The Prevalence of Insulin Resistance in Malaysia and Indonesia: An Updated Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Guidelines and Study Search
2.2. Study Filtering and Extraction of Data
2.3. Data and Statistical Analysis
3. Results
3.1. Characteristics of Studies Included
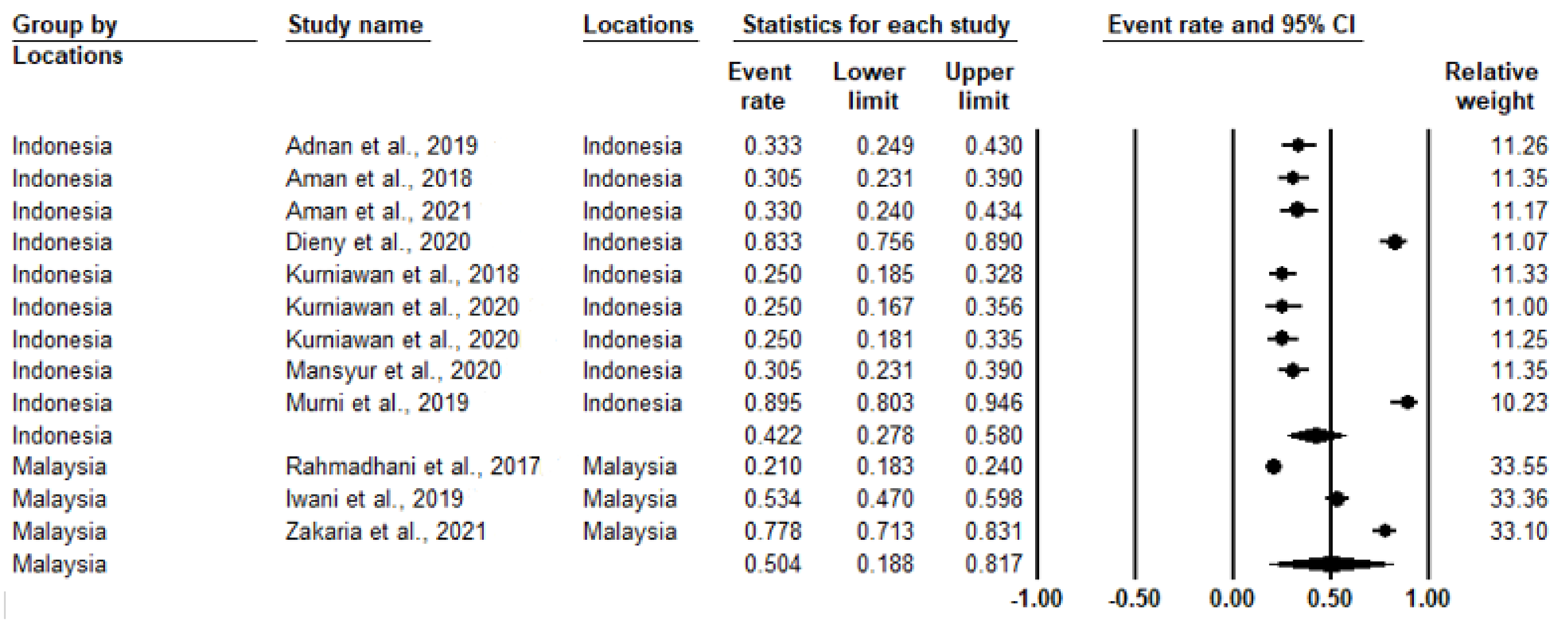
3.2. Study Heterogeneity and Prevalence of IR
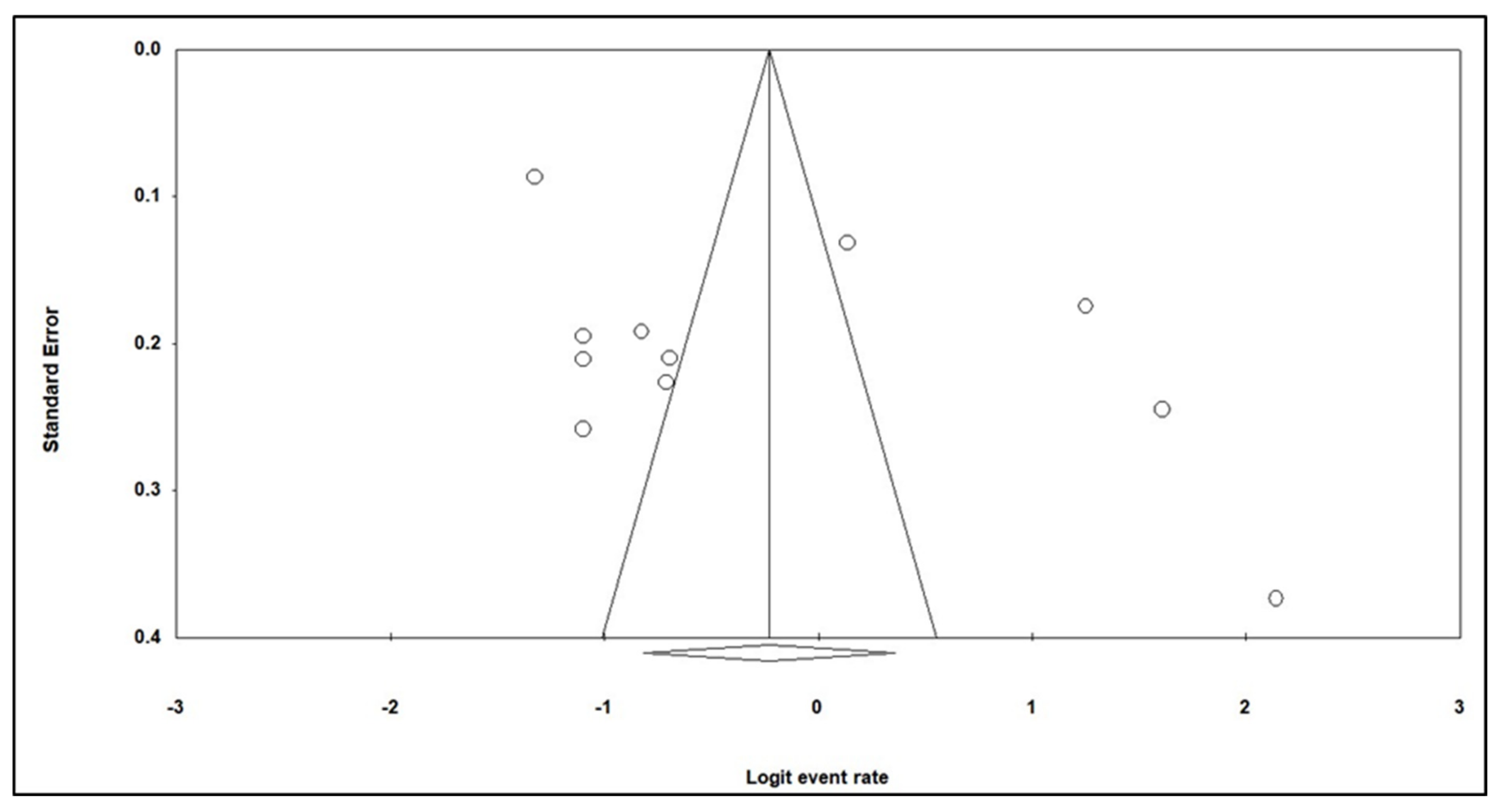
3.3. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yiengprugsawan, V.; Healy, J.; Kendig, H. Health Systems Responses to Population Ageing and Noncommunicable Diseases in Asia; Comparative Country Studies; World Health Organization: New Delhi, India, 2016; Volume 2, p. 2. Available online: https://apps.who.int/iris/handle/10665/252738?mode=simple (accessed on 18 July 2021).
- World Health Organization (WHO). World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/rest/bitstreams/835463/retrieve (accessed on 18 July 2021).
- World Health Organization (WHO). Noncommunicable Disease; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 18 July 2021).
