Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD?
Abstract
:1. Introduction
2. Evidence linking COPD to Diabetes
2.1. Epidemiological Evidence Linking COPD to T1D
2.2. Epidemiological Evidence Linking COPD to T2D
2.3. T1D Affects Specific Lung Function Parameters
2.4. T2D Affects Specific Lung Function Parameters
2.5. Metabolic Syndrome in COPD
2.6. Cigarette Smoking in Diabetics
2.7. Alpha-1 Antitrypsin and Diabetes
3. Animal Models of T1D and T2D in COPD
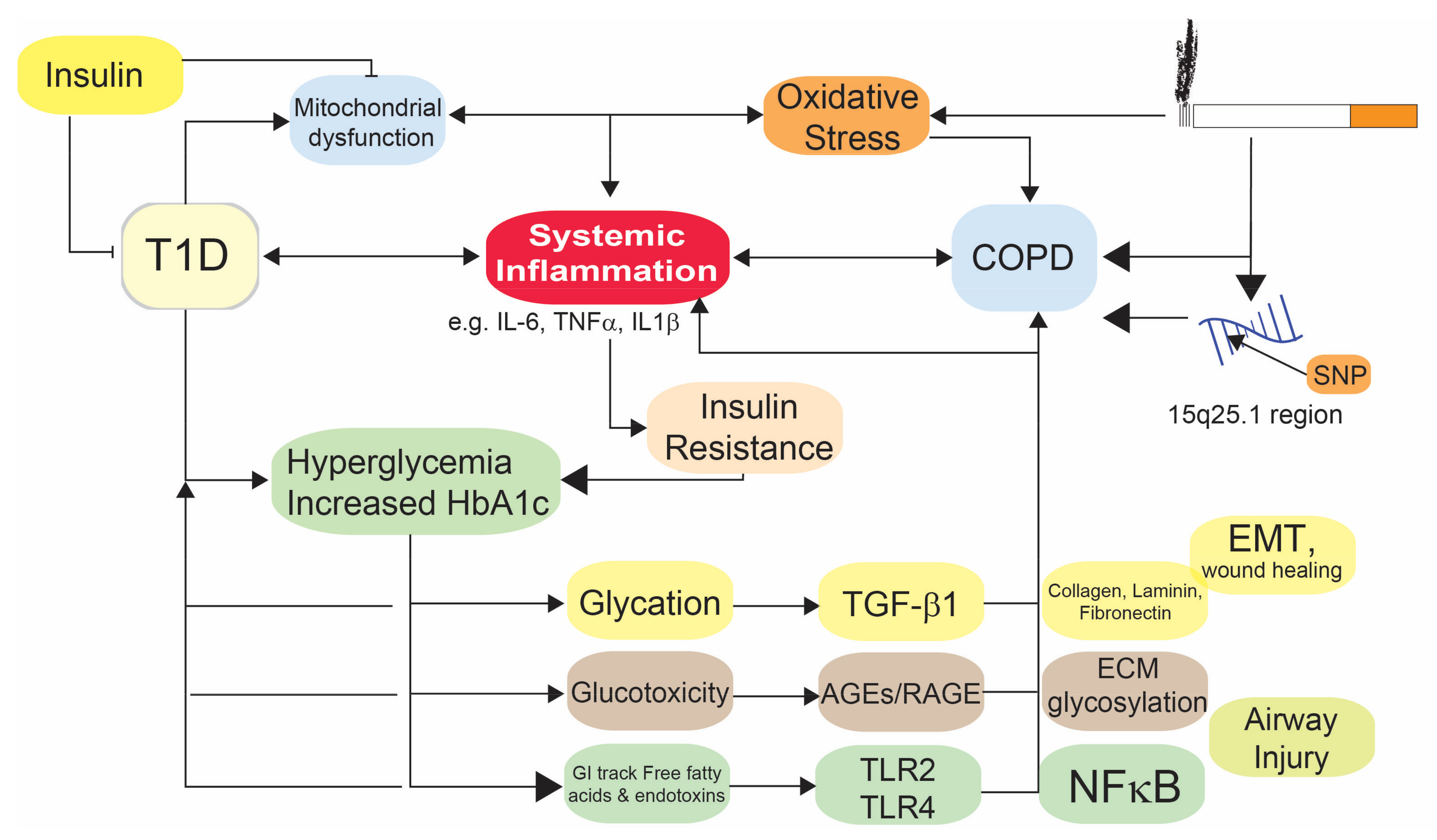
4. Mechanistic Link between COPD and T1D
4.1. Oxidative Stress
4.2. Metabolic Changes in T1D and COPD
4.3. Immune Responses in COPD and T1D
4.4. Impaired Wound Healing
4.5. Glucose and COPD
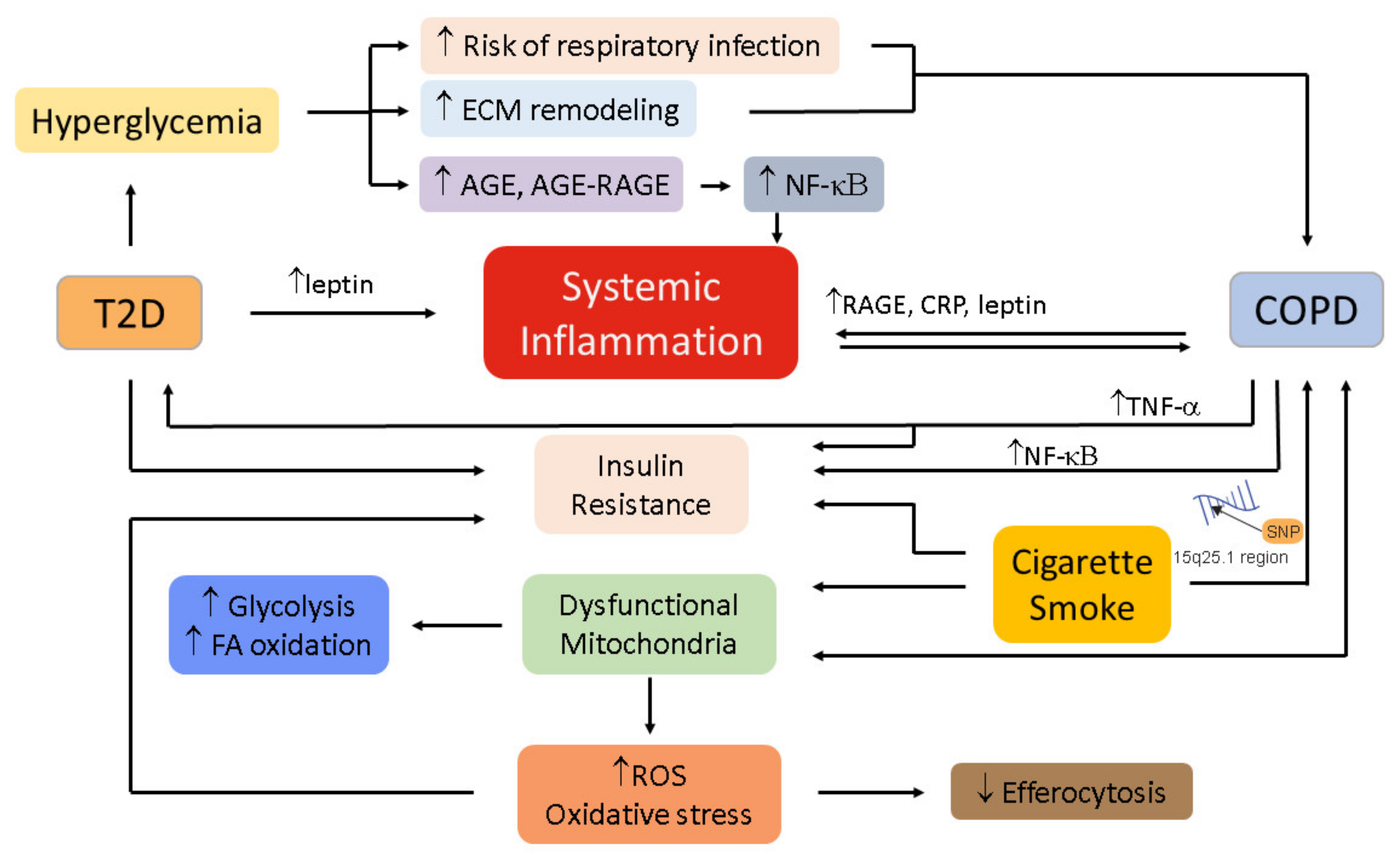
5. Mechanistic Links between COPD and T2D
5.1. Oxidative Stress
5.2. Inflammation
5.3. Immunometabolism in COPD and T2D
5.4. Hyperglycemia and Hyperinsulinemia
5.5. Autonomic Dysregulation
6. Therapy
6.1. Metformin
6.2. Additional Therapeutic Approaches
6.3. Dietary Links and Lung Function
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population health science of sedentary behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Kirsten, A.M.; Holz, O.; Mueller, K.C.; Schaper, M.; Sack, A.L.; Meyer, T.; Rabe, K.F.; Magnussen, H.; Watz, H. Disease Progression and Changes in Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, V.; McBrayer, D.G.; Ramirez, L.C.; Raskin, P.; Hsia, C.C. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am. J. Med. 1997, 103, 504–513. [Google Scholar] [CrossRef]
- Scano, G.; Filippelli, M.; Romagnoli, I.; Mancini, M.; Misuri, G.; Duranti, R.; Rosi, E. Hypoxic and hypercapnic breathlessness in patients with type I diabetes mellitus. Chest 2000, 117, 960–967. [Google Scholar] [CrossRef]
- Cazzola, M.; Bettoncelli, G.; Sessa, E.; Cricelli, C.; Biscione, G. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration 2010, 80, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.W.; Huang, C.T.; Ruan, S.Y.; Tsai, Y.J.; Lai, F.; Yu, C.J. Diabetes mellitus in patients with chronic obstructive pulmonary disease-The impact on mortality. PLoS ONE 2017, 12, e0175794. [Google Scholar] [CrossRef]
