Factors Affecting Postoperative Lung Functions in Patients Undergoing Lobectomy for Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
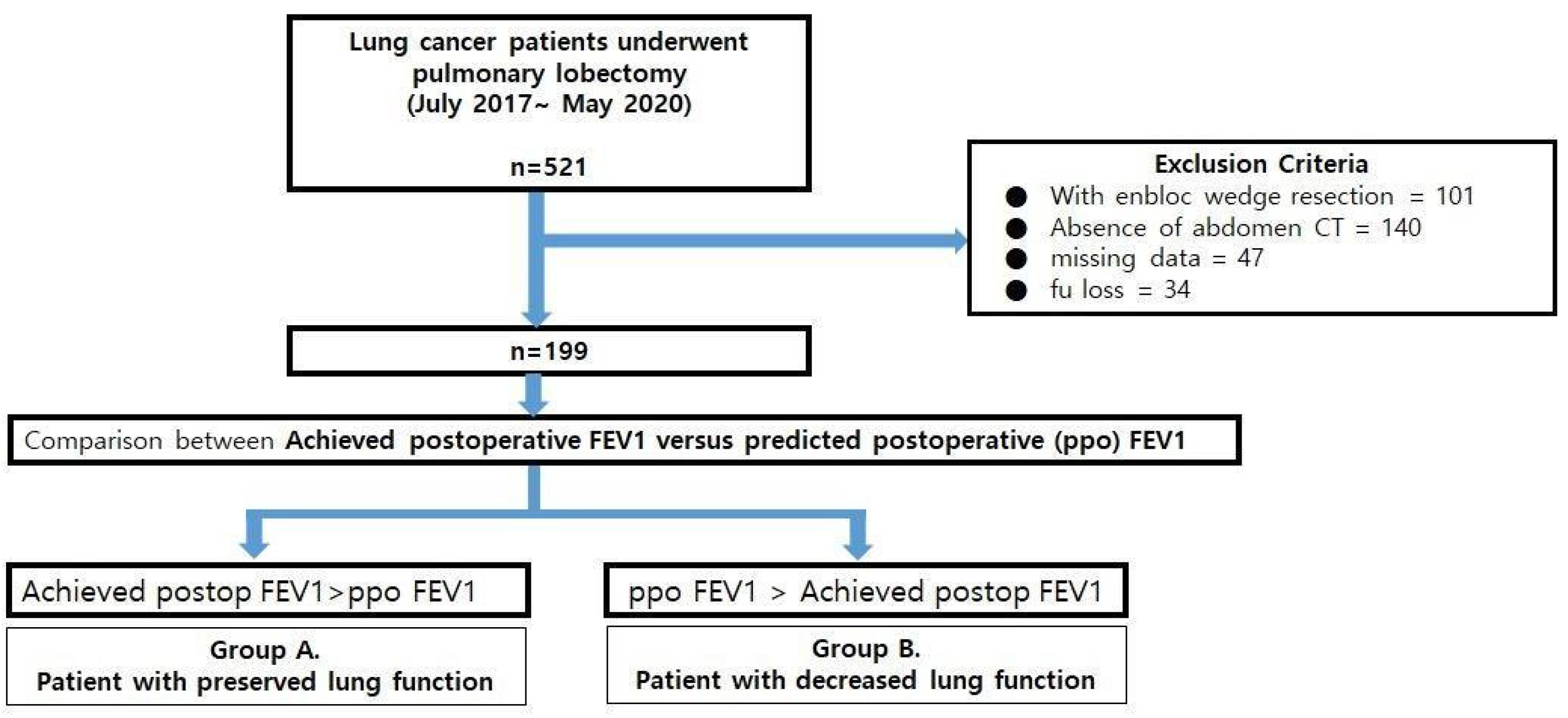
2.2. Patients
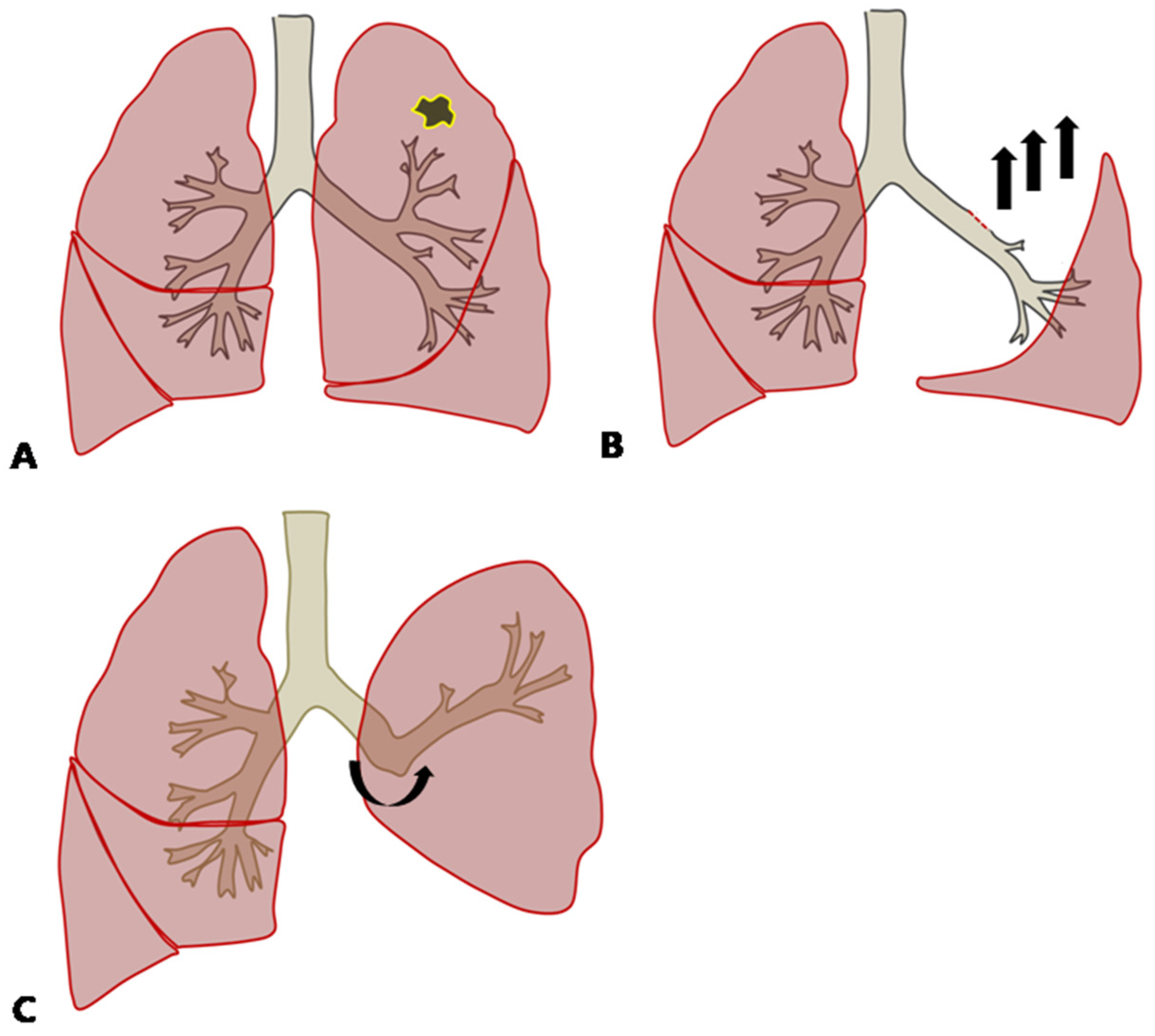
2.3. Comparison between Predicted Postoperative Lung Function after Lobectomy(ppo FEV1%) and Measured Lung Function after Lobectomy (Achieved Postoperative FEV1%: apoFEV1%)
2.4. Statistical Analysis
3. Results
3.1. Baseline Patients’ Characteristics for Group A and Group B
3.2. Comparison of Perioperative Factors between Groups A and B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, E.; Baldwin, D.; Beckles, M.; Duffy, J.; Entwisle, J.; Faivre-Finn, C.; Kerr, K.; Macfie, A.; McGuigan, J.; Padley, S.; et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010, 65, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Balduyck, B.; Hendriks, J.; Lauwers, P.; Van Schil, P. Quality of Life after Lung Cancer Surgery: A Prospective Pilot Study comparing Bronchial Sleeve Lobectomy with Pneumonectomy. J. Thorac. Oncol. 2008, 3, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, Y.; Takeda, S.-I.; Sawabata, N.; Okumura, Y.; Maeda, H. Long-Term Pulmonary Function after Lobectomy for Primary Lung Cancer. Asian Cardiovasc. Thorac. Ann. 2005, 13, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic Evaluation of the Patient With Lung Cancer Being Considered for Resectional Surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e166S–e190S. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tagayun, A.; Bogardus, A.; Qian, D.; Tiep, B.; Horak, D.; Grannis, F.W., Jr. How Accurately Can We Predict Forced Expiratory Volume in One Second after Major Pulmonary Resection? Am. Surgeon 2007, 73, 1047–1051. [Google Scholar] [CrossRef]