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Dev. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthaei, S.; Greten, H. Evidence that metformin ameliorates cellular insulin-resistance by potentiating insulin-induced translocation of glucose transporters to the plasma membrane. Diabete Metab. 1991, 17, 150–158. [Google Scholar]
- Sun, J.; Wang, Y.; Zhang, X.; He, H. The effects of metformin on insulin resistance in overweight or obese children and adolescents: A PRISMA—Compliant systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e14249. [Google Scholar] [CrossRef]
- Bermudez, V.; Salazar, J.; Martínez, M.S.; Chávez-Castillo, M.; Olivar, L.C.; Calvo, M.J.; Palmar, J.; Bautista, J.; Ramos, E.; Cabrera, M.; et al. Prevalence and Associated Factors of Insulin Resistance in Adults from Maracaibo City, Venezuela. Adv. Prev. Med. 2016, 2016, 9405105. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, N.; Thuesen, B.; Jørgensen, T.; Juul, A.; Spielhagen, C.; Wallaschofksi, H.; Linneberg, A. The Association Between IGF-I and Insulin Resistance. Diabetes Care 2012, 35, 768–773. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.-Q.; Li, Q.; Rentfro, A.R.; Fisher-Hoch, S.P.; McCormick, J.B. The Definition of Insulin Resistance Using HOMA-IR for Americans of Mexican Descent Using Machine Learning. PLoS ONE 2011, 6, e21041. [Google Scholar] [CrossRef] [Green Version]
- Penno, G.; For the Renal Insufficiency and Cardiovascular Events (RIACE) Study Group; Solini, A.; Orsi, E.; Bonora, E.; Fondelli, C.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Zerbini, G.; et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: A prospective cohort study. BMC Med. 2021, 19, 66. [Google Scholar] [CrossRef]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a Major Antioxidant: When, what for and Why It Fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H.R. Comprehensive meta-analysis version 2. In Biostat; Engelwood: Broomall, PA, USA, 2005. [Google Scholar]
- Adnan, E.; Rahman, I.A.; Faridin, H. Relationship between insulin resistance, metabolic syndrome components and serum uric acid. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2158–2162. [Google Scholar] [CrossRef]
- Aman, A.M.; Rasyid, H.; Bakri, S.; Patellongi, I.J. The Association Between Parents History of Type 2 Diabetes with Metabolic Syndrome Component and Insulin Resistance in Non-Diabetic Young Adult Male. Acta Medica Indones. 2018, 50, 309–313. [Google Scholar]
- Aman, M.; Resnawita, D.; Rasyid, H.; Kasim, H.; Bakri, S.; Umar, H.; Daud, N.A.; Seweng, A. The concordance of triglyceride glucose index (TyG index) and homeostatic model assessment for insulin resistance (Homa-IR) in non-diabetic subjects of adult Indonesian males. Clin. Epidemiol. Glob. Health 2020, 9, 227–230. [Google Scholar] [CrossRef]
- Dieny, F.F.; Tsani, A.F.A.; Setyaningsih, R.F.; Fitranti, D.Y.; Jauharany, F.F.; Putra, Y.D. Abdominal Diameter Profiles have Relationship with Insulin Resistance in Obese Female Adolescents. Electron. J. Gen. Med. 2020, 17, 219. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, L.B.; Bahrun, U.; Hatta, M.; Arif, M. Body Mass, Total Body Fat Percentage, and Visceral Fat Level Predict Insulin Resistance Better Than Waist Circumference and Body Mass Index in Healthy Young Male Adults in Indonesia. J. Clin. Med. 2018, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, L.B.; Syamsir, B.; Rahman, I.A.; Adnan, E.; Esa, T.; Widaningsih, Y.; Bahrun, U.; Arif, M. Anthropometric features in predicting insulin resistance among non-menopausal Indonesian adult females. Romanian J. Intern. Med. 2020, 58, 168–172. [Google Scholar] [CrossRef]
- Kurniawan, L.B.; Adnan, E.; Windarwati; Mulyono, B. Insulin resistance and testosterone level in Indonesian young adult males. Romanian J. Intern. Med. 2020, 58, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Mansyur, M.A.; Bakri, S.; Patellongi, I.J.; Rahman, I.A. The association between metabolic syndrome components, low-grade systemic inflammation and insulin resistance in non-diabetic Indonesian adolescent male. Clin. Nutr. ESPEN 2020, 35, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murni, I.K.; Sulistyoningrum, D.C.; Susilowati, R.; Julia, M. Risk of metabolic syndrome and early vascular markers for atherosclerosis in obese Indonesian adolescents. Paediatr. Int. Child Health 2019, 40, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Rahmadhani, R.; Zaharan, N.L.; Mohamed, Z.; Moy, F.M.; Jalaludin, M.Y. The associations between VDR BsmI polymorphisms and risk of vitamin D deficiency, obesity and insulin resistance in adolescents residing in a tropical country. PLoS ONE 2017, 12, e0178695. [Google Scholar] [CrossRef] [Green Version]
