Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Investigation
2.1.1. Clinical Evaluation of Antibacterial Effects
2.1.2. Assessment of Efficacy
2.1.3. Assessment of Tolerability and Safety
2.2. Experimental Study
2.2.1. Bacteria and Biofilm Chips
2.2.2. Evaluation of Antibacterial Effects
2.2.3. Effects of SSD on Mice Wounds
2.3. Statistical Analysis
3. Results
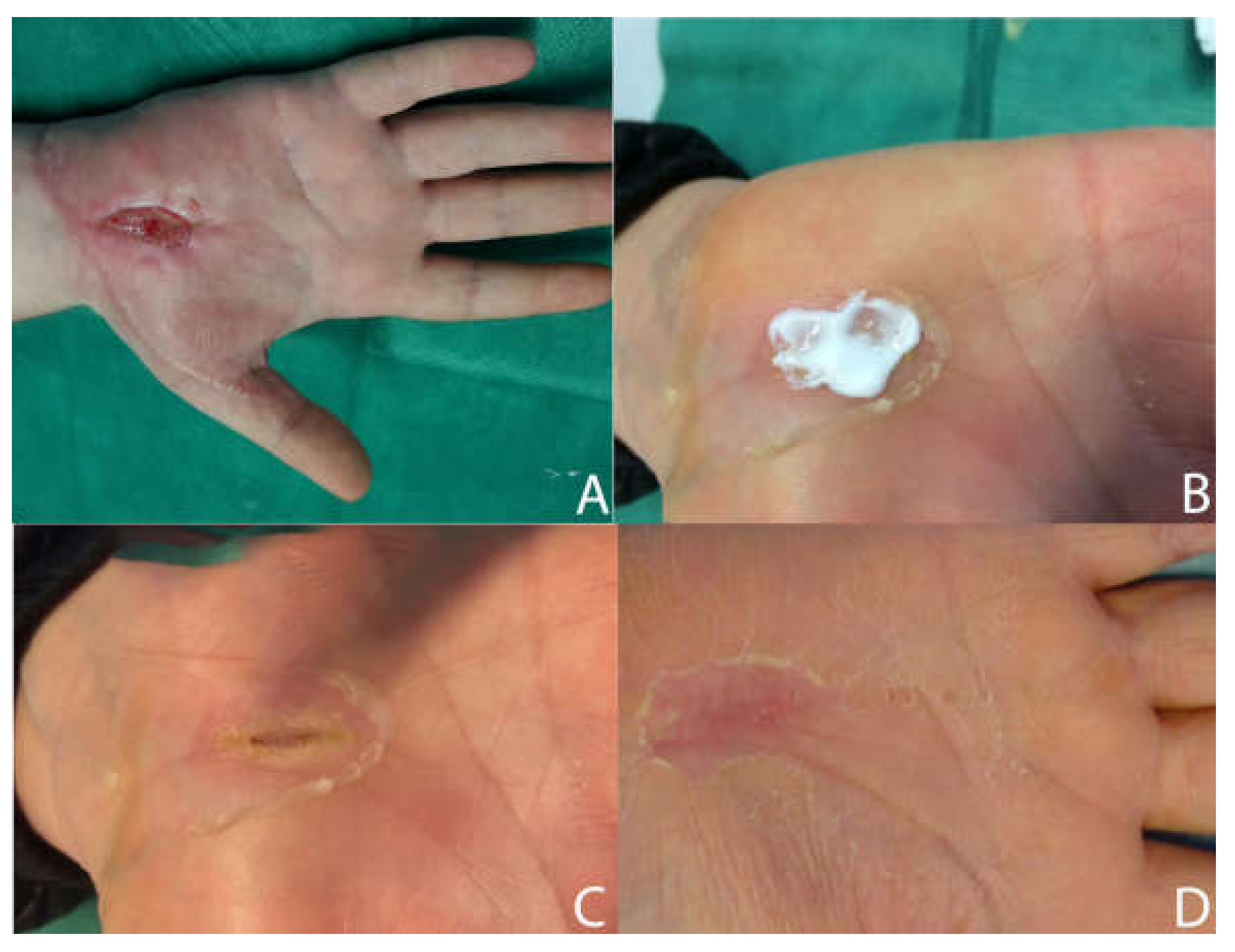
3.1. Clinical Study of Ulcers Treated with SSD + HA
3.2. Experimental Study
3.2.1. Bactericidal Effects of Vancomycin and SSD
3.2.2. SSD Effects on Wound Infection in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dow, G.; Browne, A.; Sibbald, R.G. Infection in chronic wounds: Controversies in diagnosis and treatment. Ostomy Wound Manag. 1999, 45, 23–27. [Google Scholar]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef]
- Fletcher, J. Wound bed preparation and the TIME principles. Nurs. Stand. 2005, 20, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Harries, R.L.; Bosanquet, D.C.; Harding, K.G. Wound bed preparation: TIME for an update. Int. Wound J. 2016, 13 (Suppl. 3), 8–14. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, C. Using the TIME framework in wound bed preparation. Br. J. Community Nurs. 2008, 13, S15, S16, S18, S20 passim. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Goodman, L.; Woo, K.Y.; Krasner, D.L.; Smart, H.; Tariq, G.; Ayello, E.A.; Burrell, R.E.; Keast, D.H.; Mayer, D.; et al. Special considerations in wound bed preparation 2011: An update. Adv. Skin Wound Care 2011, 24, 415–436. [Google Scholar] [CrossRef]
- De Francesco, F.; Marchesini, A.; Campodonico, A.; Neuendorf, A.D.; Pangrazi, P.P.; Riccio, M. A multistep iter for functional reconstruction in mangled upper limb: A retrospective analysis of integrated surgical and medical approach. Medicina 2020, 56, 398. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef] [Green Version]
- De Prost, N.; Lipman, J.; Mimoz, O. Therapeutic targets in necrotizing soft tissue infections. Intensiv. Care Med. 2017, 43, 1717–1719. [Google Scholar] [CrossRef] [Green Version]
- Maslova, E.; Eisaiankhongi, L.; Sjoberg, F.; McCarthy, R.R. Burns and biofilms: Priority pathogens and in vivo models. NPJ Biofilms Microbiomes 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Chari, P.S.; Singh, M.; Singh, G.; Biswal, M.; Sharma, M. Evolution of bacterial flora in burn wounds: Key role of environmental disinfection in control of infection. Int. J. Burn. Trauma 2013, 3, 102–107. [Google Scholar]
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326. [Google Scholar] [CrossRef] [PubMed]
