678–682. [Google Scholar] [CrossRef]
- Di Renzo, M.F.; Poulsom, R.; Olivero, M.; Comoglio, P.M.; Lemoine, N.R. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995, 55, 1129–1138. [Google Scholar]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Eterno, V.; Zambelli, A.; Pavesi, L.; Villani, L.; Zanini, V.; Petrolo, G.; Manera, S.; Tuscano, A.; Amato, A. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget 2014, 5, 613–633. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Hill, K.S.; Gaziova, I.; Sastry, S.K.; Qui, S.; Szaniszlo, P.; Fennewald, S.; Resto, V.A.; Elferink, L.A. Silencing Met receptor tyrosine kinase signaling decreased oral tumor growth and increased survival of nude mice. Oral Oncol. 2014, 50, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Cho, S.Y.; Ha, J.D.; Jung, H.; Kim, H.R.; Lee, C.O.; Jang, I.Y.; Chae, C.H.; Lee, H.K.; Choi, S.U. Novel c-Met inhibitor suppresses the growth of c-Met-addicted gastric cancer cells. BMC Cancer 2016, 16, 35. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Luo, Y.; Wang, Y.; Chen, Y.; Li, M.; Jiang, Y. Hepatocyte growth factor increases the invasive potential of PC-3 human prostate cancer cells via an ERK/MAPK and Zeb-1 signaling pathway. Oncol. Lett. 2016, 11, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Causado, A.; Caballero-Diaz, D.; Bertran, E.; Roncero, C.; Addante, A.; Garcia-Alvaro, M.; Fernandez, M.; Herrera, B.; Porras, A.; Fabregat, I.; et al. HGF/c-Met signaling promotes liver progenitor cell migration and invasion by an epithelial-mesenchymal transition-independent, phosphatidyl inositol-3 kinase-dependent pathway in an in vitro model. Biochim. Biophys. Acta 2015, 1853, 2453–2463. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.H.; Numata, Y.; Numata, M. Endosomal Na+/H+ exchanger NHE5 influences MET recycling and cell migration. Mol. Biol. Cell 2016, 27, 702–715. [Google Scholar] [CrossRef]
- Ip, C.K.; Cheung, A.N.; Ngan, H.Y.; Wong, A.S. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 2011, 30, 2420–2432. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.W.; Brost, B.; Zhang, D.; Ren, C.E. Aberrant Gene Regulation in Obstetric, Gynecologic, and Reproductive Diseases. Biomed. Res. Int. 2015, 2015, 187691. [Google Scholar] [CrossRef]
- Lupo, B.; Vialard, J.; Sassi, F.; Angibaud, P.; Puliafito, A.; Pupo, E.; Lanzetti, L.; Comoglio, P.M.; Bertotti, A.; Trusolino, L. Tankyrase inhibition impairs directional migration and invasion of lung cancer cells by affecting microtubule dynamics and polarity signals. BMC Biol. 2016, 14, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Flier, A.; Sonnenberg, A. Function and interactions of integrins. Cell Tissue Res. 2001, 305, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Hepatocyte growth factor disrupts tight junctions in human breast cancer cells. Cell Biol. Int. 2004, 28, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Joosten, S.P.J.; Spaargaren, M.; Clevers, H.; Pals, S.T. Hepatocyte growth factor/MET and CD44 in colorectal cancer: Partners in tumorigenesis and therapy resistance. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188437. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zeppernick, F.; Meinhold-Heerlein, I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch. Gynecol. Obstet. 2014, 290, 839–842. [Google Scholar] [CrossRef]
- Schiavone, M.B.; Herzog, T.J.; Lewin, S.N.; Deutsch, I.; Sun, X.; Burke, W.M.; Wright, J.D. Natural history and outcome of mucinous carcinoma of the ovary. Am. J. Obstet. Gynecol. 2011, 205, 480 e481–488. [Google Scholar] [CrossRef]
- van Haaften-Day, C.; Shen, Y.; Xu, F.; Yu, Y.; Berchuck, A.; Havrilesky, L.J.; de Bruijn, H.W.; van der Zee, A.G.; Bast, R.C., Jr.; Hacker, N.F. OVX1, macrophage-colony stimulating factor, and CA-125-II as tumor markers for epithelial ovarian carcinoma: A critical appraisal. Cancer 2001, 92, 2837–2844. [Google Scholar] [CrossRef]
- Prahm, K.P.; Karlsen, M.A.; Hogdall, E.; Scheller, N.M.; Lundvall, L.; Nedergaard, L.; Christensen, I.J.; Hogdall, C. The prognostic value of dividing epithelial ovarian cancer into type I and type II tumors based on pathologic characteristics. Gynecol. Oncol. 2015, 136, 205–211. [Google Scholar] [CrossRef]