- Rana, J.S.; Mittleman, M.A.; Sheikh, J.; Hu, F.B.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Barr, R.G.; Camargo, C.A., Jr. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004, 27, 2478–2484. [Google Scholar] [CrossRef] [PubMed]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Pathak, P.; Gerstein, H.C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke 2001, 32, 2426–2432. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Angus, R.M.; Agarwal, S.; Lane, S.; Calverley, P.M. Hyperglycaemia as a predictor of outcome during non-invasive ventilation in decompensated COPD. Thorax 2009, 64, 857–862. [Google Scholar] [CrossRef]
- Gudmundsson, G.; Ulrik, C.S.; Gislason, T.; Lindberg, E.; Brøndum, E.; Bakke, P.; Janson, C. Long-term survival in patients hospitalized for chronic obstructive pulmonary disease: A prospective observational study in the Nordic countries. Int. J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Edwards, L.D.; Agustí, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; Lomas, D.A.; Miller, B.E.; et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir. Med. 2013, 107, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Sandler, M.; Bunn, A.E.; Stewart, R.I. Cross-section study of pulmonary function in patients with insulin-dependent diabetes mellitus. Am. Rev. Respir. Dis. 1987, 135, 223–229. [Google Scholar] [CrossRef]
- Schuyler, M.R.; Niewoehner, D.E.; Inkley, S.R.; Kohn, R. Abnormal lung elasticity in juvenile diabetes mellitus. Am. Rev. Respir. Dis. 1976, 113, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kuziemski, K.; Słomiński, W.; Jassem, E. Impact of diabetes mellitus on functional exercise capacity and pulmonary functions in patients with diabetes and healthy persons. BMC Endocr. Disord. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.E.; Beiser, A.; Givelber, R.J.; O’Connor, G.T.; Gottlieb, D.J. Association between glycemic state and lung function: The Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2003, 167, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.C.; Punjabi, N.M.; Wang, N.Y.; Pankow, J.S.; Duncan, B.B.; Cox, C.E.; Selvin, E.; Brancati, F.L. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2008, 31, 741–746. [Google Scholar] [CrossRef]