- Courtney, M.; Townsend, R., Jr.; Daniel Beauchamp, B.; Evers, M.; Kenneth, L.M. Sabiston Textbook of Surgery, 20th ed.; Elsevier—Health Sciences Division: Amsterdam, The Netherlands, 2016; p. 42. [Google Scholar]
- Duk, H.M.; Chul, H.P.; Joon, H.J.; Tae, H.K.; Seok, J.H.; Sungsoo, L. Inferior Pulmonary Ligament Division May Be Unnecessary during Left Upper Lobectomy: Effects on Lung Volume, Bronchial Angle and Bronchial Tortuosity. J. Clin. Med. 2021, 10, 4033. [Google Scholar]
- Yamada, Y.; Yamada, M.; Chubachi, S.; Yokoyama, Y.; Matsuoka, S.; Tanabe, A.; Niijima, Y.; Murata, M.; Fukunaga, K.; Jinzaki, M. Comparison of inspiratory and expiratory lung and lobe volumes among supine, standing, and sitting positions using conventional and upright CT. Sci. Rep. 2020, 10, 16203. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Croci, M.; Ravagnan, I.; Tredici, S.; Pedoto, A.; Lissoni, A.; Gattinoni, L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth. Analg. 1998, 87, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Boriek, A.M.; Lopez, M.A.; Velasco, C.; Bakir, A.A.; Frolov, A.; Wynd, S.; Babb, T.G.; Hanania, N.A.; Hoffman, E.A.; Sharafkhaneh, A. Obesity modulates diaphragm curvature in subjects with and without COPD. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R620–R629. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Su, J.; Qiu, P.; Wang, M.; Zhou, K.; Tang, Y.; Che, G. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: A randomized trial. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Antonio, B.; Alfredo, C.; Luca, A.; Gian, L.P.; Eveline, I.; Paolo, C.; Michele, R.; Dario, O. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2008, 33, 95–98. [Google Scholar]