- Iwani, A.K.N.Z.; Jalaludin, M.Y.; Zin, R.M.W.M.; Fuziah, Z.; Hong, J.Y.H.; Abqariyah, Y.; Mokhtar, A.H.; Mohamud, W.N.W. TG: HDL-C Ratio Is a Good Marker to Identify Children Affected by Obesity with Increased Cardiometabolic Risk and Insulin Resistance. Int. J. Endocrinol. 2019, 2019, 8586167. [Google Scholar] [CrossRef]
- Zakaria, W.; Yunus, N.M.; Yaacob, N.; Omar, J.; Mohamed, W.W.; Sirajudeen, K.; Ismail, T.T. Association between Vitamin D Receptor Polymorphisms (BsmI and FokI) and Glycemic Control among Patients with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 1595. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- Freeman, A.M.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Sacks, N.; Liu, Y.; Sanyal, A.; DeFronzo, R.; Bhatt, D.; Caplan, J.; Rajagopalan, H.; Cyr, P.; Jena, A. The Economic Burden of Insulin Resistance, Obesity, and Cardiovascular Disease in Medicare Beneficiaries 65 Years of Age and Older. Circulation 2018, 136, A15099. [Google Scholar]
- American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care 2018, 41, 917–928. [CrossRef] [Green Version]
- Wang, C.-C.; Lee, W.-C. Evaluation of the Normality Assumption in Meta-Analyses. Am. J. Epidemiol. 2019, 189, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V.; Carlomagno, G.; Fazio, V.; Fazio, S. Insulin resistance: Is it time for primary prevention? World J. Cardiol. 2012, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.A.; Bremer, A.A. Insulin Resistance and Metabolic Syndrome in the Pediatric Population. Metab. Syndr. Relat. Disord. 2010, 8, 1–14. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2014, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]


| Study (References) | Method of Defining Insulin Resistance | Country | Events 1 | Total 2 |
|---|---|---|---|---|
| Adnan et al., 2019 [18] | HOMA-IR | Indonesia | 34 | 102 |
| Aman et al., 2018 [19] | HOMA-IR | Indonesia | 39 | 128 |
| Aman et al., 2021 [20] | HOMA-IR | Indonesia | 29 | 88 |
| Dieny et al., 2020 [21] | HOMA-IR | Indonesia | 100 | 120 |
| Kurniawan et al. 2018 [22] | HOMA-IR | Indonesia | 35 | 140 |
| Kurniawan et al., 2020a [23] | HOMA-IR | Indonesia | 20 | 80 |
| Kurniawan et al., 2020b [24] | HOMA-IR | Indonesia | 30 | 120 |
| Mansyur et al., 2020 [25] | HOMA-IR | Indonesia | 39 | 128 |
| Murni et al., 2019 [26] | HOMA-IR | Indonesia | 68 | 76 |
| Rahmadhani et al., 2017 [27] | HOMA-IR | Malaysia | 167 | 795 |
| Iwani et al., 2019 [28] | HOMA-IR | Malaysia | 124 | 232 |
| Zakaria et al., 2021 [29] | HOMA-IR | Malaysia | 147 | 189 |
| Total | 832 | 2198 |
| Heterogeneity | Prevalence Rate (95% CI) | Sample Size (n) | Number of Studies | Subgroups | |
|---|---|---|---|---|---|
| I2 (%) | p-Value | ||||
| 97.0 | <0.001 | 0.443 (0.306–0.589) | 2198 | 12 | Overall |
| 94.80 | <0.001 | 0.422 (0.278–0.580) | 982 | 9 | Indonesia |
| 99.06 | <0.001 | 0.504 (0.188–0.817) | 1216 | 3 | Malaysia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, L.P.W.; Sani, S.A.; Sabullah, M.K.; Gansau, J.A. The Prevalence of Insulin Resistance in Malaysia and Indonesia: An Updated Systematic Review and Meta-Analysis. Medicina 2022, 58, 826. https://doi.org/10.3390/medicina58060826
Goh LPW, Sani SA, Sabullah MK, Gansau JA. The Prevalence of Insulin Resistance in Malaysia and Indonesia: An Updated Systematic Review and Meta-Analysis. Medicina. 2022; 58(6):826. https://doi.org/10.3390/medicina58060826
Chicago/Turabian StyleGoh, Lucky Poh Wah, Suraya Abdul Sani, Mohd Khalizan Sabullah, and Jualang Azlan Gansau. 2022. "The Prevalence of Insulin Resistance in Malaysia and Indonesia: An Updated Systematic Review and Meta-Analysis" Medicina 58, no. 6: 826. https://doi.org/10.3390/medicina58060826
APA StyleGoh, L. P. W., Sani, S. A., Sabullah, M. K., & Gansau, J. A. (2022). The Prevalence of Insulin Resistance in Malaysia and Indonesia: An Updated Systematic Review and Meta-Analysis. Medicina, 58(6), 826. https://doi.org/10.3390/medicina58060826






