Predictors of Noninvasive Respiratory Support Failure in COVID-19 Patients: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
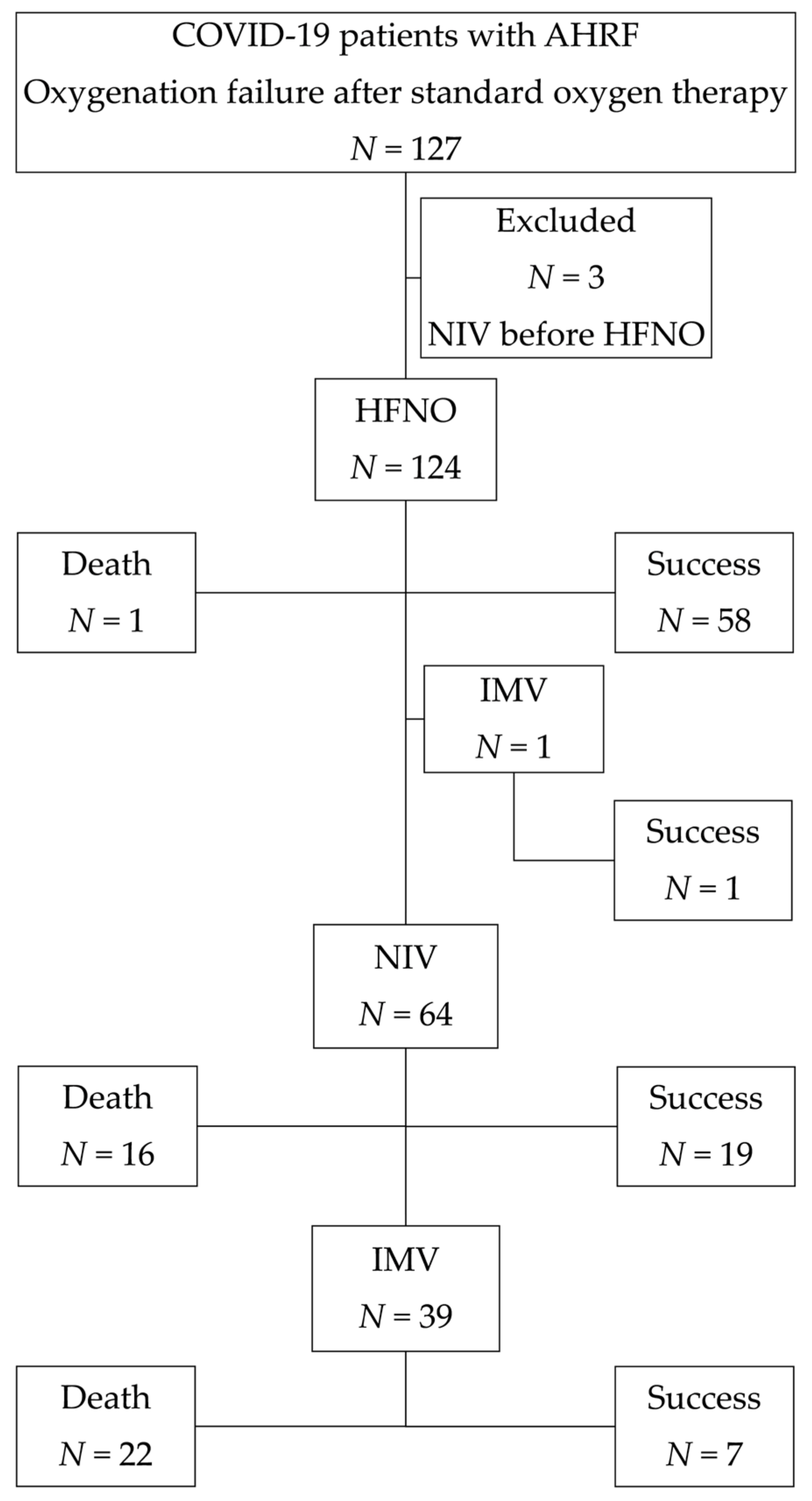
3.1. The Study Population’s Basic Characteristics and Outcomes
3.2. Predictors of HFNC and NIV Failure
3.3. The Predictive Model’s Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Tang, X.; He, D. The Disease Severity and Clinical Outcomes of the SARS-CoV-2 Variants of Concern. Front. Public Health 2021, 9, 775224. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, Y.; Ni, L.; Chen, L.; Zhou, C.; Gao, C.; Wu, X.; Duan, J.; Xie, J.; Guo, Q.; et al. High-Flow Nasal Oxygen in Coronavirus Disease 2019 Patients with Acute Hypoxemic Respiratory Failure: A Multicenter, Retrospective Cohort Study. Crit. Care Med. 2020, 48, e1079–e1086. [Google Scholar] [CrossRef] [PubMed]
- Rorat, M.; Szymanski, W.; Jurek, T.; Karczewski, M.; Zelig, J.; Simon, K. When Conventional Oxygen Therapy Fails-The Effectiveness of High-Flow Nasal Oxygen Therapy in Patients with Respiratory Failure in the Course of COVID-19. J. Clin. Med. 2021, 10, 4751. [Google Scholar] [CrossRef]
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoglu, U. Severe COVID-19 pneumonia: Pathogenesis and clinical management. BMJ 2021, 372, n436. [Google Scholar] [CrossRef] [PubMed]
- Menga, L.S.; Berardi, C.; Ruggiero, E.; Grieco, D.L.; Antonelli, M. Noninvasive respiratory support for acute respiratory failure due to COVID-19. Curr. Opin. Crit. Care 2022, 28, 25–50. [Google Scholar] [CrossRef]
- Demoule, A.; Vieillard Baron, A.; Darmon, M.; Beurton, A.; Geri, G.; Voiriot, G.; Dupont, T.; Zafrani, L.; Girodias, L.; Labbe, V.; et al. High-Flow Nasal Cannula in Critically III Patients with Severe COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, A.A.; Mylvaganam, R.J.; Agarwal, R.; Avery, R.J.; Cuttica, M.J. Timing of Intubation in Coronavirus Disease 2019: A Study of Ventilator Mechanics, Imaging, Findings, and Outcomes. Crit. Care Explor. 2021, 3, e0415. