The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis—A 10 Year Retrospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Population and Data Source
2.3. Therapeutic Management
2.4. Measurements
2.5. Outcome and Follow-Up Period
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics, Treatment Description, and Perioperative Results
3.2. Factors Associated with Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. ESC Guidelines for the diagnosis and management of pericardial diseases; The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Nistor, C.E.; Ciuche, A.; Bontaș, E.; Horvat, T. Malignant Pericardial Effusions. In Thoracic Surgery; Nistor, C.E., Tsui, S., Kirali, K., Ciuche, A., Aresu, G., Kocher, G., Eds.; Springer: Dordrecht, The Netherlands, 2020; pp. 627–644. [Google Scholar] [CrossRef]
- Imazio, M. Noninfectious pericarditis: Management challenges for cardiologists. Kardiol. Pol. 2020, 78, 396–403. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Farkas, D.K.; Ehrenstein, V.; Bhaskaran, K.; Bøtker, H.E.; Sørensen, H.T. Pericarditis as a Marker of Occult Cancer and a Prognostic Factor for Cancer Mortality. Circulation. 2017, 12, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur. Heart J. 2013, 34, 1186–1197. [Google Scholar] [CrossRef] [Green Version]
- Jamaa, G.M.; Scarcib, M.; Bowdenc, J.; Marciniaka, S. Palliative treatment for symptomatic malignant pericardial effusion. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 1019–1026. [Google Scholar] [CrossRef]
- Chahine, J.; Shekhar, S.; Mahalwar, G.; Imazio, M.; Collier, P.; Klein, A. Pericardial Involvement in Cancer. Am. J. Cardiol. 2021, 145, 151–159. [Google Scholar] [CrossRef]
- Bari, M.A.; Abdel-aal, K.M.; Mohamed, R.G.; Abdel-maboud, A.M.; Helmy, A.A. Video-assisted thoracoscopic pericardial window for massive pericardial effusion: South Egypt experience. J. Egypt. Soc. Cardio-Thorac. Surg. 2017, 25, 73–78. [Google Scholar] [CrossRef]
- Mirhosseini, S.M.; Fakhri, M.; Mozaffary, A.; Lotfaliany, M.; Behzadnia, N.; Aval, Z.A.; Ghiasi, S.M.S.; Boloursaz, M.R.; Masjedi, M.R. Risk factors affecting the survival rate in patients with symptomatic pericardial effusion undergoing surgical intervention. Interact Cardiovasc. Thorac Surg. 2013, 16, 495–500. [Google Scholar] [CrossRef]
- Muhammad, M.I.A. The pericardial window: Is a video-assisted thorascopy approach better than a surgical approach? Interact. Cardiovasc. Thorac. Surg. 2011, 12, 174–178. [Google Scholar] [CrossRef]