| Characteristics | Overall (n = 199) | Group A. (n = 127) | Group B. (n = 72) | p Value | |
|---|---|---|---|---|---|
| Age (mean (SD)) | 67.26 (7.12) | 67.28 (7.20) | 67.21 (7.04) | 0.943 | |
| Sex | Male | 126 (63.3) | 86 (67.7) | 40 (55.6) | 0.119 |
| Female | 73 (36.7) | 41 (32.3) | 32 (44.4) | ||
| Smoking habit | Never | 93 (46.7) | 53 (41.7) | 40 (55.6) | 0.170 |
| Ex smoker | 82 (41.2) | 57 (44.9) | 25 (34.7) | ||
| current | 24 (12.1) | 17 (13.4) | 7 (9.7) | ||
| clinical stage(%) | IA1 | 23 (11.6) | 13 (10.2) | 10 (13.9) | 0.291 |
| IA2 | 32 (16.1) | 18 (14.2) | 14 (19.4) | ||
| IA3 | 36 (18.1) | 28 (22.0) | 8 (11.1) | ||
| IB | 43 (21.6) | 24 (18.9) | 19 (26.4) | ||
| IIA | 20 (10.1) | 15 (11.8) | 5 (6.9) | ||
| IIB | 20 (10.1) | 11 (8.7) | 9 (12.5) | ||
| IIIA | 23 (11.6) | 17 (13.4) | 6 (8.3) | ||
| IIIB | 2 (1.0) | 1 (0.8) | 1 (1.4) | ||
| Comorbidity | COPD(%) | 13 (6.5) | 9 (7.1) | 4 (5.6) | 0.903 |
| CAOD(%) | 8 (4.0) | 6 (4.7) | 2 (2.8) | 0.767 | |
| CVS(%) | 28 (14.1) | 18 (14.2) | 10 (13.9) | 1.000 | |
| CHF(%) | 1 (0.5) | 1 (0.8) | 0 (0.0) | 1.000 | |
| DM(%) | 40 (20.1) | 28 (22.0) | 12 (16.7) | 0.468 | |
| CRF(%) | 6 (3.0) | 5 (3.9) | 1 (1.4) | 0.563 | |
| Patient Factors | Overall (n = 199) | Group A. (n = 127) | Group B. (n = 72) | p Value | |
|---|---|---|---|---|---|
| Resected location (%) | RUL | 52 (26.3) | 27 (21.4) | 25 (34.7) | 0.002 |
| RML | 10 (5.1) | 7 (5.6) | 3 (4.2) | ||
| RLL | 53 (26.8) | 40 (31.7) | 13 (18.1) | ||
| LUL | 48 (24.2) | 23 (18.3) | 25 (34.7) | ||
| LLL | 35 (17.7) | 29 (23.0) | 6 (8.3) | ||