- Jneid, J.; Lavigne, J.P.; La Scola, B.; Cassir, N. The diabetic foot microbiota: A review. Hum. Microbiome J. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Hill, K.E.; Davies, C.E.; Wilson, M.J.; Stephens, P.; Harding, K.G.; Thomas, D.W. Molecular analysis of the microflora in chronic venous leg ulceration. J. Med. Microbiol. 2003, 52, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the third millenium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Riccio, M.; Marchesini, A.; Senesi, L.; Skrami, E.; Gesuita, R.; De Francesco, F. Managing pathological scars by injecting auto-cross-linked hyaluronic acid: A preliminary prospective clinical study. Aesthetic Plast. Surg. 2019, 43, 480–489. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Clement, D.; Ajayan, P.; Mousa, S.; Linhardt, R.J. Hyaluronan- and heparin-reduced silver nanoparticles with antimicrobial properties. Nanomedicine 2009, 4, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Xie, Z.; Hu, J.; Liu, Y. Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing application. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112319. [Google Scholar] [CrossRef]
- Chang, S.M. The Agency for Healthcare Research and Quality (AHRQ) effective health care (EHC) program methods guide for comparative effectiveness reviews: Keeping up-to-date in a rapidly evolving field. J. Clin. Epidemiol. 2011, 64, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.S.; Lindberg, R.B.; Mason, A.D., Jr.; Pruitt, B.A., Jr. The quantitative swab culture and smear: A quick, simple method for determining the number of viable aerobic bacteria on open wounds. J. Trauma 1976, 16, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.K.R.; Chong, S.S.; Othman, A.M. Validation of Harikrishna Periwound Skin Classification for wound assessment. J. Wound Care 2020, 29, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Miyazaki, M.; Mashima, K.; Takagi, S.; Hara, S.; Kamimura, H.; Jimi, S. The Effects of Silver Sulfadiazine on Methicillin-Resistant Staphylococcus aureus Biofilms. Microorganisms 2020, 8, 1551. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Jimi, S.; Oyama, T.; Nakano, Y.; Hamamoto, K.; Mamishin, K.; Yahiro, T.; Hara, S.; Takata, T.; Ohjimi, H. Infection mechanism of biofilm-forming Staphylococcus aureus on indwelling foreign materials in mice. Int. Wound J. 2015, 12, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Y.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef]
- Onesti, M.; Fioramonti, P.; Carella, S.; Fino, P.; Sorvillo, V.; Scuderi, N. A new association between hyaluronic acid and collagenase in wound repair: An open study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 210–216. [Google Scholar]
