Specific MRP4 Inhibitor Ceefourin-1 Enhances Apoptosis Induced by 6-Mercaptopurine in Jurkat Leukemic Cells, but Not in Normal Lymphoblast Cell Line CRL-1991
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Cell Culture
2.3. Proliferation Assay and Viability
2.4. Apoptosis Assay
2.5. Molecular Docking
2.6. Umbrella Sampling
3. Results
3.1. Effect of Ceefourin-1 and 6-MP on the Cellular Proliferation
3.2. Apoptotic Interaction between Ceefourin-1 and 6-MP
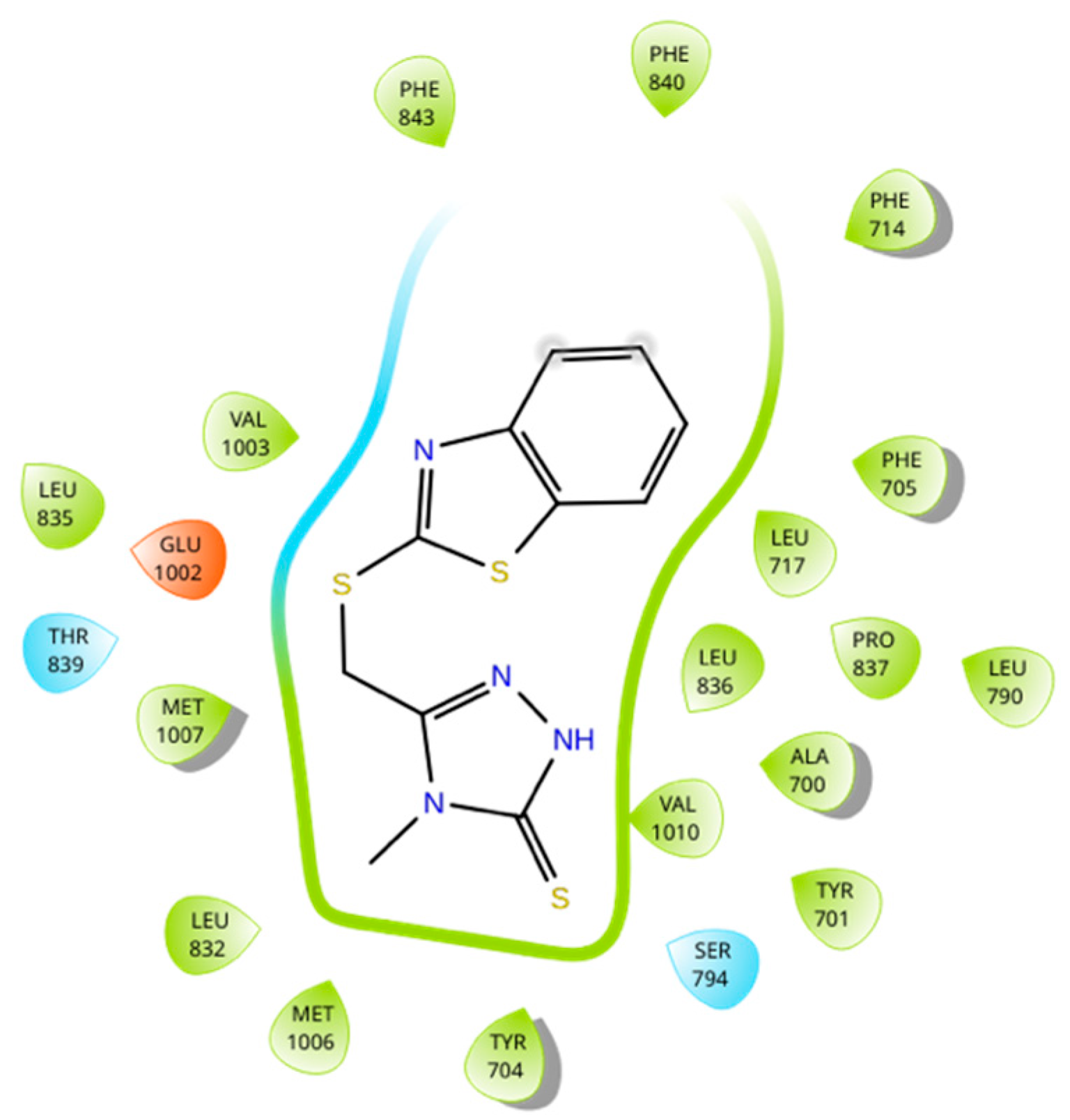
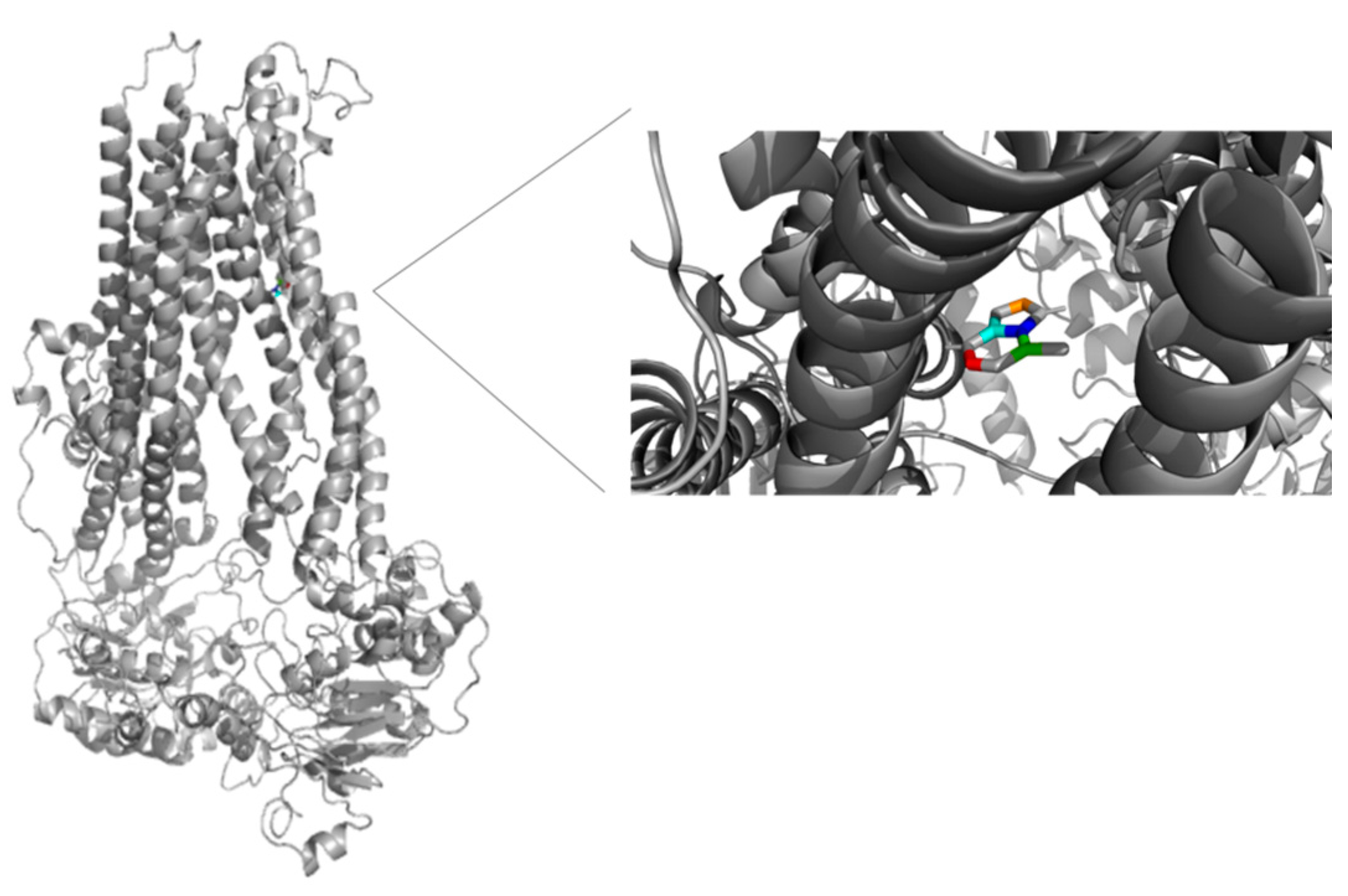
3.3. Molecular Docking and Umbrella Sampling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berthier, J.; Arnion, H.; Saint-Marcoux, F.; Picard, N. Multidrug resistance-associated protein 4 in pharmacology: Overview of its contribution to pharmacokinetics, pharmacodynamics and pharmacogenetics. Life Sci. 2019, 231, 116540. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-X.; Shi, Z.; Damaraju, V.L.; Huang, X.-C.; Kruh, G.D.; Wu, H.-C.; Zhou, Y.; Tiwari, A.; Fu, L.; Cass, C.E.; et al. Up-regulation of MRP4 and down-regulation of influx transporters in human leukemic cells with acquired resistance to 6-mercaptopurine. Leuk. Res. 2008, 32, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.D.; Smith, J.; Tanabe, K.; Tobin, P.; Flemming, C.; Scheffer, G.L.; Wielinga, P.; Cohn, S.L.; London, W.B.; Marshall, G.N.; et al. Expression of multidrug transporter MRP4/ABCC4 is a marker of poor prognosis in neuroblastoma and confers resistance to irinotecan in vitro. Mol. Cancer Ther. 2005, 4, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Copsel, S.; Garcia, C.; Diez, F.; Vermeulem, M.; Baldi, A.; Bianciotti, L.G.; Russel, F.G.M.; Shayo, C.; Davio, C. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J. Biol. Chem. 2011, 286, 6979–6988. [Google Scholar] [CrossRef]
- Perez, D.R.; Smagley, Y.; Garcia, M.; Carter, M.B.; Evangelisti, A.; Matlawska-Wasowska, K.; Winter, S.S.; Sklar, L.A.; Chigaev, A. Cyclic AMP efflux inhibitors as potential therapeutic agents for leukemia. Oncotarget 2016, 7, 33960. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Guo, Y.; Yue, W.; Zhang, L.; Gu, M.; Wang, Y. ABCC4 is required for cell proliferation and tumorigenesis in non-small cell lung cancer. OncoTargets Ther. 2014, 7, 343–351. [Google Scholar] [CrossRef][Green Version]
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249. [Google Scholar] [CrossRef]
