Surgical Significance of Berry’s Posterolateral Ligament and Frequency of Recurrent Laryngeal Nerve Injury into the Last 2 cm of Its Caudal Extralaryngeal Part(P1) during Thyroidectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Techniques and Neurostimulation Procedure
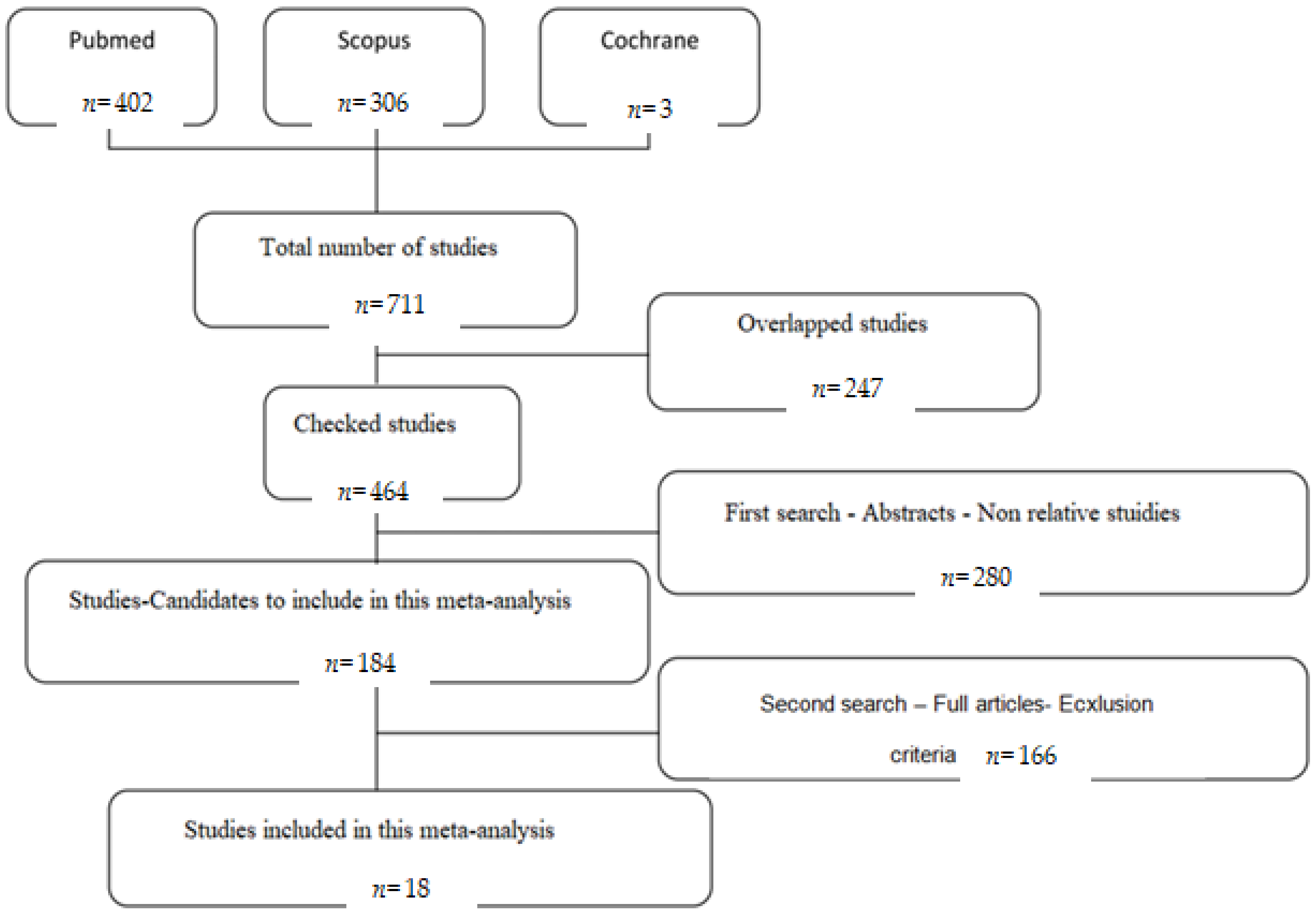