- Trimbos, J.B.; Vergote, I.; Bolis, G.; Vermorken, J.B.; Mangioni, C.; Madronal, C.; Franchi, M.; Tateo, S.; Zanetta, G.; Scarfone, G.; et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J. Natl. Cancer Inst. 2003, 95, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Szubert, M.; Suzin, J.; Wierzbowski, T.; Kowalczyk-Amico, K. CA-125 concentration in serum and peritoneal fluid in patients with endometriosis—Preliminary results. Arch. Med. Sci. 2012, 8, 504–508. [Google Scholar] [CrossRef]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef]
- Raja, F.A.; Chopra, N.; Ledermann, J.A. Optimal first-line treatment in ovarian cancer. Ann. Oncol. 2012, 23 (Suppl. 10), x118–x127. [Google Scholar] [CrossRef]
- Ho, G.Y.; Woodward, N.; Coward, J.I. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, K.N. Microtubule-targeted anticancer agents and apoptosis. Oncogene 2003, 22, 9075–9086. [Google Scholar] [CrossRef] [Green Version]
- Blagosklonny, M.V.; Schulte, T.; Nguyen, P.; Trepel, J.; Neckers, L.M. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway. Cancer Res. 1996, 56, 1851–1854. [Google Scholar]
- Ferlini, C.; Cicchillitti, L.; Raspaglio, G.; Bartollino, S.; Cimitan, S.; Bertucci, C.; Mozzetti, S.; Gallo, D.; Persico, M.; Fattorusso, C.; et al. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res. 2009, 69, 6906–6914. [Google Scholar] [CrossRef] [Green Version]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev. Anticancer Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef]
- Cristea, M.; Han, E.; Salmon, L.; Morgan, R.J. Practical considerations in ovarian cancer chemotherapy. Ther. Adv. Med. Oncol. 2010, 2, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Luvero, D.; Milani, A.; Ledermann, J.A. Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Martin, A.; Gladieff, L.; Tholander, B.; Stroyakovsky, D.; Gore, M.; Scambia, G.; Kovalenko, N.; Oaknin, A.; Ronco, J.P.; Freudensprung, U.; et al. Efficacy and safety results from OCTAVIA, a single-arm phase II study evaluating front-line bevacizumab, carboplatin and weekly paclitaxel for ovarian cancer. Eur. J. Cancer 2013, 49, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Dai, X. The relationship between DNA single-stranded damage response and double-stranded damage response. Cell Cycle 2018, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Penson, R.T.; Domchek, S.M.; Kaufman, B.; Shapira-Frommer, R.; Audeh, M.W.; Kaye, S.; Molife, L.R.; Gelmon, K.A.; Robertson, J.D.; et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: A multistudy analysis of response rates and safety. Ann. Oncol. 2016, 27, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Tinker, A.V.; Oaknin, A.; Shapira-Frommer, R.; McNeish, I.A.; Swisher, E.M.; Ray-Coquard, I.; Bell-McGuinn, K.; Coleman, R.L.; O’Malley, D.M.; et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 2017, 147, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Coleman, R.L.; Sill, M.W.; Bell-McGuinn, K.; Aghajanian, C.; Gray, H.J.; Tewari, K.S.; Rubin, S.C.; Rutherford, T.J.; Chan, J.K.; Chen, A.; et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation—An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015, 137, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.; Godwin, A.K. Regulation of HGF and c-MET Interaction in Normal Ovary and Ovarian Cancer. Reprod. Sci. 2017, 24, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, M.F.; Olivero, M.; Katsaros, D.; Crepaldi, T.; Gaglia, P.; Zola, P.; Sismondi, P.; Comoglio, P.M. Overexpression of the Met/HGF receptor in ovarian cancer. Int. J. Cancer 1994, 58, 658–662. [Google Scholar] [CrossRef]
- Sawada, K.; Radjabi, A.R.; Shinomiya, N.; Kistner, E.; Kenny, H.; Becker, A.R.; Turkyilmaz, M.A.; Salgia, R.; Yamada, S.D.; Vande Woude, G.F.; et al. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007, 67, 1670–1679. [Google Scholar] [CrossRef] [Green Version]