- Pilling, J.E.; Dusmet, M.E.; Ladas, G.; Goldstraw, P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J. Thorac. Oncol. 2010, 5, 1544–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laisaar, T.; Palmiste, V.; Vooder, T.; Umbleja, T. Life expectancy of patients with malignant pleural effusion treated with video-assisted thoracoscopic talc pleurodesis. Interact. Cardiovasc. Thorac. Surg. 2006, 5, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.W.; Cho, J.H.; Choi, Y.S.; Kim, J.; Kim, H.K.; Zo, J.I.; Shim, Y.M. Predictors of survival in patients who underwent vide-oassisted thoracic surgery talc pleurodesis for malignant pleural effusion. Thoracic Cancer. 2016, 7, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Bussani, R.; De Giorgio, F.; Abbate, A.; Silvestri, F. Cardiac metastases. J. Clin. Pathol. 2007, 60, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Numico, G.; Cristofano, A.; Occelli, M.; Sicuro, M.; Mozzicafreddo, A.; Fea, E.; Colantonio, I.; Merlano, M.; Piovano, P.; Silvestris, N. Prolonged Drainage and Intrapericardial Bleomycin Administration for Cardiac Tamponade Secondary to Cancer-Related Pericardial Effusion. Medicine 2016, 95, e3273. [Google Scholar] [CrossRef]
- Nistor, C.E.; Ranetti, A.E.; Ciuche, A.; Pantile, D.; Constantin, L.M.; Brîncoveanu, R. BETADINE® in chemical pleurodesis. Farmacia 2014, 62, 897–906. (In Romanian) [Google Scholar]
- Inoue, T.; Ishida, A.; Nakamura, M.; Nishine, H.; Mineshita, M.; Miyazawa, T. Talc Pleurodesis for the Management of Malignant Pleural Effusions in Japan. Intern. Med. 2013, 52, 1173–1176. [Google Scholar] [CrossRef] [Green Version]
- Ciuche, A.; Nistor, C.; Motaş, C.; Horvat, T. Minimally invasive surgery in the treatment of malignant pleuro-pericardial effusions. Chirurgia (Bucuresti) 2012, 107, 206–212. (In Romanian) [Google Scholar]
- O’Brien, P.K.; Kucharczuk, J.C.; Marshall, M.B.; Friedberg, J.S.; Chen, Z.; Kaiser, L.R.; Shrager, J.B. Comparative study of subxiphoid versus video-thoracoscopic pericardial “window”. Ann. Thorac. Surg. 2005, 80, 2013–2019. [Google Scholar] [CrossRef]
- Loizzi, D.; Sollitto, F.; Piazzolla, M.; Ardò, N.P. Thoracoscopic pleurodesis using talc poudrage versus cytotoxic drug in malignant pleural effusion: Narrative review. J. Xiangya Med. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Colak, A.; Becit, N.; Kaya, U.; Ceviz, M.; Kocak, H. Treatment of Pericardial Effusion Through Subxiphoid Tube Pericardiostomy and Computerized Tomography- or Echocardiography-Guided Percutaneous Catheter Drainage Methods. Braz. J. Cardiovasc. Surg. 2019, 34, 194–202. [Google Scholar] [CrossRef]