2.2. Search Strategy
2.3. Criteria for Selection of Studies
2.4. Data Extraction
2.5. Statistical Analysis
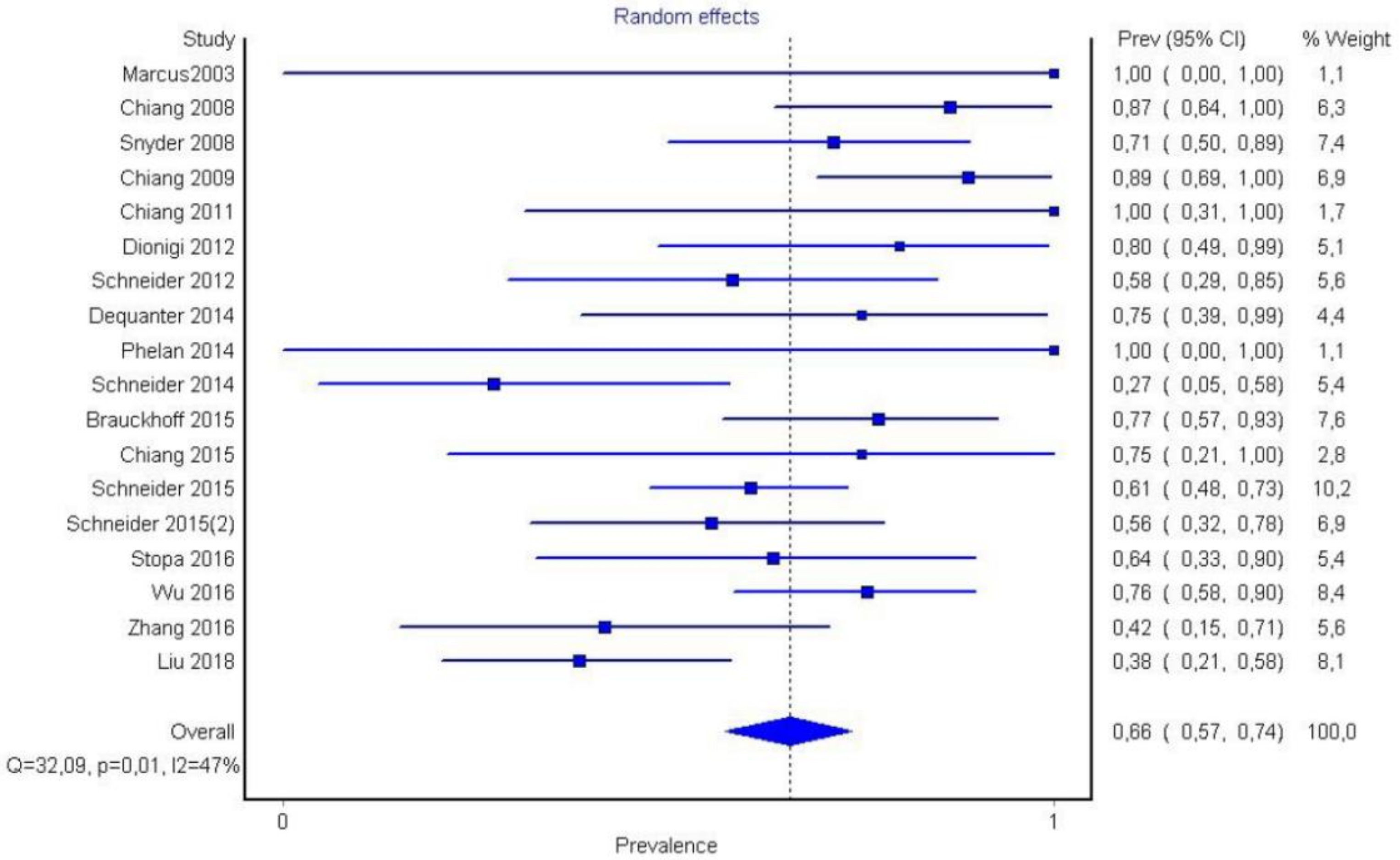
3. Results
4. Discussion
5. Conclusions
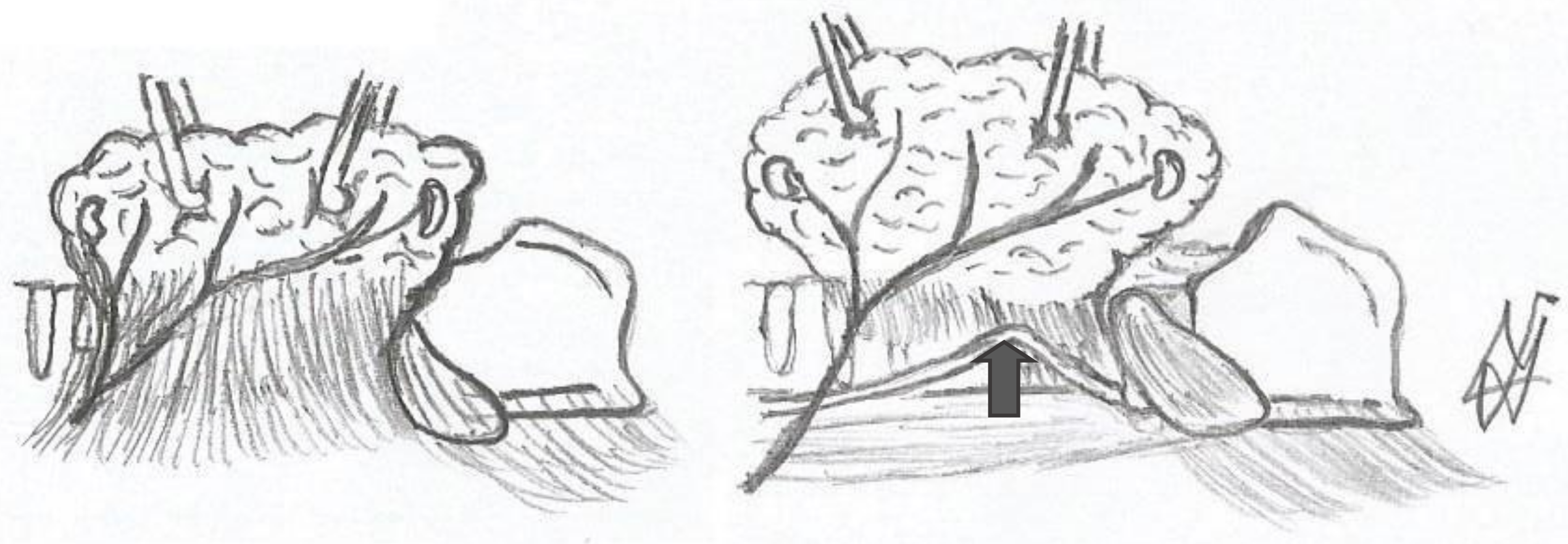
- Berry’s ligament is a stable anatomical structure in relation to the recurrent laryngeal nerve because it has the most consistent link with the nerve (in 75% of cases, the nerve passes superficially to the ligament).
- Berry’s ligament is surgically significant since the majority of recurrent laryngeal nerve injuries (functional or non-functional) are detected in that anatomical location (66%).