- Cole, S.P.C. Targeting Multidrug Resistance Protein 1 (MRP1, ABCC1): Past, Present and Future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef]
- Leier, I.; Jedlitschky, G.; Buchholz, U.; Cole, S.P.; Deeley, R.G.; Keppler, D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994, 269, 27807–27810. [Google Scholar] [CrossRef]
- Keppler, D. Multidrug Resistance Proteins (MRPs, ABCCs): Importance for Pathophysiology and Drug Therapy. Drug Transp. 2011, 201, 299–323. [Google Scholar] [CrossRef]
- Xie, M.; Rich, T.C.; Scheitrum, C.; Conti, M.; Richter, W. Inactivation of Multidrug Resistance Proteins Disrupts Both Cellular Extrusion and Intracellular Degradation of cAMP. Mol. Pharmacol. 2011, 80, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.; Flemming, C.L.; Watt, F.; Masada, N.; Yu, D.M.T.; Huynh, T.; Conseil, G.; Tivnan, A.; Polinsky, A.; Gudkov, A.V.; et al. High-throughput screening identifies Ceefourin 1 and Ceefourin 2 as highly selective inhibitors of multidrug resistance protein 4 (MRP4). Biochem. Pharmacol. 2014, 91, 97–108. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.; van den Heuvel, J.J.M.W.; Krieger, E.; Russel, F.G.M.; Koenderink, J.B. Functional role of arginine 375 in transmembrane helix 6 of multidrug resistance protein 4 (MRP4/ABCC4). Mol. Pharmacol. 