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Z.; Liang, J.; Lin, H.; Yang, Z.; Wang, Y.; Liang, H.; Wu, H.; Chen, R.; Ou, Y.; et al. Critical Review of the Scientific Evidence and Recommendations in COVID-19 Management Guidelines. Open Forum Infect. Dis. 2021, 8, ofab376. [Google Scholar] [CrossRef]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies with All-Cause Mortality in Adults with Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-analysis. JAMA 2020, 324, 57–67. [Google Scholar] [CrossRef]
- Mellado-Artigas, R.; Ferreyro, B.L.; Angriman, F.; Hernandez-Sanz, M.; Arruti, E.; Torres, A.; Villar, J.; Brochard, L.; Ferrando, C.; COVID-19 Spanish ICU Network. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit. Care 2021, 25, 58. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Ewig, S.; Voshaar, T.; Randerath, W.J.; Bauer, T.; Geiseler, J.; Dellweg, D.; Westhoff, M.; Windisch, W.; Schonhofer, B.; et al. Position Paper for the State-of-the-Art Application of Respiratory Support in Patients with COVID-19. Respiration 2020, 99, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Rabec, C.; Gonzalez-Bermejo, J.; Respiratory Support Chronic Care Group AVO2 of the French Society of Respiratory Diseases SPLF; GAVO2 Collaborators. Respiratory support in patients with COVID-19 (outside intensive care unit). A position paper of the Respiratory Support and Chronic Care Group of the French Society of Respiratory Diseases. Respir. Med. Res. 2020, 78, 100768. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Barbieri, C.; Fontana, M.; Scelfo, C.; Castagnetti, C.; Ghidoni, G.; Ruggiero, P.; Livrieri, F.; Piro, R.; Ghidorsi, L.; et al. Effectiveness of noninvasive ventilation in COVID-19 related-acute respiratory distress syndrome. Clin. Respir. J. 2021, 15, 779–787. [Google Scholar] [CrossRef]
- Tobin, M.J.; Laghi, F.; Jubran, A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, C.; Bouadma, L.; de Montmollin, E.; Goldgran-Toledano, D.; Schwebel, C.; Reignier, J.; Neuville, M.; Ursino, M.; Siami, S.; Ruckly, S.; et al. Association Between Early Invasive Mechanical Ventilation and Day-60 Mortality in Acute Hypoxemic Respiratory Failure Related to Coronavirus Disease-2019 Pneumonia. Crit. Care Explor. 2021, 3, e0329. [Google Scholar] [CrossRef]