| BMI (%) | underweight | 4 (2.0) | 1 (0.8) | 3 (4.2) | 0.012 |
| normal | 120 (60.3) | 86 (67.7) | 34 (47.2) | ||
| overweight | 70 (35.2) | 36 (28.3) | 34 (47.2) | ||
| obese | 5 (2.5) | 4 (3.1) | 1 (1.4) | ||
| Total muscle area (mean (SD)) | 119.34 (26.02) | 121.47 (27.03) | 115.06 (23.87) | 0.320 | |
| muscle mass index (mean (SD)) | 44.45 (9.86) | 45.31 (8.84) | 42.82 (11.61) | 0.363 | |
| Patient Factors | Overall (n = 199) | Group A. (n = 127) | Group B. (n = 72) | p Value | |
|---|---|---|---|---|---|
| performance status (%) | 0 | 192 (96.5) | 122 (96.1) | 70 (97.2) | 0.743 |
| 1 | 6 (3.0) | 4 (3.1) | 2 (2.8) | ||
| 2 | 1 (0.5) | 1 (0.8) | 0 (0.0) | ||
| Approach modality (%) | VATS | 183 (92.0) | 118 (92.9) | 65 (90.3) | 0.700 |
| open | 16 (8.0) | 9 (7.1) | 7 (9.7) | ||
| conversion to open (%) | 1 (0.5) | 1 (0.8) | 0 (0.0) | 1.000 | |
| operative time(mean (SD)) | 3.09 (1.36) | 3.06 (1.16) | 3.14 (1.67) | 0.705 | |
| ICU stay (days(%)) | none | 13 (6.5) | 3 (4.2) | 3 (4.2) | 0.325 |
| 0 ≤ ≤ 1 | 172 (86.4) | 107 (84.3) | 65 (90.3) | ||
| 1 < ≤ 2 | 10 (5.0) | 8 (6.3) | 2 (2.8) | ||
| 2 < ≤ 4 | 1 (0.5) | 0 (0.0) | 1 (1.4) | ||
| 4 < ≤ 5 | 1 (0.5) | 1 (0.8) | 0 (0.0) | ||
| 6 < ≤ 8 | 1 (0.5) | 1 (0.8) | 0 (0.0) | ||
| 9 < ≤ 16 | 1 (0.5) | 0 (0.0) | 1 (1.4) | ||
| Hospital stay (mean (SD)) | 7.66 (5.19) | 7.51 (4.85) | 7.93 (5.76) | 0.603 | |
| postoperative complication | pulmonary | 10 (5.0) | 7 (5.5) | 3 (4.2) | 0.936 |
| cardiovascular | 0 (0) | 0 (0) | 0 (0) | - | |
| infectious | 0 (0) | 0 (0) | 0 (0) | - | |
| other | 4 (2.0) | 2 (1.6) | 2 (2.8) | 0.956 | |
| 30-day mortality | 10 (5.0) | 7 (5.5) | 3 (4.2) | 0.936 | |
|
Overall
(n = 199) |
