Long-Term Outcomes of Laparoscopic Liver Resection for Centrally Located Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Surgical Techniques
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.M.; Kim, D.G.; Kim, J.; Lee, K.; Lee, K.W.; Ryu, J.H.; Kim, B.W.; Choi, D.L.; You, Y.K.; Kim, D.S.; et al. Outcomes after liver transplantation in korea: Incidence and risk factors from korean transplantation registry. Clin. Mol. Hepatol. 2021, 27, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Torimura, T.; Iwamoto, H. Optimizing the management of intermediate-stage hepatocellular carcinoma: Current trends and prospects. Clin. Mol. Hepatol. 2021, 27, 236–245. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Marrero, J.A.; Rudolph, L.; Reddy, K.R. Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2008, 134, 1752–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavien, P.P.; Lesurtel, M.; Patrick, P.; Bossuyt, M.M.; Gores, P.G.J.; Langer, P.B.; Perrier, P.A.; Consensus, H.C.C. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012, 13, e11–e22. [Google Scholar] [CrossRef] [Green Version]
- Guro, H.; Cho, J.Y.; Han, H.S.; Yoon, Y.S.; Choi, Y.R.; Periyasamy, M. Current status of laparoscopic liver resection for hepatocellular carcinoma. Clin. Mol. Hepatol. 2016, 22, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, T.; Zhang, Y.; Xie, Z.B.; Wang, X.B.; Wu, F.X.; Li, L.Q. The safety and efficacy of laparoscopic and open hepatectomy in hepatocellular carcinoma patients with liver cirrhosis: A systematic review. Int. J. Clin. Exp. Med. 2015, 8, 20679–20689. [Google Scholar] [PubMed]
- Franken, C.; Lau, B.; Putchakayala, K.; DiFronzo, L.A. Comparison of short-term outcomes in laparoscopic vs open hepatectomy. JAMA Surg. 2014, 149, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Han, H.; Yoon, Y.; Cho, J.Y. Liver Comparison of laparoscopic liver resection for hepatocellular carcinoma located in the posterosuperior segments or anterolateral segments: A case-matched analysis. Surgery 2016, 160, 1219–1226. [Google Scholar] [CrossRef]
- Ciria, R.; Gomez-Luque, I.; Ocaña, S.; Cipriani, F.; Halls, M.; Briceño, J.; Okuda, Y.; Troisi, R.; Rotellar, F.; Soubrane, O.; et al. A Systematic Review and Meta-Analysis Comparing the Short- and Long-Term Outcomes for Laparoscopic and Open Liver Resections for Hepatocellular Carcinoma: Updated Results from the European Guidelines Meeting on Laparoscopic Liver Surgery, Southampton, UK. Ann. Surg. Oncol. 2019, 26, 252–263. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. Position on laparoscopic liver surgery. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Han, H.; Cho, J.Y. Laparoscopic liver resection for centrally located tumors close to the hilum, major hepatic veins, or inferior vena cava. Surgery 2010, 153, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, J.; Xie, G.; Xiao, Y.; Sun, K.; Yuan, K.; Wu, H. Laparoscopic versus open mesohepatectomy for patients with centrally located hepatocellular carcinoma: A propensity score matched analysis. Surg. Endosc. 2019, 33, 2916–2926. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, K.H.; Kim, S.H.; Kang, W.H.; Lee, S.G. Laparoscopic Versus Open Liver Resection for Centrally Located Hepatocellular Carcinoma in Patients with Cirrhosis: A Propensity Score-matching Analysis. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 394–400. [Google Scholar] [CrossRef]
- Yu, W.B.; Rao, A.; Vu, V.; Xu, L.; Rao, J.Y.; Wu, J.X. Management of centrally located hepatocellular carcinoma: Update 2016. World J. Hepatol. 2017, 9, 627–634. [Google Scholar] [CrossRef]
- Kim, Y.K.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Lee, W. Total anatomical laparoscopic liver resection of segment 4 (S4), extended s4, and subsegments s4a and s4b for hepatocellular carcinoma. J. Laparoendosc. Adv. Surg. Tech. 2015, 25, 375–379. [Google Scholar] [CrossRef]
- Lee, W.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Choi, Y.; Shin, H.K. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 65–68. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Strasberg, S.M. Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 system. J. Hepato-Biliary-Pancreat. Surg. 2005, 12, 351–355. [Google Scholar] [CrossRef]
- Han, H.S.; Cho, J.Y.; Yoon, Y.S. Techniques for performing laparoscopic liver resection in various hepatic locations. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 427–432. [Google Scholar] [CrossRef]
- Guro, H.; Young, J.; Han, C.H. Laparoscopic liver resection of hepatocellular carcinoma located in segments 7 or 8. Surg. Endosc. 2018, 32, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.I.; Adachi, E.; Shimada, M.; Taketomi, A.; Shirabe, K.; Tanaka, S.; Maeda, T.; Ikeda, K.; Higashi, H.; Maehara, Y. Clinical Usefulness of Biliary Scope for Pringle’s Maneuver in Laparoscopic Hepatectomy. J. Am. Coll. Surg. 2007, 205, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Fan, S.T.; Ng, I.O.L.; Lo, C.M.; Liu, C.L.; Wong, J. Prospective evaluation of pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann. Surg. 1997, 226, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Mostaedi, R. Laparoscopic liver resection: Current role and limitations. World J. Gastrointest. Oncol. 2012, 4, 187. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, M.; Yazici, P.; Yigitbas, H.; Dural, C.; Okoh, A.; Aliyev, S.; Aucejo, F.; Quintini, C.; Fung, J.; Berber, E. Oncologic results of laparoscopic liver resection for malignant liver tumors. J. Surg. Oncol. 2016, 113, 127–129. [Google Scholar] [CrossRef] [PubMed]