- Kinney, G.L.; Black-Shinn, J.L.; Wan, E.S.; Make, B.; Regan, E.; Lutz, S.; Soler, X.; Silverman, E.K.; Crapo, J.; Hokanson, J.E. Pulmonary function reduction in diabetes with and without chronic obstructive pulmonary disease. Diabetes Care 2014, 37, 389–395. [Google Scholar] [CrossRef]
- Davis, W.A.; Knuiman, M.; Kendall, P.; Grange, V.; Davis, T.M. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: The Fremantle Diabetes Study. Diabetes Care 2004, 27, 752–757. [Google Scholar] [CrossRef]
- Gutiérrez-Carrasquilla, L.; Sánchez, E.; Barbé, F.; Dalmases, M.; López-Cano, C.; Hernández, M.; Rius, F.; Carmona, P.; Hernández, C.; Simó, R.; et al. Effect of Glucose Improvement on Spirometric Maneuvers in Patients With Type 2 Diabetes: The Sweet Breath Study. Diabetes Care 2019, 42, 617–624. [Google Scholar] [CrossRef]
- Ahmadi-Abhari, S.; Kaptoge, S.; Luben, R.N.; Wareham, N.J.; Khaw, K.T. Longitudinal association of C-reactive protein and lung function over 13 years: The EPIC-Norfolk study. Am. J. Epidemiol. 2014, 179, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hancox, R.J.; Poulton, R.; Greene, J.M.; Filsell, S.; McLachlan, C.R.; Rasmussen, F.; Taylor, D.R.; Williams, M.J.; Williamson, A.; Sears, M.R. Systemic inflammation and lung function in young adults. Thorax 2007, 62, 1064–1068. [Google Scholar] [CrossRef]
- Antonelli Incalzi, R.; Fuso, L.; Giordano, A.; Pitocco, D.; Maiolo, C.; Calcagni, M.L.; Ghirlanda, G. Neuroadrenergic denervation of the lung in type I diabetes mellitus complicated by autonomic neuropathy. Chest 2002, 121, 443–451. [Google Scholar] [CrossRef]
- Williams, J.G.; Morris, A.I.; Hayter, R.C.; Ogilvie, C.M. Respiratory responses of diabetics to hypoxia, hypercapnia, and exercise. Thorax 1984, 39, 529–534. [Google Scholar] [CrossRef]
- Bernardi, L.; Bianchi, L. Integrated Cardio-Respiratory Control: Insight in Diabetes. Curr. Diabetes Rep. 2016, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Chance, W.W.; Rhee, C.; Yilmaz, C.; Dane, D.M.; Pruneda, M.L.; Raskin, P.; Hsia, C.C. Diminished alveolar microvascular reserves in type 2 diabetes reflect systemic microangiopathy. Diabetes Care 2008, 31, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Kahnert, K.; Lucke, T.; Huber, R.M.; Behr, J.; Biertz, F.; Vogt, A.; Watz, H.; Alter, P.; Fahndrich, S.; Bals, R.; et al. Relationship of hyperlipidemia to comorbidities and lung function in COPD: Results of the COSYCONET cohort. PLoS ONE 2017, 12, e0177501. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Lam, K.B.; Jordan, R.E.; Jiang, C.Q.; Thomas, G.N.; Miller, M.R.; Zhang, W.S.; Lam, T.H.; Cheng, K.K.; Adab, P. Airflow obstruction and metabolic syndrome: The Guangzhou Biobank Cohort Study. Eur. Respir. J. 2010, 35, 317–323. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Kirsten, A.; Müller, K.C.; Kretschmar, G.; Meyer, T.; Holz, O.; Magnussen, H. The metabolic syndrome in patients with chronic bronchitis and COPD: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009, 136, 1039–1046. [Google Scholar] [CrossRef]
- Vujic, T.; Nagorni, O.; Maric, G.; Popovic, L.; Jankovic, J. Metabolic syndrome in patients with chronic obstructive pulmonary disease: Frequency and relationship with systemic inflammation. Hippokratia 2016, 20, 110–114. [Google Scholar] [PubMed]
- Breyer, M.K.; Spruit, M.A.; Hanson, C.K.; Franssen, F.M.; Vanfleteren, L.E.; Groenen, M.T.; Bruijnzeel, P.L.; Wouters, E.F.; Rutten, E.P. Prevalence of metabolic syndrome in COPD patients and its consequences. PLoS ONE 2014, 9, e98013. [Google Scholar] [CrossRef] [PubMed]
- Piazzolla, G.; Castrovilli, A.; Liotino, V.; Vulpi, M.R.; Fanelli, M.; Mazzocca, A.; Candigliota, M.; Berardi, E.; Resta, O.; Sabbà, C.; et al. Metabolic syndrome and Chronic Obstructive Pulmonary Disease (COPD): The interplay among smoking, insulin resistance and vitamin D. PLoS ONE 2017, 12, e0186708. [Google Scholar] [CrossRef]
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007, 7, 220. [Google Scholar] [CrossRef]
- Díez-Manglano, J.; Barquero-Romero, J.; Almagro, P.; Cabrera, F.J.; López García, F.; Montero, L.; Soriano, J.B. COPD patients with and without metabolic syndrome: Clinical and functional differences. Intern. Emerg. Med. 2014, 9, 419–425. [Google Scholar] [CrossRef]