- Maytin, E.V. Hyaluronan: More than just a wrinkle filler. Glycobiology 2016, 26, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Roehrs, H.; Stocco, J.G.D.; Pott, F.; Blanc, G.; Crozeta, K.; Meier, M.J.; Dias, F.A.L. Dressings and topical agents containing hyaluronic acid for chronic wound. Cochrane Syst. Rev. 2016, CD012215. [Google Scholar] [CrossRef]
- Hunter, S.; Langemo, D.; Thompson, P.; Hanson, D.; Anderson, J.; Oh, I.E.; Paulson, R.; Rustvang, D.; Dorman, S.; Roth, D.L. Observations of periwound skin protection in venous ulcers: A comparison of treatments. Adv. Skin Wound Care 2013, 26, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J. Protecting the integrity of the periwound skin. Wound Essent. 2012, 1, 58–64. [Google Scholar]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. Functions of hyaluronan. Ann. Rheum. Dis. 1995, 54, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Price, R.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. J. Cosmet. Laser Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef]
- Akila, S.; Nanda, A. In-vivo wound healing activity of silver nanoparticles: An investigation. Int. J. Sci. Res. 2014, 3, 1208–1212. [Google Scholar]
- Dai, T.; Huang, Y.Y.; Sharma, S.K.; Hashmi, J.T.; Kurup, D.B.; Hamblin, M.R. Topical antimicrobials for burn wound infections. Recent Pat. Antiinfect. Drug Discov. 2010, 5, 124–151. [Google Scholar] [CrossRef] [Green Version]
- Marx, D.E.; Barillo, D.J. Silver in medicine: The basic science. Burns 2014, 40 (Suppl. 1), S9–S18. [Google Scholar] [CrossRef]
- Russell, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar]
- Jimi, S.; Miyazaki, M.; Takata, T.; Ohjimi, H.; Akita, S.; Hara, S. Increased drug resistance of meticillin-reistant Staphylococcus aureus biofilms formed on a mouse dermal chip model. J. Med. Microbiol. 2017, 66, 542–550. [Google Scholar] [CrossRef] [PubMed]







| Characteristic | Value |
|---|---|
| Age [mean ± SD] | 67 ± 15 |
| Gender [n (%)] | |
| Male | 56 (70) |
| Female | 24 (30) |
| Weight (Kg) [mean ± SD] | 62.5 ± 14.5 |
| Height (cm) [mean ± SD] | 166 ± 4 |
| Nutritional status [n] | |
| Good | 62 |
| Correct | 16 |
| Bad | 2 |
| Associated pathologies | |
| Respiratory system | 6 |
| Cardiovascular system | 22 |
| Cancer | 10 |
| Trauma (fractures) | 60 |
| Diabetes mellitus | 10 |
| Neurological disorders | 5 |
| Smoker | 21 |
| Ulcer Etiologies | |
| Diabetes | 10 |
| Post-traumatic ulcers | 45 |
| I- or II-degree Burns | 15 |
| Superficial abrasion | 10 |
| Duration [n (%)] | |