2008, 74, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Van Aubel, R.A.M.H.; Smeets, P.H.E.; van den Heuvel, J.J.M.W.; Russel, F.G.M. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am. J. Physiol.-Ren. Physiol. 2005, 288, F327–F333. [Google Scholar] [CrossRef] [PubMed]
- Maeno, K.; Nakajima, A.; Conseil, G.; Rothnie, A.; Deeley, R.G.; Cole, S.P.C. Molecular Basis for Reduced Estrone Sulfate Transport and Altered Modulator Sensitivity of Transmembrane Helix (TM) 6 and TM17 Mutants of Multidrug Resistance Protein 1 (ABCC1). Drug Metab. Dispos. 2009, 37, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 21, A3.B.1–A3.B.2. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Becerra, E.; Aguilera-Durán, G.; Berumen, L.; Romo-Mancillas, A.; García-Alcocer, G. Study of Endogen Substrates, Drug Substrates and Inhibitors Binding Conformations on MRP4 and Its Variants by Molecular Docking and Molecular Dynamics. Molecules 2021, 26, 1051. [Google Scholar] [CrossRef]
- Ravna, A.W.; Sager, G. Molecular modeling studies of ABC transporters involved in multidrug resistance. Mini Rev. Med. Chem. 2009, 9, 186–193. [Google Scholar] [CrossRef]