- Virk, S.A.; Chandrakumar, D.; Villanueva, C.; Wolfenden, H.; Liou, K.; Cao, C. Systematic review of percutaneous interventions for malignant pericardial effusion. Heart (Br. Card. Soc.) 2015, 101, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Dokhan, A.L.; El-Sessy, A.A.; Eltaweel, M.F. Povidone-iodine pleurodesis versus talc pleurodesis in preventing recurrence of malignant pleural effusion. J. Cardiothorac. Surg. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, M.E.; Awad, G.; Sanad, M. Chemical pleurodesis for malignant pleural effusion: Which agent is perfect? Cardiothorac. Surg. 2020, 28, 1–7. [Google Scholar] [CrossRef]
| Variables | Thoracoscopic Pleuropericardial Window (N = 288) | Subxiphoid Pleuropericardial Window through Mediastinoscopy (N = 50) | p Value |
|---|---|---|---|
| Event | |||
| Alive/Censored | 25 (8.68%) | 3 (6.00%) | 0.526 |
| Death | 263 (91.32%) | 47 (94.00%) | |
| Sex | 0.594 | ||
| Males | 138 (47.92%) | 26 (52.00%) | |
| Females | 150 (52.08%) | 24 (51.48%) | |
| Age (y) | 61 (13.54), 19–94 | 57 (13.40), 28–80 | |
| Imaging Diagnosis | <0.001 | ||
| Echocardiography | 0 (0.00%) | 39 (78.00%) | |
| Computed tomography | 288 (100%) | 11 (22.00%) | |
| Clinical Diagnosis | 0.162 | ||
| Pericarditis and right pleural effusion | 114 (39.58%) | 27 (54.00%) | |
| Pericarditis and left pleural effusion | 143 (49.65%) | 19 (28.00%) | |
| Pericarditis and bilateral pleural effusion | 31 (10.76%) | 4 (8.00%) | |
| Comorbidities | 0.449 | ||
| No comorbidity | 57 (19.86%) | 14 (28.47%) | |
| Hypertension | 118 (41.11%) | 19 (38.78%) | |
| Diabetes | 22 (7.67%) | 4 (8,16%) | |
| Atrial fibrillation | 11 (3.83%) | 4 (8.16%) | |
| Renal insufficiency | 17 (5.92%) | 0 (0.00%) | |
| Heart failure | 48 (16.72%) | 6 (12.24%) | |
| Hepatic insufficiency | 7 (2.44%) | 1 (2.04%) | |
| Chronic obstructive bronchopneumpathy | 7 (2.44%) | 1 (2.04%) | |
| Presence of pericardial tamponade | <0.001 | ||
| Yes | 10 (3.47%) | 25 (50.00%) | |
| No | 278 (96.53%) | 25 (50.00%) | |
| Presence of hypodiastolia | |||
| Yes | 0 (0.00%) | 40 (80.00%) | <0.001 |
| No | 288 (100%) | 10 (20.00%) |