Group A.
(n = 127) |
Group B.
(n = 72) | Multivariate Analysis | ||
|---|---|---|---|---|---|
| OR [95% CI] | p Value | ||||
| BMI (%) | normal | 86 (67.7) | 34 (47.2) | Ref. | |
| underweight | 1 (0.8) | 3 (4.2) | 9.459 [0.839, 106.693] | 0.069 | |
| overweight | 36 (28.3) | 34 (47.2) | 2.278 [1.185, 4.380] | 0.014 | |
| obese | 4 (3.1) | 1 (1.4) | 0.659 [0.066, 6.568] | 0.722 | |
| Location (%) | LLL | 29 (23.0) | 6 (8.3) | Ref. | |
| RUL | 27 (21.4) | 25 (34.7) | 4.691 [1.613, 13.647] | 0.005 | |
| RML | 7 (5.6) | 3 (4.2) | 2.067 [0.396, 10.784] | 0.389 | |
| RLL | 40 (31.7) | 13 (18.1) | 1.929 [0.629, 5.913] | 0.250 | |
| LUL | 23 (18.3) | 25 (34.7) | 5.871 [1.987, 17.345] | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Ahn, H.-Y.; Park, J.-H.; Cho, J.-S. Factors Affecting Postoperative Lung Functions in Patients Undergoing Lobectomy for Non-Small Cell Lung Cancer. Medicina 2022, 58, 1021. https://doi.org/10.3390/medicina58081021
Lee S-J, Ahn H-Y, Park J-H, Cho J-S. Factors Affecting Postoperative Lung Functions in Patients Undergoing Lobectomy for Non-Small Cell Lung Cancer. Medicina. 2022; 58(8):1021. https://doi.org/10.3390/medicina58081021
Chicago/Turabian StyleLee, Soo-Jin, Hyo-Yeong Ahn, Jong-Hwan Park, and Jeong-Su Cho. 2022. "Factors Affecting Postoperative Lung Functions in Patients Undergoing Lobectomy for Non-Small Cell Lung Cancer" Medicina 58, no. 8: 1021. https://doi.org/10.3390/medicina58081021
APA StyleLee, S.-J., Ahn, H.-Y., Park, J.-H., & Cho, J.-S. (2022). Factors Affecting Postoperative Lung Functions in Patients Undergoing Lobectomy for Non-Small Cell Lung Cancer. Medicina, 58(8), 1021. https://doi.org/10.3390/medicina58081021