- Usuki, F.; Fujimura, M.; Yamashita, A. Endoplasmic reticulum stress preconditioning modifies intracellular mercury content by upregulating membrane transporters. Sci. Rep. 2017, 7, 12390. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sarli, V.N.; Kinne, H.E.; Shamsnia, A.; Lucci, A. Evaluation of 6-mercaptopurine in a cell culture model of adaptable triple-negative breast cancer with metastatic potential. Oncotarget 2019, 10, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Mei, D.; Yuan, J.; Han, J.; Xu, J.; Sun, N.; He, H.; Yang, C.; Zhao, L. Preparation, Characterization, Pharmacokinetic and Therapeutic Potential of Novel 6-Mercaptopurine-Loaded Oral Nanomedicines for Acute Lymphoblastic Leukemia. Int. J. Nanomed. 2021, 16, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Haglund, S.; Vikingsson, S.; Almer, S.; Söderman, J. Combination treatment with 6-mercaptopurine and allopurinol in HepG2 and HEK293 cells—Effects on gene expression levels and thiopurine metabolism. PLoS ONE 2017, 12, e0173825. [Google Scholar] [CrossRef]
- Karran, P.; Attard, N. Thiopurines in current medical practice: Molecular mechanisms and contributions to therapy-related cancer. Nat. Rev. Cancer 2008, 8, 24–36. [Google Scholar] [CrossRef]