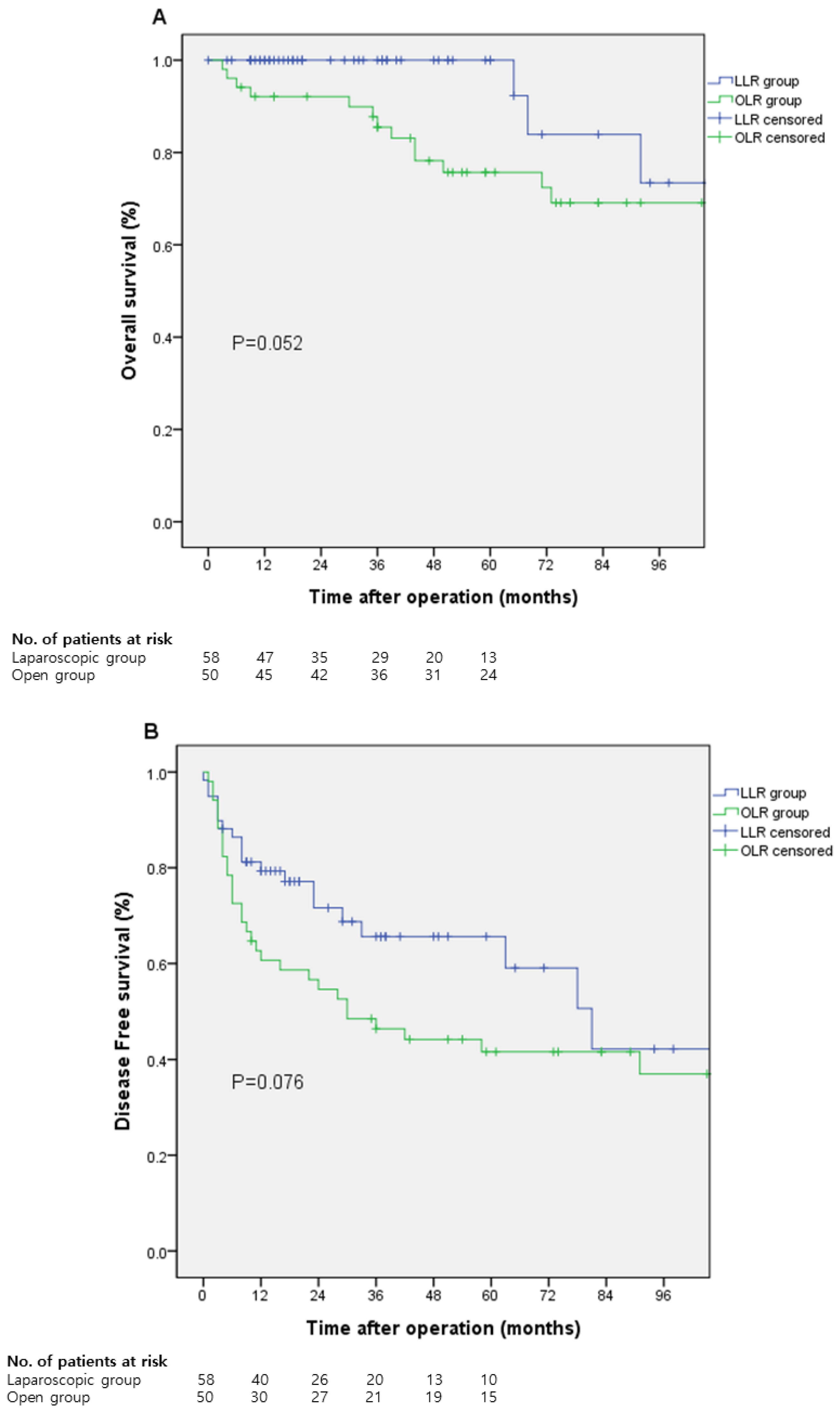
| LLR (n = 59) | OLR (n = 51) | p-Value | |
|---|---|---|---|
| Age, (years), median (range) | 57 (26–74) | 57 (30–85) | 0.926 |
| Gender | 0.036 | ||
| Male | 41 (69.5) | 44 (86.3) | |
| Female | 18 (30.5) | 7 (13.7) | |
| BMI (kg/m2), median (range) | 24.4 (16.36–31.61) | 24.2 (16.73–32.06) | 0.242 |
| Tumor size (cm), median (range) | 3.0 (0.9–10.3) | 5.0 (1.5–13.0) | 0.000 |
| Location of tumor | |||
| Segment 1 | 7 (11.9) | 2 (3.9) | |
| Segment 4 | 15 (25.4) | 11 (21.6) | |
| Segment 5 | 10 (16.9) | 14 (27.5) | |
| Segment 8 | 15 (25.4) | 11 (21.6) | |
| Segment 1 and 8 | 0 | 1 (2.0) | |
| Segment 4 and 5 | 1 (1.7) | 2 (4.0) | |
| Segment 4 and 8 | 2 (3.4) | 6 (11.8) | |
| Segment 5 and 8 | 6 (10.2) | 3 (5.9) | |
| Segment 4, 5, 8 | 3 (5.1) | 1 (2.0) | |
| Albumin (g/dL), median (range) | 4.3 (1.3–4.9) | 4.1 (2.5–5.1) | 0.011 |
| Bilirubin (mg/dL), median (range) | 0.7 (0.2–2.4) | 0.8 (0.3–2.8) | 0.149 |
| PT-INR, median (range) | 1.05 (0.9–1.24) | 1.1 (0.9–1.45) | 0.005 |
| PLT (1000/µL), median (range) | 179 (73–334) | 176 (38–424) | 0.590 |
| SGOT (IU/L), median (range) | 37.0 (14–176) | 36 (20–118) | 0.563 |
| SGPT (IU/L), median (range) | 33.0 (11–256) | 36 (7–260) | 0.500 |
| AFP (ng/mL), median (range) | 7.6 (1.2–6540) | 16.0 (1.4–35,000) | 0.096 |
| Child Pugh class, n (%) | 0.002 | ||
| A | 58 (98.3) | 40 (78.4) | |
| B | 1 (1.8) | 7 (13.7) | |
| C | 0 | 4 (7.8) | |
| Hepatitis, n (%) | 1.000 | ||
| Hepatitis B | 44 (74.6) | 38 (74.5) | |
| Hepatitis C | 3 (5.4) | 2 (3.9) | |
| Both positive | 0 | 0 | |
| Both negative | 12 (20.3) | 11 (21.6) | |
| Prior RFA, n (%) | 4 (6.9) | 1 (2.0) | 0.369 |
| Prior TACE, n (%) | 13 (22.4) | 17 (33.3) | 0.203 |
| LLR (n = 59) | OLR (n = 51) | p-Value | |
|---|---|---|---|
| Operation type, n (%) | 0.013 | ||
| Caudate lobectomy | 5 (8.5) | 3 (5.9) | |
| Segmentectomy | 28 (47.5) | 9 (17.6) | |
| Bi-segmentectomy | 2 (3.4) | 2 (3.9) | |
| Extended segmentectomy | 3 (5.1) | 0 | |
| Left hemihepatectomy | 4 (6.8) | 5 (9.8) | |
| Right anterior sectionectomy | 5 (8.5) | 6 (11.8) | |
| Right posterior sectionectomy | 2 (3.4) | 1 (2.0) | |
| Right hepatectomy | 6 (10.2) | 12 (23.5) | |
| Extended right hepatectomy | 1 (1.7) | 4 (7.8) | |
| Central bisectionectomy | 3 (5.1) | 8 (15.7) | |
| Operation time (min), median (range) | 285 (70–790) | 280 (105–745) | 0.938 |
| Blood loss (mL), median (range) | 500 (10–5900) | 700 (150–7000) | 0.000 |
| Transfusion, n (%) | 6 (10.2) | 16 (31.4) | 0.006 |
| LLR (n = 59) | OLR (n = 51) | p-Value | |
|---|---|---|---|
| Resection margin (cm), median (range) | 0.4 (0.0–5.0) | 0.5 (0.0–3.5) | 0.398 |
| Cirrhosis, n (%) | 35 (59.3) | 30 (58.8) | 1.000 |