| <1 month | 10 (12.5) |
| 1–2 months | 40 (50) |
| 2–5 months | 20 (25) |
| 5–12 months | 10 (12.5) |
| Location [n (%)] | |
| Heel | 5 (6.25) |
| Sacrum | 5 (6.25) |
| Tibia | 10 (12.5) |
| Malleolus | 25 (31.25) |
| Thigh | 2 (2.5) |
| Finger | 13 (16.25) |
| Hand | 18 (22.5) |
| Forearm | 2 (2.5) |
| Chronic Ulcer Characteristic | Value |
|---|---|
| Etiologies | |
| Diabetes | 10 |
| Post-traumatic ulcers | 45 |
| Burns | 15 |
| Superficial abrasion | 10 |
| Duration [n (%)] | |
| <1 month | 10 (12.5) |
| 1–2 months | 40 (50) |
| 2–5 months | 20 (25) |
| 5–12 months | 10 (12.5) |
| Location [n (%)] | |
| Heel | 5 (6.25) |
| Sacrum | 5 (6.25) |
| Tibia | 10 (12.5) |
| Malleolus | 25 (31.25) |
| Thigh | 2 (2.5) |
| Finger | 13 (16.25) |
| Hand | 18 (22.5) |
| Forearm | 2 (2.5) |
| Granulation Tissue (%) [mean ± SD] | 43 ± 8.6 |
| Estimated surface area (cm2) [median (range)] | 7.45 (3–45) |
| Estimated depth (cm) [median (range)] | 0.35 (0–0.5) |
| Appearance of surrounding skin [n (%)] | |
| Inflammation | |
| Nil | 0 (0) |
| Slight | 29 (36.25) |
| Moderate | 51 (63.75) |
| Oedema | |
| Nil | 26 (32.5) |
| Slight | 34 (42.5) |
| Moderate | 20 (25) |
| Purpura | |
| Nil | 0 (0) |
| Slight | 15 (18.75) |
| Moderate | 65 (81.25) |
| Erythema | |
| Nil | 0 (0) |
| Slight | 57 (71.25) |
| Moderate | 23 (28.75) |
| Harikrishna Periwound Skin (HPS) classification [n (%)] | |
| Class 0 | 0 (0) |
| Class 1 | 0 (0) |
| Class 2a | 0 (0) |
| Class 2b | 0 (0) |
| Class 2c | 0 (0) |
| Class 3 | 0 (0) |
| Class 4 | 80 (100) |
| Class 5 | 0 (0) |
| Pain (10 mm VAS) [mean ± SD] | 4.5 ± 3.5 |
| State | MIC | MBC | MBEC | MBC/MIC | MBEC/MIC | |
|---|---|---|---|---|---|---|
| Vancomycin (μg/mL) | Planktonic | 1.56 | 1.56 | NA | 1 | NA |
| Biofilm | 1.56 | 50 | 50 | 32 | 32 | |
| SSD (μg/mL) | Planktonic | 125 | 250 | NA | 2 | NA |
| Biofilm | 125 | 500 | 1000 | 4 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francesco, F.; Riccio, M.; Jimi, S. Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm. Medicina 2022, 58, 835. https://doi.org/10.3390/medicina58060835
De Francesco F, Riccio M, Jimi S. Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm. Medicina. 2022; 58(6):835. https://doi.org/10.3390/medicina58060835
Chicago/Turabian StyleDe Francesco, Francesco, Michele Riccio, and Shiro Jimi. 2022. "Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm" Medicina 58, no. 6: 835. https://doi.org/10.3390/medicina58060835
APA StyleDe Francesco, F., Riccio, M., & Jimi, S. (2022). Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm. Medicina, 58(6), 835. https://doi.org/10.3390/medicina58060835