| Variables | Thoracoscopic Pleuropericardial Window (N = 288) | Subxiphoid Pleuropericardial Window through Mediastinoscopy (N = 50) | p Value |
|---|---|---|---|
| Malignancies | 0.209 | ||
| Lung Cancer | 152 (52.78%) | 29 (58.00%) | |
| Esophageal/Gastric Cancer | 5 (1.74%) | 1 (2.00%) | |
| Leukemia/Lymphoma | 40 (13.89%) | 3 (6.00%) | |
| Breast Cancer | 48 (16.67%) | 7 (14.00%) | |
| Pleural Mesothelioma | 19 (6.60%) | 2 (4.00%) | |
| Ovarian Cancer | 8 (2.78%) | 3 (6.00%) | |
| Cervical Cancer | 10 (3.47%) | 1 (2.00%) | |
| Other types of cancers (Sarcoma, renal and colorectal Cancer) | 6 (2.08%) | 4 (8.00%) | |
| Metastases | 0.012 | ||
| No metastases | 265 (92.01%) | 43 (86.00%) | |
| Heart | 0 (0.00%) | 2 (4.00%) | |
| Liver | 6 (2.08%) | 0 (0.00%) | |
| Peritoneum | 3 (1.04%) | 2 (4.00%) | |
| Bone | 11 (3.82%) | 2 (4.00%) | |
| Upper kidney | 2 (0.69%) | 1 (2.00%) | |
| Kidney | 1 (0.35%) | 0 (0.00%) |
| Variables | Thoracoscopic Pleuropericardial Window (N = 288) | Subxiphoid Pleuropericardial Window through Mediastinoscopy (N = 50) | p Value |
|---|---|---|---|
| Pleurodesis treatment | 0.002 | ||
| Talc | 49 (17.01%) | 0 (0.00%) | |
| Betadine | 239 (82.99%) | 50 (100%) | |
| Recurrence of pleuropericarditis | 0.638 | ||
| Recurrence | 10 (3.47%) | 0 (0.00%) | |
| No recurrence | 280 (96.53%) | 50 (100%) |
| Pleuropericarditis Recurrence after Surgery | Talc (N = 49) | Betadine (N = 289) | p Value |
|---|---|---|---|
| Recurrence | 0 (0.00%) | 10 (3.46%) | 0.186 |
| No recurrence | 49 (100%) | 279 (96.54%) |
| Variable | Univariate HR | 95% CI | p-Value | Multivariate HR | 95% CI | p Value | |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| Age | Age | 1.03 | 1.02–1.03 | <0.001 | 1.03 | 1.02–1.04 | <0.001 |
| Sex | Females | 1 | 1 | ||||
| Males | 1.04 | 0.83–1.31 | 0.705 | 0.99 | 0.76–1.28 | 0.930 | |
| Clinical diagnosis | Pericarditis and left pleural effusion | 1 | 1 | ||||
| Pericarditis and right pleural effusion | 0.89 | 0.71–1.13 | 0.362 | 0.76 | 0.60–0.98 | 0.038 | |
| Pericarditis and bilateral pleural effusion | 1.17 | 0.80–1.70 | 0.401 | 1.01 | 0.69–1.47 | 0.975 | |
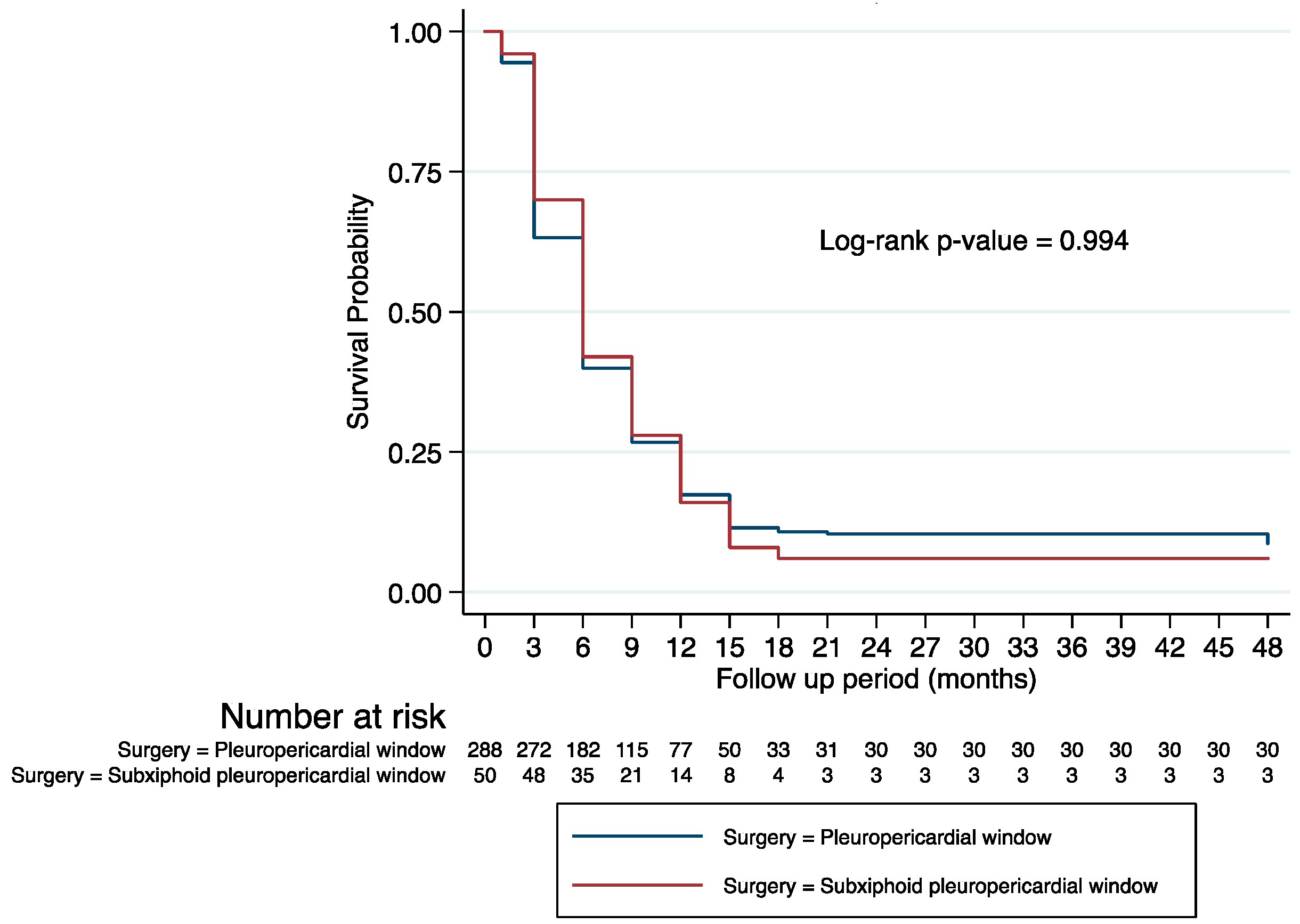
| Surgery | Thoracoscopic pleuropericardial window | 1 | 1 | ||||
| Subxiphoid pleuropericardial window through mediastinoscopy | 1.00 | 0.73–1.37 | 0.996 | 0.88 | 0.64–1.23 | 0.469 | |
| Malignancies | Lung Cancer | 1 | 1 | ||||
| Esophageal/Gastric Cancer | 1.16 | 0.451–2.62 | 0.716 | 1.13 | 0.49–2.57 | 0.768 | |