| Satellite nodule, n (%) | 3 (5.1) | 3 (5.9) | 1.000 |
| Microvascular invasion, n (%) | 30 (51.7) | 25 (49.0) | 0.778 |
| Resection, n (%) | 0.043 | ||
| R0 | 59 (100) | 47 (92.2) | |
| R1 | 0 | 4 (7.8) | |
| Postoperative complication, n (%) | 13 (22.0) | 14 (27.5) | 0.510 |
| Complication type, n (%) | 0.319 | ||
| General | 3 (5.1) | 2 (3.9) | |
| Surgical | 7 (11.9) | 5 (9.8) | |
| Liver related | 3 (5.1) | 3 (5.9) | |
| Mixed | 0 | 4 (7.8) | |
| Clavien–Dindo grade, n (%) | 0.798 | ||
| I | 2 (3.4) | 1 (2.0) | |
| II | 1 (1.7) | 3 (5.9) | |
| IIIa | 6 (10.2) | 7 (13.7) | |
| IIIb | 4 (6.8) | 3 (5.9) | |
| IVa | 0 | 0 | |
| IVb | 0 | 0 | |
| V | 0 | 0 | |
| Hospital stay (days), median (range) | 6 (2–59) | 10 (4–64) | 0.000 |
| Mortality within 3 months, n (%) | 0 | 0 |
| Group 1 (n = 19) | Group 2 (n = 40) | p-Value | |
|---|---|---|---|
| Operation type, n (%) | 0.264 | ||
| Segmentectomy | 8 (42.1) | 23 (57.5) | |
| Bi-segmentectomy | 0 | 2 (5.0) | |
| Left hemihepatectomy | 2 (10.5) | 2 (5.0) | |
| Right anterior sectionectomy | 1 (5.3) | 4 (10.5) | |
| Right posterior sectionectomy | 2 (10.5) | 0 | |
| Right hepatectomy | 4 (21.1) | 2 (5.0) | |
| Extended right hepatectomy | 0 | 1 (2.5) | |
| Caudate Lobectomy | 1 (5.3) | 4 (10.0) | |
| Central bisectionectomy | 1 (5.3) | 2 (5.0) | |
| Operation time (min), median (range) | 360 (80–790) | 280 (70–755) | 0.036 |
| Blood loss (mL), median (range) | 500 (200–5900) | 455 (5–2000) | 0.075 |
| Transfusion, n (%) | 4 (21.1) | 2 (5.0) | 0.078 |
| Hospital stay (days), median (range) | 8 (4–59) | 6 (2–19) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Cho, J.Y.; Han, H.-S.; Yoon, Y.-S.; Lee, H.W.; Lee, J.S.; Lee, B.; Jo, Y.; Kang, M.; Park, Y.; et al. Long-Term Outcomes of Laparoscopic Liver Resection for Centrally Located Hepatocellular Carcinoma. Medicina 2022, 58, 737. https://doi.org/10.3390/medicina58060737
Kim HJ, Cho JY, Han H-S, Yoon Y-S, Lee HW, Lee JS, Lee B, Jo Y, Kang M, Park Y, et al. Long-Term Outcomes of Laparoscopic Liver Resection for Centrally Located Hepatocellular Carcinoma. Medicina. 2022; 58(6):737. https://doi.org/10.3390/medicina58060737
Chicago/Turabian StyleKim, Hyo Jun, Jai Young Cho, Ho-Seong Han, Yoo-Seok Yoon, Hae Won Lee, Jun Suh Lee, Boram Lee, Yeongsoo Jo, Meeyouong Kang, Yeshong Park, and et al. 2022. "Long-Term Outcomes of Laparoscopic Liver Resection for Centrally Located Hepatocellular Carcinoma" Medicina 58, no. 6: 737. https://doi.org/10.3390/medicina58060737
APA StyleKim, H. J., Cho, J. Y., Han, H.-S., Yoon, Y.-S., Lee, H. W., Lee, J. S., Lee, B., Jo, Y., Kang, M., Park, Y., & Lee, E. (2022). Long-Term Outcomes of Laparoscopic Liver Resection for Centrally Located Hepatocellular Carcinoma. Medicina, 58(6), 737. https://doi.org/10.3390/medicina58060737