- Fernández-Ramos, A.A.; Poindessous, V.; Marchetti-Laurent, C.; Pallet, N.; Loriot, M.-A. The effect of immunosuppressive molecules on T-cell metabolic reprogramming. Biochimie 2016, 127, 23–36. [Google Scholar] [CrossRef]
- Abrol, S.; Bodla, B.R.; Goswami, C. A comprehensive review on benzothiazole derivatives for their biological activities. Int. J. Pharm. Sci. Res. 2019, 10, 3196–3209. [Google Scholar] [CrossRef]
- Copsel, S.; Bruzzone, A.; May, M.; Beyrath, J.; Wargon, V.; Cany, J.; Russel, F.G.M.; Shayo, C.; Davio, C. Multidrug resistance protein 4/ ATP binding cassette transporter 4: A new potential therapeutic target for acute myeloid leukemia. Oncotarget 2014, 5, 9308–9321. [Google Scholar] [CrossRef]
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893. [Google Scholar] [CrossRef]
- Ledderose, C.; Woehrle, T.; Ledderose, S.; Strasser, K.; Seist, R.; Bao, Y.; Zhang, J.; Junger, W.G. Cutting off the power: Inhibition of leukemia cell growth by pausing basal ATP release and P2X receptor signaling? Purinergic Signal. 2016, 12, 439–451. [Google Scholar] [CrossRef]