- This systematic review and meta-analysis provide a higher quality of evidence in the aforementioned anatomical relationship. It is the first meta-analysis in this era referring to the anatomical regions where recurrent laryngeal nerve injuries are observed (P1, P2, and P3) after the widespread use of intraoperative neurostimulation in head and neck surgery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fundakowski, C.E.; Hales, N.W.; Agrawal, N.; Barczyński, M.; Camacho, P.M.; Hartl, D.M.; Kandil, E.; Liddy, W.E.; McKenzie, T.J.; Morris, J.C.; et al. Surgical management of the recurrent laryngeal nerve in thyroidectomy: American Head and Neck Society Consensus Statement. Otolaryngol. Neck Surg. 2018, 40, 663–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeannon, J.-P.; Orabi, A.A.; Bruch, G.A.; Abdalsalam, H.A.; Simo, R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: A systematic review. Int. J. Clin. Pract. 2009, 63, 624–629. [Google Scholar] [CrossRef]
- Francis, D.O.; Pearce, E.C.; Ni, S.; Garrett, C.G.; Penson, D. Epidemiology of Vocal Fold Paralyses after Total Thyroidectomy for Well-Differentiated Thyroid Cancer in a Medicare Population. Otolaryngol. Neck Surg. 2014, 150, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasou, S.; Nakamura, S.; Kurihara, H. Suspensory Ligament of Berry: Its Relationship to Recurrent Laryngeal Nerve and Anatomic Examination of 24 Autopsies. Head Neck 1998, 20, 695–698. [Google Scholar] [CrossRef]
- Lore, J.M. Practical Anatomical Considerations in Thyroid Tumor Surgery. Arch. Otolaryngol. Head Neck Surg. 1983, 109, 568–574. [Google Scholar] [CrossRef]
- Berlin, D.D. The Recurrent Laryngeal Nerves in Total Ablation of the Normal Thyroid Gland. J. Gynecol. Obstet. 1935, 60, 19–26. [Google Scholar]
- Lahey, F.B.D. Dissections of the recurrent and superior laryngeal nerves. Surg. Gynecol. Obstet. 1929, 49, 102–104. [Google Scholar]
- Reeve, T.S.; Coupland, G.A.E.; Johnson, D.C.; Buddee, F.W. THE RECURRENT AND EXTERNAL LARYNGEAL NERVES IN THYROIDECTOMY. Med. J. Aust. 1969, 1, 380–382. [Google Scholar] [CrossRef]
- Thompson, N.W.; Olsen, W.R.; Hoffman, G.L. The continuing development of the technique of thyroidectomy. Surgery 1973, 73, 913–927. [Google Scholar]
- Loré, J.M.; Kim, D.J.; Elias, S. Preservation of the Laryngeal Nerves during Total Thyroid Lobectomy. Ann. Otol. Rhinol. Laryngol. 1977, 86, 777–788. [Google Scholar] [CrossRef]
- Marcus, B.; Edwards, B.; Yoo, S.; Byrne, A.; Gupta, A.; Kandrevas, J.; Bradford, C.; Chepeha, D.B.; Teknos, T.N. Recurrent Laryngeal Nerve Monitoring in Thyroid and Parathyroid Surgery: The University of Michigan Experience. Laryngoscope 2003, 113, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.K.; Lairmore, T.C.; Hendricks, J.C.; Roberts, J.W. Elucidating Mechanisms of Recurrent Laryngeal Nerve Injury during Thyroidectomy and Parathyroidectomy. J. Am. Coll. Surg. 2008, 206, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chiang, F.-Y.; Lee, K.-W.; Chen, H.-C.; Chen, H.-Y.; Lu, I.-C.; Kuo, W.-R.; Hsieh, M.-C.; Wu, C.-W. Standardization of Intraoperative Neuromonitoring of Recurrent Laryngeal Nerve in Thyroid Operation. World J. Surg. 2009, 34, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chiang, F.-Y.; Lu, I.-C.; Tsai, C.-J.; Hsiao, P.-J.; Hsu, C.-C.; Wu, C.-W. Does extensive dissection of recurrent laryngeal nerve during thyroid operation increase the risk of nerve injury? Evidence from the application of intraoperative neuromonitoring. Am. J. Otolaryngol. 2011, 32, 499–503. [Google Scholar] [CrossRef]