- Fayed, M.; Patel, N.; Yeldo, N.; Nowak, K.; Penning, D.H.; Vasconcelos Torres, F.; Natour, A.K.; Chhina, A. Effect of Intubation Timing on the Outcome of Patients with Severe Respiratory Distress Secondary to COVID-19 Pneumonia. Cureus 2021, 13, e19620. [Google Scholar] [CrossRef]
- Daniel, P.; Mecklenburg, M.; Massiah, C.; Joseph, M.A.; Wilson, C.; Parmar, P.; Rosengarten, S.; Maini, R.; Kim, J.; Oomen, A.; et al. Non-invasive positive pressure ventilation versus endotracheal intubation in treatment of COVID-19 patients requiring ventilatory support. Am. J. Emerg. Med. 2021, 43, 103–108. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, A.; Gigliotti, C.; Saavedra, S.; Zamarron-Lopez, E.I.; Guerrero-Gutierrez, M.A.; Perez-Nieto, O.R. Strategies for monitoring and predicting failure to high-flow nasal cannula therapy in the ED. Am. J. Emerg. Med. 2021, 57, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Bhattacharya, P.K.; Yadav, A.K.; Kumar, A.; Tudu, L.C.; Prasad, K. ROX index as a good predictor of high flow nasal cannula failure in COVID-19 patients with acute hypoxemic respiratory failure: A systematic review and meta-analysis. J. Crit. Care 2021, 66, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.A.; Erne, P.; Investigators, A.P. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuty Kuswardhani, R.A.; Henrina, J.; Pranata, R.; Anthonius Lim, M.; Lawrensia, S.; Suastika, K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 2103–2109. [Google Scholar] [CrossRef]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The Impact of Pre-existing Comorbidities and Therapeutic Interventions on COVID-19. Front. Immunol. 2020, 11, 1991. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Chi, J.; Dong, B.; Lv, W.; Shen, L.; Wang, Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 99, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Tipih, T.; Makoah, N.A.; Vermeulen, J.G.; Goedhals, D.; Sempa, J.B.; Burt, F.J.; Taylor, A.; Mahalingam, S. Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. mBio 2021, 12, e03647-20. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Demaria, M.; Gnjidic, J.; Rob, Z.; Filipovic, D.; Penovic, T.; Jordan, A.; Barisic-Jaman, M.; Pastrovic, F.; Lucijanic, D.; et al. Higher ferritin levels in COVID-19 patients are associated with hyperinflammation, worse prognosis, and more bacterial infections without pronounced features of hemophagocytosis. Ann. Hematol. 2022, 101, 1119–1121. [Google Scholar] [CrossRef]
- Qeadan, F.; Tingey, B.; Gu, L.Y.; Packard, A.H.; Erdei, E.; Saeed, A.I. Prognostic Values of Serum Ferritin and D-Dimer Trajectory in Patients with COVID-19. Viruses 2021, 13, 419. [Google Scholar] [CrossRef]
- Carubbi, F.; Salvati, L.; Alunno, A.; Maggi, F.; Borghi, E.; Mariani, R.; Mai, F.; Paoloni, M.; Ferri, C.; Desideri, G.; et al. Ferritin is associated with the severity of lung involvement but not with worse prognosis in patients with COVID-19: Data from two Italian COVID-19 units. Sci. Rep. 2021, 11, 4863. [Google Scholar] [CrossRef] [PubMed]
- Plays, M.; Muller, S.; Rodriguez, R. Chemistry and biology of ferritin. Metallomics 2021, 13, mfab021. [Google Scholar] [CrossRef]
- Colafrancesco, S.; Alessandri, C.; Conti, F.; Priori, R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun. Rev. 2020, 19, 102573. [Google Scholar] [CrossRef]
- Shoenfeld, Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun. Rev. 2020, 19, 102538. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Rosario, C.; Zandman-Goddard, G.; Meyron-Holtz, E.G.; D’Cruz, D.P.; Shoenfeld, Y. The hyperferritinemic syndrome: Macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Alroomi, M.; Rajan, R.; Omar, A.A.; Alsaber, A.; Pan, J.; Fatemi, M.; Zhanna, K.D.; Aboelhassan, W.; Almutairi, F.; Alotaibi, N.; et al. Ferritin level: A predictor of severity and mortality in hospitalized COVID-19 patients. Immun. Inflamm. Dis. 2021, 9, 1648–1655. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin—From iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef] [PubMed]