- Insel, P.A.; Zhang, L.; Murray, F.; Yokouchi, H.; Zambon, A.C. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol. 2012, 204, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zambon, A.C.; Vranizan, K.; Pothula, K.; Conklin, B.R.; Insel, P.A. Gene expression signatures of cAMP/protein kinase A (PKA)-promoted, mitochondrial-dependent apoptosis. Comparative analysis of wild-type and cAMP-deathless S49 lymphoma cells. J. Biol. Chem. 2008, 283, 4304–4313. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Hibi, Y.; Cueno, M.; Asamitsu, K.; Okamoto, T. A-kinase-interacting protein 1 (AKIP1) acts as a molecular determinant of PKA in NF-kappaB signaling. J. Biol. Chem. 2010, 285, 28097–28104. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, J.T.; Strack, S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007, 8, 939–944. [Google Scholar] [CrossRef] [PubMed]
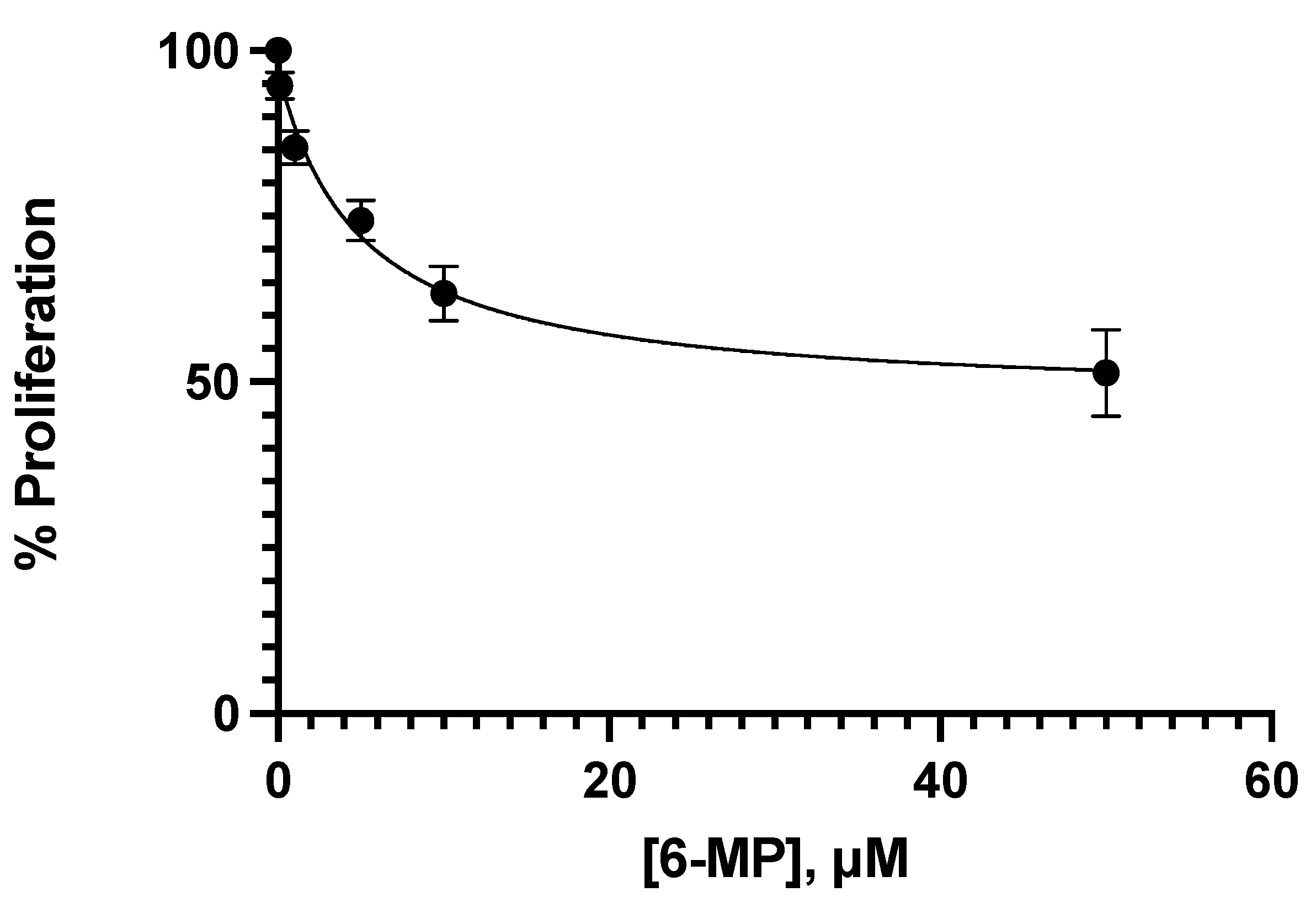
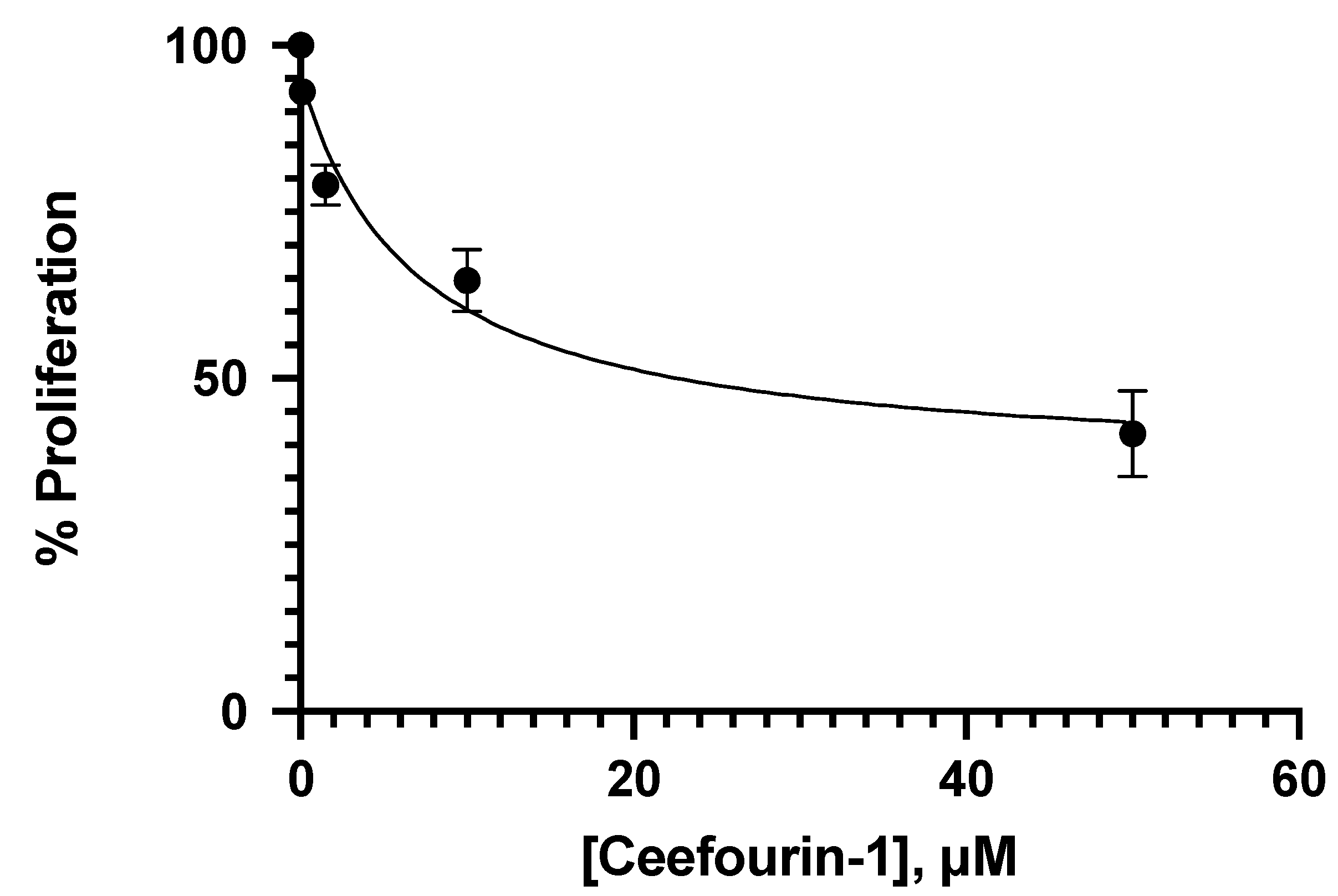

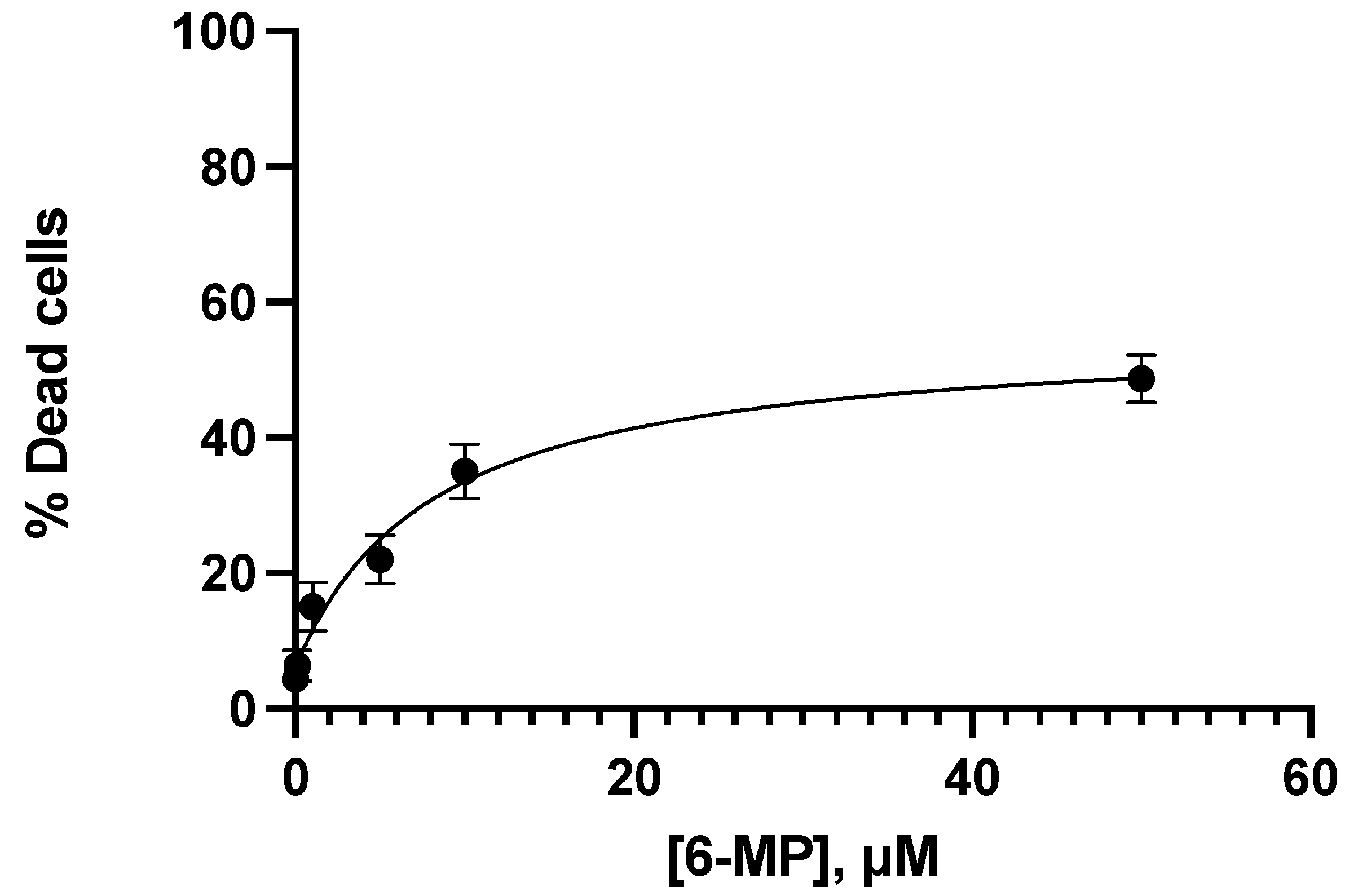
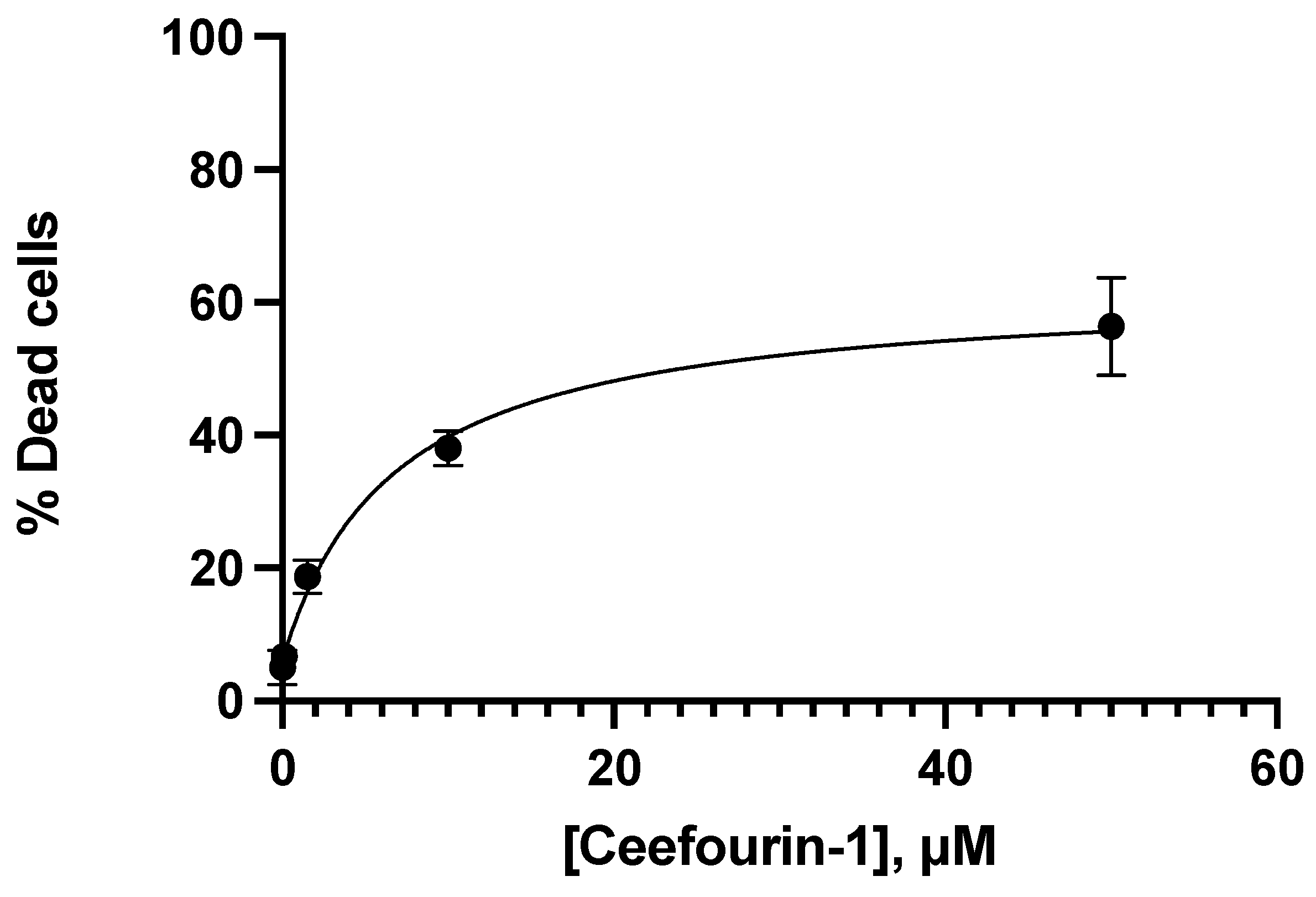



| Concentration μM | |||||
|---|---|---|---|---|---|
| 6-MP | 0.1 | 1.0 | 5.0 | 10 | 50 |
| Ceefourin-1 | 0.1 | 1.5 | 10 | 50 | -- |
| Complex | Docking Score (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|
| 6-MP-MRP4 | −4.79 | −17.86 |