- Dionigi, G.; Chiang, F.Y.; Hui, S.; Wu, C.W.; Xiaoli, L.; Ferrari, C.C.; Mangano, A.; Lianos, G.D.; Leotta, A.; Lavazza, M.; et al. Continuous Intraoperative Neuromonitoring (C-IONM) Technique with the Au-tomatic Periodic Stimulating (APS) Accessory for Conventional and Endoscopic Thyroid Surgery. Surg. Technol. Int. 2015, 26, 101–114. [Google Scholar] [PubMed]
- Schneider, R.; Randolph, G.W.; Sekulla, C.; Phelan, E.; Thanh, P.N.; Bucher, M.; Machens, A.; Dralle, H.; Lorenz, K. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2012, 35, 1591–1598. [Google Scholar] [CrossRef]
- Dequanter, D.; Charara, F.; Shahla, M.; Lothaire, P. Usefulness of neuromonitoring in thyroid surgery. Eur. Arch. Oto-Rhino-Laryngol. 2014, 272, 3039–3043. [Google Scholar] [CrossRef]
- Schneider, R.; Lorenz, K.; Sekulla, C.; Machens, A.; Nguyen-Thanh, P.; Dralle, H. Operative Strategie bei geplanter totaler Thyreoidektomie und Verlust des Neuromonitoring-Signals auf der erstoperierten Seite. Der Chir. 2014, 86, 154–163. [Google Scholar] [CrossRef]
- Brauckhoff, K.; Vik, R.; Sandvik, L.; Heimdal, J.-H.; Aas, T.; Biermann, M.; Brauckhoff, M. Impact of EMG Changes in Continuous Vagal Nerve Monitoring in High-Risk Endocrine Neck Surgery. World J. Surg. 2016, 40, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Chiang, F.-Y.; Lu, I.-C.; Chang, P.-Y.; Sun, H.; Wang, P.; Lu, X.-B.; Chen, H.-C.; Chen, H.-Y.; Kim, H.Y.; Dionigi, G.; et al. Stimulating dissecting instruments during neuromonitoring of RLN in thyroid surgery. Laryngoscope 2015, 125, 2832–2837. [Google Scholar] [CrossRef]
- Schneider, R.; Sekulla, C.; Machens, A.; Lorenz, K.; Thanh, P.N.; Dralle, H. Dynamics of loss and recovery of the nerve monitoring signal during thyroidectomy predict early postoperative vocal fold function. Head Neck 2015, 38, E1144–E1151. [Google Scholar] [CrossRef] [PubMed]
- Stopa, M.; Barczyński, M. Prognostic value of intraoperative neural monitoring of the recurrent laryngeal nerve in thyroid surgery. Langenbecks Arch. Surg. 2016, 402, 957–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-W.; Hao, M.; Tian, M.; Dionigi, G.; Tufano, R.P.; Kim, H.Y.; Jung, K.Y.; Liu, X.; Sun, H.; Lu, I.-C.; et al. Recurrent laryngeal nerve injury with incomplete loss of electromyography signal during monitored thyroidectomy—evaluation and outcome. Langenbecks Arch Surg. 2016, 402, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, F.; Wu, C.-W.; Liu, X.; Xin, J.; Chiang, F.-Y.; Sun, H. Percutaneous probe stimulation for intraoperative neuromonitoring in total endoscopic thyroidectomy: A preliminary experience. Head Neck 2017, 39, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, D.; Zhang, G.; Zhao, L.; Zhou, L.; Fu, Y.; Li, S.; Zhao, Y.; Li, C.; Wu, C.-W.; et al. Laryngeal nerve morbidity in 1.273 central node dissections for thyroid cancer. Surg. Oncol. 2018, 27, A21–A25. [Google Scholar] [CrossRef] [PubMed]
- Phelan, E.; Schneider, R.; Lorenz, K.; Dralle, H.; Kamani, D.; Potenza, A.; Sritharan, N.; Shin, J.; Randolph, G.W. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: A prospective, multicenter study. Laryngoscope 2014, 124, 1498–1505. [Google Scholar] [CrossRef]
- Chiang, F.-Y.; Lu, I.-C.; Kuo, W.-R.; Lee, K.-W.; Chang, N.-C.; Wu, C.-W. The mechanism of recurrent laryngeal nerve injury during thyroid surgery—The application of intraoperative neuromonitoring. Surgery 2008, 143, 743–749. [Google Scholar] [CrossRef]
