Early Use of ECMO for Refractory Kounis Syndrome Concealed by General Anesthesia—A Case Report
Abstract
:1. Introduction
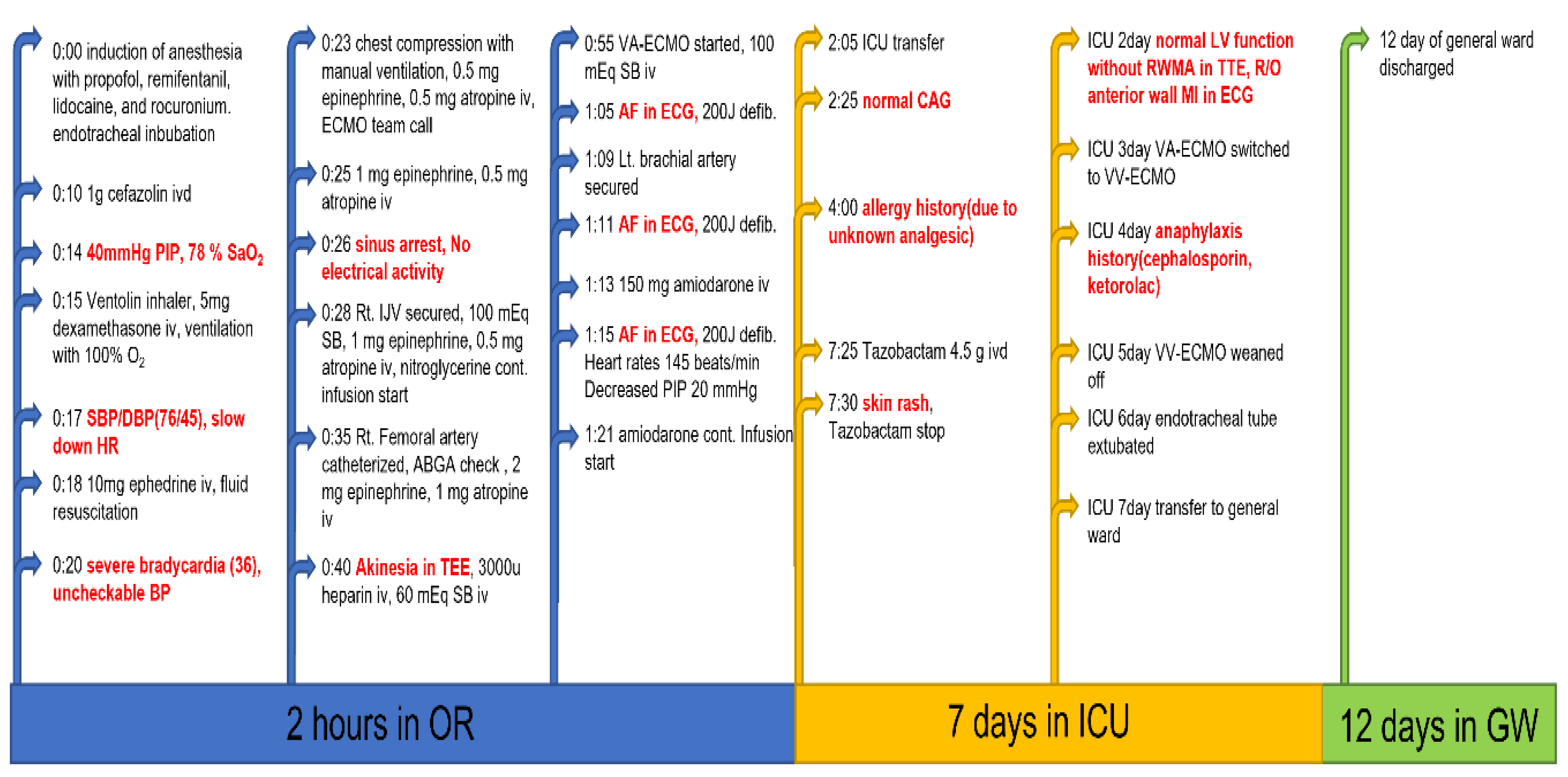
2. Case Presentation
3. Discussion
- Anesthesiologists should remember that various anesthetics, antibiotics, and other drugs administered together during anesthesia induction can induce severe anaphylaxis and cause KS (ACS can cause fatal acute coronary syndrome).
- KS should be managed in both allergic reactions and acute coronary syndrome. According to the World Allergy Organization guidelines, intramuscular epinephrine (0.01 mg/kg of body weight, to the maximum total dose of 0.5 mg by 1 mg/mL (1:1000) epinephrine) is the first-line drug for anaphylaxis treatment [1]. Epinephrine as the first drug of choice in anaphylaxis should be cautiously administered with close hemodynamic monitoring, especially when injected intravenously. Intravenous epinephrine should be cautiously used at a dilution of 1:10,000 to 1:100,000 [17].