- Deying, W.; Feng, G.; Shumei, L.; Hui, Z.; Ming, L.; Hongqing, W. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci. Rep. 2017, 37, BSR20160470. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, H.; Zhao, S.; Shi, Y.; Yao, J.; Zhang, Y.; Guo, H.; Liu, X. Overexpression of MACC1 and the association with hepatocyte growth factor/c-Met in epithelial ovarian cancer. Oncol. Lett. 2015, 9, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, M.; Zhang, S.; Li, X.; Gao, J.; Zhang, D.; Hao, Y.; Gao, S.; Liu, J.; Lin, B. Expression and significance of CD44, CD47 and c-met in ovarian clear cell carcinoma. Int. J. Mol. Sci. 2015, 16, 3391–3404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matte, I.; Lane, D.; Laplante, C.; Garde-Granger, P.; Rancourt, C.; Piche, A. Ovarian cancer ascites enhance the migration of patient-derived peritoneal mesothelial cells via cMet pathway through HGF-dependent and -independent mechanisms. Int. J. Cancer 2015, 137, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.M.; Link, T.; Wimberger, P.; Kuhlmann, J.D. Prognostic relevance of longitudinal HGF levels in serum of patients with ovarian cancer. Mol. Oncol. 2021, 15, 3626–3638. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, Z.; Lin, L.; Yang, M.; Li, C.; Li, Z.; Yu, X.; Lizaso, A.; Han-Zhang, H.; Li, B.; et al. Characterization of MET exon 14 alteration and association with clinical outcomes of crizotinib in Chinese lung cancers. Lung Cancer 2020, 148, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Rudin, C.M. Crizotinib in the treatment of non-small-cell lung cancer. Expert Opin. Pharmacother. 2012, 13, 1195–1201. [Google Scholar] [CrossRef]
- Roberts, P.J. Clinical use of crizotinib for the treatment of non-small cell lung cancer. Biologics 2013, 7, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.; Prabhash, K.; Noronha, V.; Joshi, A.; Desai, S. Crizotinib: A comprehensive review. South Asian J. Cancer 2013, 2, 91–97. [Google Scholar] [CrossRef]
- Rothenstein, J.M.; Letarte, N. Managing treatment-related adverse events associated with Alk inhibitors. Curr. Oncol. 2014, 21, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Adjei, A.A.; Schwartz, B.; Garmey, E. Early clinical development of ARQ 197, a selective, non-ATP-competitive inhibitor targeting MET tyrosine kinase for the treatment of advanced cancers. Oncologist 2011, 16, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Su, X.; Li, Z.; Deng, L.; Liu, X.; Feng, X.; Peng, J. HGF/c-MET pathway in cancer: From molecular characterization to clinical evidence. Oncogene 2021, 40, 4625–4651. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Bessudo, A.; Hart, L.L.; Severtsev, A.; Gladkov, O.; Muller, L.; Kopp, M.V.; Vladimirov, V.; Langdon, R.; Kotiv, B.; et al. A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy. Int. J. Cancer 2016, 139, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iveson, T.; Donehower, R.C.; Davidenko, I.; Tjulandin, S.; Deptala, A.; Harrison, M.; Nirni, S.; Lakshmaiah, K.; Thomas, A.; Jiang, Y.; et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: An open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014, 15, 1007–1018. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef]
- D’Arcangelo, M.; Cappuzzo, F. Focus on the potential role of ficlatuzumab in the treatment of non-small cell lung cancer. Biologics 2013, 7, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Mok, T.S.; Geater, S.L.; Su, W.C.; Tan, E.H.; Yang, J.C.; Chang, G.C.; Han, M.; Komarnitsky, P.; Payumo, F.; Garrus, J.E.; et al. A Randomized Phase 2 Study Comparing the Combination of Ficlatuzumab and Gefitinib with Gefitinib Alone in Asian Patients with Advanced Stage Pulmonary Adenocarcinoma. J. Thorac. Oncol. 2016, 11, 1736–1744. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.K.; Kang, J.H.; Kim, B.; Park, B.H.; Shin, K.J.; Song, S.W.; Kim, J.J.; Kim, H.M.; Lee, S.J.; Oh, S.H. Humanized anti-hepatocyte growth factor (HGF) antibody suppresses innate irinotecan (CPT-11) resistance induced by fibroblast-derived HGF. Oncotarget 2015, 6, 24047–24060. [Google Scholar] [CrossRef]
- Zillhardt, M.; Park, S.M.; Romero, I.L.; Sawada, K.; Montag, A.; Krausz, T.; Yamada, S.D.; Peter, M.E.; Lengyel, E. Foretinib (GSK1363089), an orally available multikinase inhibitor of c-Met and VEGFR-2, blocks proliferation, induces anoikis, and impairs ovarian cancer metastasis. Clin. Cancer Res. 2011, 17, 4042–4051. [Google Scholar] [CrossRef] [Green Version]