- Khodeir, M.M.; Shabana, H.A.; Alkhamiss, A.S.; Rasheed, Z.; Alsoghair, M.; Alsagaby, S.A.; Khan, M.I.; Fernandez, N.; Al Abdulmonem, W. Early prediction keys for COVID-19 cases progression: A meta-analysis. J. Infect. Public Health 2021, 14, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Jafarzadeh, A.; Jafarzadeh, S.; Nozari, P.; Mokhtari, P.; Nemati, M. Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms. Scand. J. Immunol. 2021, 93, e12967. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Prajapati, M.; Li, Y. Lymphopenia Caused by Virus Infections and the Mechanisms Beyond. Viruses 2021, 13, 1876. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Sareli, C.; Mayer, D.; Visbal, A.; Sareli, A. Lymphopenia as a Predictor for Adverse Clinical Outcomes in Hospitalized Patients with COVID-19: A Single Center Retrospective Study of 4485 Cases. J. Clin. Med. 2022, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Zhao, J.; Li, F.; Lu, S.; Liu, P.; Liu, X.H.; Huang, Q.; Wang, H.; Xu, Q.N.; et al. The predictive role of lymphocyte subsets and laboratory measurements in COVID-19 disease: A retrospective study. Ther. Adv. Respir. Dis. 2021, 15, 17534666211049739. [Google Scholar] [CrossRef]
| Characteristics | HFNC (N = 124) | NIV (N = 64) | p-Value |
|---|---|---|---|
| Age, years | 64.0 (57.0–70.0) | 67.0 (61.2–74.0) | 0.039 |
| Gender, male, % | 69 (55.6) | 34 (53.1) | 0.759 |
| BMI > 30, kg/m2, % | 17 (13.7) | 10 (15.6) | 0.827 |
| CCI | 2.0 (2.0–5.0) | 4.0 (2.0–5.7) | 0.012 |
| PaO2/FiO2 | 107.0 (86.0–133.5) | 102.5 (78.2–127.0) | 0.344 |
| ROX index at 24 h | 6.2 (4.9–7.9) | 5.6 (4.4–6.8) | 0.043 |
| WBC, count per mm3 | 6.7 (5.3–9.1) | 6.8 (5.5–9.6) | 0.910 |
| Lymphocyte, count per mm3 | 0.8 (0.5–1.1) | 0.8 (0.5–1.0) | 0.679 |
| NLR | 6.6 (4.0–10.4) | 6.8 (4.4–11.9) | 0.660 |
| CRP, mg/dL | 124.2 (63.4–182.2) | 131.2 (94.5–187.4) | 0.252 |
| Ferritin, ng/mL | 902.0 (378.0–2118.4) | 581.9 (316.9–1927.6) | 0.317 |
| IL-6, pg/mL | 47.8 (19.4–96.7) | 51.7 (30.7–106.7) | 0.301 |
| LDH, IU/L | 462.0 (319.0–594.0) | 481.0 (334.7–620.2) | 0.702 |
| D-dimer, ng/mL | 660.0 (490.0–995.0) | 717.5 (527.5–1210.0) | 0.258 |
| Dexamethasone, % | 81 (65.3) | 42 (65.6) | 0.613 |
| Remdesivir, % | 35 (28.2) | 18 (28.1) | 0.583 |
| Antibiotics, % | 112 (90.3) | 59 (92.2) | 0.792 |