| Ceefourin-1-MRP4 | −7.63 | −26.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra, E.; Berumen, L.; Soto-Ontiveros, V.; García-Alcocer, G. Specific MRP4 Inhibitor Ceefourin-1 Enhances Apoptosis Induced by 6-Mercaptopurine in Jurkat Leukemic Cells, but Not in Normal Lymphoblast Cell Line CRL-1991. Medicina 2022, 58, 695. https://doi.org/10.3390/medicina58060695
Becerra E, Berumen L, Soto-Ontiveros V, García-Alcocer G. Specific MRP4 Inhibitor Ceefourin-1 Enhances Apoptosis Induced by 6-Mercaptopurine in Jurkat Leukemic Cells, but Not in Normal Lymphoblast Cell Line CRL-1991. Medicina. 2022; 58(6):695. https://doi.org/10.3390/medicina58060695
Chicago/Turabian StyleBecerra, Edgardo, Laura Berumen, Valeria Soto-Ontiveros, and Guadalupe García-Alcocer. 2022. "Specific MRP4 Inhibitor Ceefourin-1 Enhances Apoptosis Induced by 6-Mercaptopurine in Jurkat Leukemic Cells, but Not in Normal Lymphoblast Cell Line CRL-1991" Medicina 58, no. 6: 695. https://doi.org/10.3390/medicina58060695
APA StyleBecerra, E., Berumen, L., Soto-Ontiveros, V., & García-Alcocer, G. (2022). Specific MRP4 Inhibitor Ceefourin-1 Enhances Apoptosis Induced by 6-Mercaptopurine in Jurkat Leukemic Cells, but Not in Normal Lymphoblast Cell Line CRL-1991. Medicina, 58(6), 695. https://doi.org/10.3390/medicina58060695