- Schneider, R.; Randolph, G.; Dionigi, G.; Barczyński, M.; Chiang, F.-Y.; Triponez, F.; Vamvakidis, K.; Brauckhoff, K.; Musholt, T.J.; Almquist, M.; et al. Prospective study of vocal fold function after loss of the neuromonitoring signal in thyroid surgery: The International Neural Monitoring Study Group’s POLT study. Laryngoscope 2015, 126, 1260–1266. [Google Scholar] [CrossRef]
- Serpell, J.W. New Operative Surgical Concept of Two Fascial Layers Enveloping the Recurrent Laryngeal Nerve. Ann. Surg. Oncol. 2010, 17, 1628–1636. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Henry, B.M.; Tomaszewski, K.; Walocha, J.A. Methods of Evidence-Based Anatomy: A guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann. Anat.-Anat. Anz. 2016, 205, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, F.-Y.; Wang, L.-F.; Huang, Y.-F.; Lee, K.-W.; Kuo, W.-R. Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery 2005, 137, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, G.; Alesina, P.F.; Barczyński, M.; Boni, L.; Chiang, F.Y.; Kim, H.Y.; Materazzi, G.; Randolph, G.W.; Terris, D.J.; Wu, C.-W.; et al. Recurrent laryngeal nerve injury in video-assisted thyroidectomy: Lessons learned from neuromonitoring. Surg. Endosc. 2012, 26, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.B.; McGrath, P. Recurrent Laryngeal Nerve and the Posterior Fascial Attachment of the Thyroid Gland. ANZ J. Surg. 1992, 62, 444–449. [Google Scholar] [CrossRef]
- Negus, V.E. The Comparative Anatomy and Physiology of the Larynx; Hafner Pub. Co.: New York, NY, USA, 1962. [Google Scholar]
- Wadie, M.; Adam, S.I.; Sasaki, C.T. Development, Anatomy, and Physiology of the Larynx. In Principles of Deglutition; Shaker, R., Belafsky, P.C., Postma, G.N., Easterling, C., Eds.; Springer: New York, NY, USA, 2013; pp. 175–197. [Google Scholar] [CrossRef]
- Henry, B.M.; Sanna, B.; Graves, M.J.; Sanna, S.; Vikse, J.; Tomaszewska, I.M.; Tubbs, R.S.; Tomaszewski, K.A. The Reliability of the Tracheoesophageal Groove and the Ligament of Berry as Landmarks for Identifying the Recurrent Laryngeal Nerve: A Cadaveric Study and Meta-Analysis. BioMed. Res. Int. 2017, 2017, 4357591. [Google Scholar] [CrossRef]
- Yalcin, B.; Ozan, H.; Yalçın, B. Detailed Investigation of the Relationship between the Inferior Laryngeal Nerve Including Laryngeal Branches and Ligament of Berry. J. Am. Coll. Surg. 2006, 202, 291–296. [Google Scholar] [CrossRef]
- Çakir, B.; Ercan, I.; Şam, B.; Turgut, S. Reliable surgical landmarks for the identification of the recurrent laryngeal nerve. Otolaryngol. Neck Surg. 2006, 135, 299–302. [Google Scholar] [CrossRef]
- Pelizzo, M.R.; Toniato, A.; Gemo, G. Zuckerkandl’s Tuberculum: An Arrow Pointing to the Recurrent Laryngeal Nerve (Constant Anatomical Landmark). J. Am. Coll. Surg. 1998, 187, 333–336. [Google Scholar] [CrossRef]
- Shindo, M.L.; Wu, J.C.; Park, E.E. Surgical Anatomy of the Recurrent Laryngeal Nerve Revisited. Otolaryngol. Neck Surg. 2005, 133, 514–519. [Google Scholar] [CrossRef]
- Ellis, H. Gray’s anatomy. 37th ed. P.L. Williams, R. Warwick, M. Dyson, L.H. Bannister. 305 × 235 mm. Pp. Illustrated. Edinburgh: Churchill Livingstone. £70. Br. J. Surg. 1989, 76, 1359. [Google Scholar] [CrossRef]
- Stranding, S. Gray’s Anatomy. Available online: https://www.bookdepository.com/Grays-Anatomy-Susan-Standring/9780443066849 (accessed on 20 March 2021).