| Leukemia/Lymphoma | 0.08 | 0.04–0.13 | <0.001 | 0.08 | 0.04–0.13 | <0.001 | |
| Breast Cancer | 0.43 | 0.32–0.60 | <0.001 | 0.37 | 0.26–0.52 | <0.001 | |
| Pleural Mesothelioma | 0.41 | 0.26–0.66 | <0.001 | 0.32 | 0.20–0.52 | <0.001 | |
| Ovarian Cancer | 0.63 | 0.34–1.16 | 0.134 | 0.79 | 0.41–1.51 | 0.485 | |
| Cervical Cancer | 0.46 | 0.25–0.86 | 0.014 | 0.52 | 0.27–0.97 | 0.042 | |
| Other types of cancer | 0.78 | 0.41–1.48 | 0.453 | 0.74 | 0.39–1.42 | 0.373 | |
| Pleuropericarditis recurrence | No recurrence | 1 | 1 | ||||
| Recurrence | 2.08 | 1.10–3.94 | 0.023 | 1.22 | 0.64–2.34 | 0.534 | |
| Model 2 | |||||||
| Pleuropericarditis recurrence | No recurrence | 1 | 1 | ||||
| Recurrence | 2.08 | 1.10–3.94 | 0.023 | 1.42 | 0.75–2.70 | 0.278 | |
| Malignancies | Lung Cancer | 1 | 1 | ||||
| Esophageal/Gastric Cancer | 1.16 | 0.451–2.62 | 0.716 | 1.18 | 0.52–2.67 | 0.685 | |
| Leukemia/Lymphoma | 0.08 | 0.04–0.13 | <0.001 | 0.08 | 0.04–0.13 | <0.001 | |
| Breast Cancer | 0.43 | 0.32–0.60 | <0.001 | 0.44 | 0.32–0.60 | <0.001 | |
| Pleural Mesothelioma | 0.41 | 0.26–0.66 | <0.001 | 0.42 | 0.26–0.66 | <0.001 | |
| Ovarian Cancer | 0.63 | 0.34–1.16 | 0.134 | 0.63 | 0.34–1.17 | 0.149 | |
| Cervical Cancer | 0.46 | 0.25–0.86 | 0.014 | 0.46 | 0.25–0.87 | 0.016 | |
| Other types of cancer | 0.78 | 0.41–1.48 | 0.453 | 0.79 | 0.41–1.50 | 0.483 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, C.-E.; Găvan, C.S.; Ciritel, A.-A.; Nemes, A.F.; Ciuche, A. The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis—A 10 Year Retrospective Observational Study. Medicina 2022, 58, 718. https://doi.org/10.3390/medicina58060718
Nistor C-E, Găvan CS, Ciritel A-A, Nemes AF, Ciuche A. The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis—A 10 Year Retrospective Observational Study. Medicina. 2022; 58(6):718. https://doi.org/10.3390/medicina58060718
Chicago/Turabian StyleNistor, Claudiu-Eduard, Camelia Stanciu Găvan, Alexandra-Andreea Ciritel, Alexandra Floriana Nemes, and Adrian Ciuche. 2022. "The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis—A 10 Year Retrospective Observational Study" Medicina 58, no. 6: 718. https://doi.org/10.3390/medicina58060718
APA StyleNistor, C.-E., Găvan, C. S., Ciritel, A.-A., Nemes, A. F., & Ciuche, A. (2022). The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis—A 10 Year Retrospective Observational Study. Medicina, 58(6), 718. https://doi.org/10.3390/medicina58060718