- Moran-Jones, K. The Therapeutic Potential of Targeting the HGF/cMET Axis in Ovarian Cancer. Mol. Diagn. Ther. 2016, 20, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.P.; Sill, M.; Shahin, M.S.; Powell, M.; DiSilvestro, P.; Landrum, L.M.; Gaillard, S.L.; Goodheart, M.J.; Hoffman, J.; Schilder, R.J. A phase II evaluation of AMG 102 (rilotumumab) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2014, 132, 526–530. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.S.; Sweeney, C.S.; Mendelson, D.S.; Eckhardt, S.G.; Anderson, A.; Beaupre, D.M.; Branstetter, D.; Burgess, T.L.; Coxon, A.; Deng, H.; et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin. Cancer Res. 2010, 16, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Lee, S.; Oh, Y.S.; Chang, H.K.; Kim, Y.S.; Hong, S.H.; Kim, J.Y.; Park, Y.W.; Lee, S.J.; Song, S.W.; et al. Humanized Anti-hepatocyte Growth Factor Monoclonal Antibody (YYB-101) Inhibits Ovarian Cancer Progression. Front. Oncol. 2019, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Heo, K. YYB-101, a Humanized Antihepatocyte Growth Factor Monoclonal Antibody, Inhibits Ovarian Cancer Cell Motility and Proliferation. Anticancer Res. 2021, 41, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Hong, J.Y.; Park, S.H.; Park, J.O.; Park, Y.W.; Park, N.; Lee, H.; Hong, S.H.; Lee, S.J.; Song, S.W.; et al. First-in-human phase I trial of anti-hepatocyte growth factor antibody (YYB101) in refractory solid tumor patients. Ther. Adv. Med. Oncol. 2020, 12, 1758835920926796. [Google Scholar] [CrossRef]
- Ozols, R.F.; Bookman, M.A.; Connolly, D.C.; Daly, M.B.; Godwin, A.K.; Schilder, R.J.; Xu, X.; Hamilton, T.C. Focus on epithelial ovarian cancer. Cancer Cell 2004, 5, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [Green Version]
- Eisenkop, S.M.; Spirtos, N.M. The clinical significance of occult macroscopically positive retroperitoneal nodes in patients with epithelial ovarian cancer. Gynecol. Oncol. 2001, 82, 143–149. [Google Scholar] [CrossRef]

| Inhibitor | Cancer Type | Characteristic | gov Identifier |
|---|---|---|---|
| Crizotinib (PF-02341066) | NSCLC | Efficacy and safety test of PF-02341066 in cancer patients with alterations in ALK, MET, or ROS1. | NCT02034981 |
| NSCLC | To analyze PK and PD in patients with NSCLC, c-MET-dependent. | NCT00585195 | |
| NSCLC | Comparison of safety and anti-cancer efficacy of PF-02341066 versus pemetrexed or docetaxel in patients with NSCLC involving the ALK gene. | NCT00932893 | |
| Rilotumumab (AMG-102) | CRC | To test the safety and efficacy of AMG-102 or ganitumab in combination with panitumumab in patients with metastatic wild-type KRAS CRC. | NCT00788957 |
| NSCLC | To evaluate AMG-102 and erlotinib in previously treated subjects with advanced NSCLC. | NCT01233687 | |
| Ficlatuzumab | PC | To identify the maximally tolerated dose of ficlatuzumab when combined with nab-paclitaxel and gemcitabine in patients with previously untreated pancreatic cancer. | NCT03316599 |
| SCCHN | To find the recommended dose of the combination of ficlatuzumab and cetuximab in patients with recurrent/metastatic SCCHN. | NCT02277197 | |
| YYB-101 | CRC | To evaluate the safety, tolerability, pharmacokinetics, and anti-tumor activity of YYB-101 with irinotecan, patients who are metastatic or recurrent colorectal cancer patients. | NCT04368507 |
| AST | To evaluate the safety, tolerability, pharmacokinetics, and maximum tolerated dose of YYB-101 in advanced solid tumor patients who are refractory to standard therapy. | NCT02499224 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J. Therapeutic Strategies for Ovarian Cancer in Point of HGF/c-MET Targeting. Medicina 2022, 58, 649. https://doi.org/10.3390/medicina58050649
Kim HJ. Therapeutic Strategies for Ovarian Cancer in Point of HGF/c-MET Targeting. Medicina. 2022; 58(5):649. https://doi.org/10.3390/medicina58050649
Chicago/Turabian StyleKim, Hyun Jung. 2022. "Therapeutic Strategies for Ovarian Cancer in Point of HGF/c-MET Targeting" Medicina 58, no. 5: 649. https://doi.org/10.3390/medicina58050649
APA StyleKim, H. J. (2022). Therapeutic Strategies for Ovarian Cancer in Point of HGF/c-MET Targeting. Medicina, 58(5), 649. https://doi.org/10.3390/medicina58050649