| P1 | P2 | P3 | Summa | Country | Study | NAR | Technique | |
|---|---|---|---|---|---|---|---|---|
| Marcus (2003) [11] | 1 | 0 | 0 | 1 | USA | P | 81 | Classic |
| Snyder (2008) [12] | 15 | 4 | 2 | 21 | USA | P | 666 | Classic |
| Chiang (2009) [13] | 16 | 1 | 1 | 18 | Taiwan | P | 435 | Classic |
| Chiang (2011) [14] | 2 | 0 | 0 | 2 | Taiwan | P | 506 | Classic |
| Schneider (2012) [16] | 7 | 2 | 3 | 12 | Germany | R | 52 | Classic |
| Dequanter (2014) [17] | 6 | 1 | 1 | 8 | Belgium | P | 175 | Classic |
| Schneider (2014) [18] | 3 | 6 | 2 | 11 | Germany | R | 2086 | Classic |
| Brauckhoff (2015) [19] | 17 | 3 | 2 | 22 | Norway | P | 87 | Classic |
| Chiang (2015) [20] | 3 | 1 | 0 | 4 | Taiwan | P | 168 | Classic |
| Schneider (2015) [21] | 34 | 10 | 12 | 56 | Germany | P | 115 | Classic |
| Stopa (2016) [22] | 7 | 4 | 0 | 11 | Poland | P | 1000 | Classic |
| Wu (2016) [23] | 22 | 5 | 2 | 29 | Taiwan | P | 522 | Classic |
| Zhang (2017) [24] | 5 | 7 | 0 | 12 | China | P | 156 | Endo |
| Liu (2018) [25] | 10 | 10 | 6 | 26 | China | P | 1273 | CND |
| Phelan (2014) [26] | 1 | 0 | 0 | 1 | Germany | P | 204 | Classic |
| Chiang (2008) [27] | 13 | 2 | 0 | 15 | Taiwan | P | 173 | Classic |
| Schneider (2015) (2) [28] | 10 | 6 | 2 | 18 | Germany | R | 1291 | Classic |
| Dionigi (2012) [33] | 8 | 1 | 1 | 10 | Italy | P | 201 | VA |
| Regions | Mean | S.D. |
|---|---|---|
| Region P1 | 70.88 | 21.22 |
| Region P2 | 20.66 | 17.39 |
| Region P3 | 8.47 | 8.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantalovas, S.; Sapalidis, K.; Manaki, V.; Magra, V.; Laskou, S.; Pantea, S.; Lagopoulos, V.; Kesisoglou, I. Surgical Significance of Berry’s Posterolateral Ligament and Frequency of Recurrent Laryngeal Nerve Injury into the Last 2 cm of Its Caudal Extralaryngeal Part(P1) during Thyroidectomy. Medicina 2022, 58, 755. https://doi.org/10.3390/medicina58060755
Mantalovas S, Sapalidis K, Manaki V, Magra V, Laskou S, Pantea S, Lagopoulos V, Kesisoglou I. Surgical Significance of Berry’s Posterolateral Ligament and Frequency of Recurrent Laryngeal Nerve Injury into the Last 2 cm of Its Caudal Extralaryngeal Part(P1) during Thyroidectomy. Medicina. 2022; 58(6):755. https://doi.org/10.3390/medicina58060755
Chicago/Turabian StyleMantalovas, Stylianos, Konstantinos Sapalidis, Vasiliki Manaki, Vasiliki Magra, Styliani Laskou, Stelian Pantea, Vasileios Lagopoulos, and Isaak Kesisoglou. 2022. "Surgical Significance of Berry’s Posterolateral Ligament and Frequency of Recurrent Laryngeal Nerve Injury into the Last 2 cm of Its Caudal Extralaryngeal Part(P1) during Thyroidectomy" Medicina 58, no. 6: 755. https://doi.org/10.3390/medicina58060755
APA StyleMantalovas, S., Sapalidis, K., Manaki, V., Magra, V., Laskou, S., Pantea, S., Lagopoulos, V., & Kesisoglou, I. (2022). Surgical Significance of Berry’s Posterolateral Ligament and Frequency of Recurrent Laryngeal Nerve Injury into the Last 2 cm of Its Caudal Extralaryngeal Part(P1) during Thyroidectomy. Medicina, 58(6), 755. https://doi.org/10.3390/medicina58060755