| LMWH, % | 120 (96.8) | 62 (96.9) | 0.668 |
| The number of days since the start of symptoms | 7.0 (5.0–9.0) | 7.0 (5.0–9.0) | 0.492 |
| Treatment failure, % | 64 (51.6) | 45 (70.3) | 0.019 |
| In-hospital mortality, % | 39 (31.5) | 38 (59.4) | 0.001 |
| Hospitalization duration, days | 21.0 (13.2–30.0) | 20.5 (13.2–31.0) | 0.875 |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, years | 1.05 (1.02–1.09) | 0.002 | 0.98 (0.93–1.03) | 0.431 |
| Gender, male | 0.81 (0.39–1.65) | 0.560 | ||
| BMI > 30, kg/m2 | 1.40 (0.49–3.96) | 0.523 | ||
| CCI | 1.52 (1.24–1.86) | 0.001 | 1.60 (1.18–2.18) | 0.003 |
| PaO2/FiO2 | 0.99 (0.95–1.00) | 0.078 | 1.00 (0.99–1.01) | 0.405 |
| ROX index at 24 h | 0.77 (0.65–0.92) | 0.003 | 0.74 (0.58–0.95) | 0.018 |
| WBC count, per mm3 | 1.02 (0.93–1.13) | 0.632 | ||
| Lymphocyte count, per mm3 | 0.88 (0.48–1.62) | 0.692 | ||
| NLR | 1.03 (0.96–1.09) | 0.405 | ||
| CRP, mg/dL | 1.00 (1.00–1.01) | 0.074 | 1.00 (0.99–1.01) | 0.198 |
| Ferritin, ng/mL | 1.00 (1.00–1.01) | 0.700 | ||
| IL-6, pg/mL | 1.00 (0.98–1.01) | 0.292 | ||
| LDH, IU/L | 1.00 (0.99–1.01) | 0.438 | ||
| D-dimer, ng/mL | 1.00 (0.99–1.01) | 0.203 | ||
| Dexamethasone | 1.08 (0.25–4.60) | 0.920 | ||
| Remdesivir | 1.07 (0.99–1.08) | 0.719 | ||
| Antibiotics | 0.64 (0.19–2.14) | 0.471 | ||
| LMWH | 1.07 (0.15–7.84) | 0.948 | ||
| The number of days since the start of symptoms | 0.97 (0.88–1.07) | 0.502 | ||
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, years | 1.00 (0.96–1.05) | 0.733 | ||
| Gender, male | 2.57 (0.85–7.77) | 0.094 | 1.73 (0.46–6.46) | 0.262 |
| BMI > 30, kg/m2 | 4.73 (1.15–19.4) | 0.031 | 0.10 (0.10–2.32) | 0.096 |
| CCI | 1.11 (0.87–1.41) | 0.384 | ||
| PaO2/FiO2 | 0.99 (0.97–1.01) | 0.243 | ||
| ROX index at 24 h | 0.83 (0.65–1.06) | 0.145 | 0.63 (0.25–1.64) | 0.540 |
| WBC count, per mm3 | 0.93 (0.81–1.06) | 0.256 | ||
| Lymphocyte count, per mm3 | 0.55 (0.23–1.29) | 0.172 | 0.23 (0.10–0.86) | 0.041 |
| NLR | 1.04 (0.95–1.14) | 0.366 | ||
| CRP, mg/dL | 0.99 (0.99–1.01) | 0.268 | ||
| Ferritin, ng/mL | 1.00 (0.99–1.01) | 0.184 | 1.03 (1.01–1.05) | 0.015 |