- Early initiation of ECMO in refractory KS with conventional treatment and CPCR should be considered because ECMO can maintain coronary circulation more effectively [14], and intravenous heparin with ECMO may be helpful owing to its anticoagulation effect during myocardial infarction.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ivd | intravenous dropping |
| PIP | peak inspiratory pressure |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| HR | heart rate |
| BP | blood pressure |
| ECMO | extracorporeal membrane oxygenation |
| Iv | intravenous injection |
| SB | sodium bicarbonate |
| IJV | internal jugular vein |
| ABGA | arterial blood gas analysis |
| TEE | transesophageal echocardiography |
| VA-ECMO | veno-arterial ECMO |
| AF | atrial fibrillation |
| Defib | defibrillation |
| ECG | electrocardiogram |
| ICU | intensive care unit |
| CAG | coronary angiogram |
| LV | left ventricle |
| RWMA | regional wall motion abnormality |
| TTE | transthoracic echocardiography |
| MI | myocardial infarction |
| VV-ECMO | veno-venous ECMO |
| SaO2 | oxygen saturation |
| OR | operating room |
| GW | general ward |
| Right | Rt |
| Left | Lt |
| Rule out | R/O |
References
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- De Feo, G.; Parente, R.; Triggiani, M. Pitfalls in anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Zavras, G.M. Histamine-induced coronary artery spasm: The concept of allergic angina. Br. J. Clin. Pract. 1991, 45, 121–128. [Google Scholar]
- Fassio, F.; Losappio, L.; Antolin-Amerigo, D.; Peveri, S.; Pala, G.; Preziosi, D.; Massaro, I.; Giuliani, G.; Gasperini, C.; Caminati, M.; et al. Kounis syndrome: A concise review with focus on management. Eur. J. Intern. Med. 2016, 30, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Alonso, M.A.; Moro-Moro, M.; Múgica-García, M.V. Epidemiology of anaphylaxis: Contributions from the last 10 years. J. Investig. Allergol. Clin. Immunol. 2015, 25, 163–175. [Google Scholar]
- Dewachter, P.; Mouton-Faivre, C.; Hepner, D.L. Perioperative anaphylaxis: What should be known? Curr. Allergy Asthma Rep. 2015, 15, 21. [Google Scholar] [CrossRef]
- Dewachter, P.; Mouton-Faivre, C.; Emala, C.W. Anaphylaxis and anesthesia: Controversies and new insights. Anesthesiology 2009, 111, 1141–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerschow, E.; Lin, R.Y.; Scaperotti, M.M.; McGinn, A.P. Fatal anaphylaxis in the United States, 1999–2010: Temporal patterns and demographic associations. J. Allergy Clin. Immunol. 2014, 134, 1318–1328.e1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pumphrey, R.S. Lessons for management of anaphylaxis from a study of fatal reactions. Clin. Exp. Allergy 2000, 30, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, M.; Subedi, R.; Shah, S.; Kozman, H. Kounis syndrome: A review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int. J. Cardiol. 2017, 232, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, A.; Nakayama, T.; Takahara, M.; Sugimoto, K.; Hattori, N.; Abe, R.; Fujimoto, Y.; Oda, S.; Kobayashi, Y. Combined use of ECMO and hemodialysis in the case of contrast-induced biphasic anaphylactic shock. Am. J. Emerg. Med. 2016, 34, 1919.e1911–1919.e1912. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kanaya, Y.; Komuro, K.; Sugawara, S.; Ishikawa, Y.; Onodera, M.; Goto, I.; Fusazaki, T.; Nakamura, M. Kounis syndrome caused by protamine shock after coronary intervention: A case report. J. Cardiol. Cases 2022, 25, 23–25. [Google Scholar] [CrossRef]
- Prisco, A.R.; Allen, J.; Gutierrez, A.; Zanotto, A.; Yannopoulos, D.; Markowitz, J.; Bartos, J.A. Kounis Syndrome Leading to Cardiac Arrest After Iodinated Contrast Exposure. JACC Case Rep. 2020, 2, 626–629. [Google Scholar] [CrossRef]
- Stub, D.; Byrne, M.; Pellegrino, V.; Kaye, D.M. Extracorporeal Membrane Oxygenation to Support Cardiopulmonary Resuscitation in a Sheep Model of Refractory Ischaemic Cardiac Arrest. Heart Lung Circ. 2013, 22, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ridella, M.; Bagdure, S.; Nugent, K.; Cevik, C. Kounis syndrome following beta-lactam antibiotic use: Review of literature. Inflamm. Allergy Drug Targets 2009, 8, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Panesar, S.S.; Javad, S.; de Silva, D.; Nwaru, B.I.; Hickstein, L.; Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Cardona, V.; et al. The epidemiology of anaphylaxis in Europe: A systematic review. Allergy 2013, 68, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.; Irvin, C.B.; Frank, J.J.; Weber, K.; Rosman, H. Confusion about epinephrine dosing leading to iatrogenic overdose: A life-threatening problem with a potential solution. Ann. Emerg. Med. 2010, 55, 341–344. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.K.; Park, M.; Lee, S.H.; Woo, J.-W.; Kang, D.-H.; Byun, J.H.; Ok, S.-H. Early Use of ECMO for Refractory Kounis Syndrome Concealed by General Anesthesia—A Case Report. Medicina 2022, 58, 759. https://doi.org/10.3390/medicina58060759
Yu HK, Park M, Lee SH, Woo J-W, Kang D-H, Byun JH, Ok S-H. Early Use of ECMO for Refractory Kounis Syndrome Concealed by General Anesthesia—A Case Report. Medicina. 2022; 58(6):759. https://doi.org/10.3390/medicina58060759
Chicago/Turabian StyleYu, Ho Kyung, Miyeong Park, Soo Hee Lee, Jung-Woo Woo, Dong-Hoon Kang, Joung Hun Byun, and Seong-Ho Ok. 2022. "Early Use of ECMO for Refractory Kounis Syndrome Concealed by General Anesthesia—A Case Report" Medicina 58, no. 6: 759. https://doi.org/10.3390/medicina58060759
APA StyleYu, H. K., Park, M., Lee, S. H., Woo, J.-W., Kang, D.-H., Byun, J. H., & Ok, S.-H. (2022). Early Use of ECMO for Refractory Kounis Syndrome Concealed by General Anesthesia—A Case Report. Medicina, 58(6), 759. https://doi.org/10.3390/medicina58060759