| IL-6, pg/mL | 0.99 (0.99–1.01) | 0.506 | ||
| LDH, IU/L | 1.00 (0.99–1.01) | 0.754 | ||
| D-dimer, ng/mL | 1.00 (0.99–1.01) | 0.417 | ||
| Dexamethasone | 0.78 (0.07–7.99) | 0.833 | ||
| Remdesivir | 0.39 (0.12–1.24) | 0.111 | 0.43 (0.03–6.92) | 0.554 |
| Antibiotics | 0.61 (0.09–3.96) | 0.602 | ||
| LMWH | 1.00 (0.99–1.01) | 0.990 | ||
| The number of days since the start of symptoms | 0.93 (0.81–1.06) | 0.250 | ||
| Treatment Group | Characteristics | Sensitivity (%) | Specificity (%) | Cut-Off Value | AUC (95% CI) | p-Value |
|---|---|---|---|---|---|---|
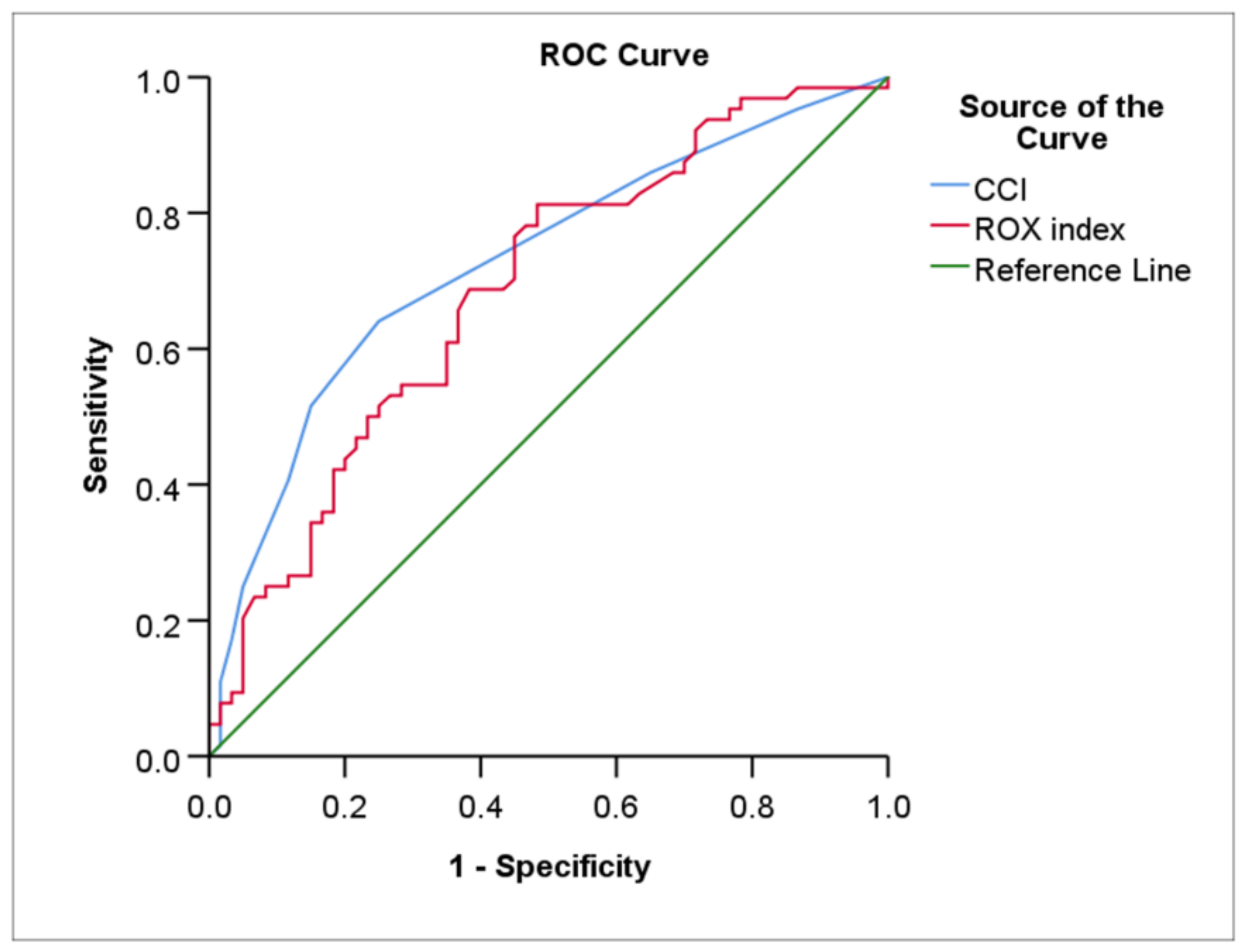
| HFNC (N = 124) | CCI | 64.1 | 75.0 | 2.5 | 0.73 (0.64–0.82) | <0.001 |
| ROX index at 24 h | 81.2 | 51.7 | 7.1 | 0.68 (0.59–0.78) | <0.001 | |
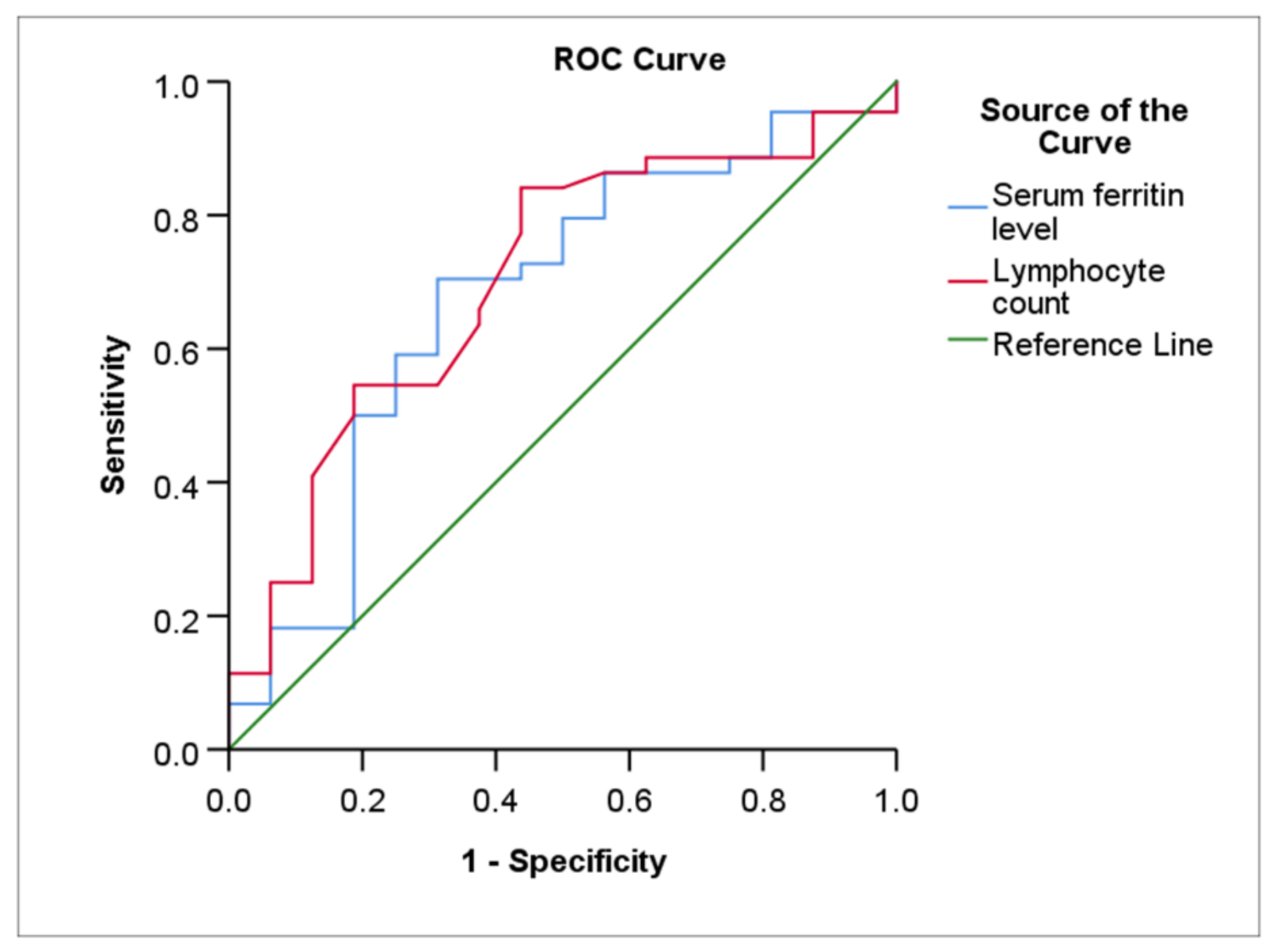
| NIV (N = 64) | Lymphocyte count, per mm3 | 84.1 | 56.2 | 1.0 | 0.70 (0.55–0.85) | 0.009 |
| Ferritin, ng/mL | 70.5 | 68.7 | 456.2 | 0.67 (0.51–0.84) | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zablockis, R.; Šlekytė, G.; Mereškevičienė, R.; Kėvelaitienė, K.; Zablockienė, B.; Danila, E. Predictors of Noninvasive Respiratory Support Failure in COVID-19 Patients: A Prospective Observational Study. Medicina 2022, 58, 769. https://doi.org/10.3390/medicina58060769
Zablockis R, Šlekytė G, Mereškevičienė R, Kėvelaitienė K, Zablockienė B, Danila E. Predictors of Noninvasive Respiratory Support Failure in COVID-19 Patients: A Prospective Observational Study. Medicina. 2022; 58(6):769. https://doi.org/10.3390/medicina58060769
Chicago/Turabian StyleZablockis, Rolandas, Goda Šlekytė, Rūta Mereškevičienė, Karolina Kėvelaitienė, Birutė Zablockienė, and Edvardas Danila. 2022. "Predictors of Noninvasive Respiratory Support Failure in COVID-19 Patients: A Prospective Observational Study" Medicina 58, no. 6: 769. https://doi.org/10.3390/medicina58060769
APA StyleZablockis, R., Šlekytė, G., Mereškevičienė, R., Kėvelaitienė, K., Zablockienė, B., & Danila, E. (2022). Predictors of Noninvasive Respiratory Support Failure in COVID-19 Patients: A Prospective Observational Study. Medicina, 58(6), 769. https://doi.org/10.3390/medicina58060769