- Hersh, C.P.; Make, B.J.; Lynch, D.A.; Barr, R.G.; Bowler, R.P.; Calverley, P.M.; Castaldi, P.J.; Cho, M.H.; Coxson, H.O.; DeMeo, D.L.; et al. Non-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitus. BMC Pulm. Med. 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Kazerooni, E.A.; Lynch, D.A.; Liu, L.X.; Murray, S.; Curtis, J.L.; Criner, G.J.; Kim, V.; Bowler, R.P.; Hanania, N.A.; et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: Associated radiologic phenotypes. Radiology 2011, 261, 274–282. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Ducatman, A.M.; Conway, B.N. Increased risk of respiratory diseases in adults with Type 1 and Type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 142, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Rhee, E.J.; Sung, K.C. Metabolic syndrome, insulin resistance and systemic inflammation as risk factors for reduced lung function in Korean nonsmoking males. J. Korean Med. Sci. 2010, 25, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, X.; Li, X.; Lin, Y.; Shen, S.; Liu, C.L.; Hobbs, B.D.; Hasegawa, K.; Liang, L.; International, C.G.C.; et al. Genetic overlap of chronic obstructive pulmonary disease and cardiovascular disease-related traits: A large-scale genome-wide cross-trait analysis. Respir. Res. 2019, 20, 64. [Google Scholar] [CrossRef]
- Hobbs, B.D.; de Jong, K.; Lamontagne, M.; Bosse, Y.; Shrine, N.; Artigas, M.S.; Wain, L.V.; Hall, I.P.; Jackson, V.E.; Wyss, A.B.; et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat. Genet. 2017, 49, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Erlich, P.M.; Hoffman, S.N.; Rukstalis, M.; Han, J.J.; Chu, X.; Linda Kao, W.H.; Gerhard, G.S.; Stewart, W.F.; Boscarino, J.A. Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Hum. Genet. 2010, 128, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Diabasana, Z.; Perotin, J.M.; Belgacemi, R.; Ancel, J.; Mulette, P.; Launois, C.; Delepine, G.; Dubernard, X.; Merol, J.C.; Ruaux, C.; et al. Chr15q25 Genetic Variant rs16969968 Alters Cell Differentiation in Respiratory Epithelia. Int. J. Mol. Sci. 2021, 22, 6657. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.; Steenaard, R.V.; Peters, M.J.; van Meurs, J.B.; Sijbrands, E.J.; Uitterlinden, A.G.; Bonder, M.J.; Consortium, B.; Hofman, A.; Franco, O.H.; et al. Tobacco smoking is associated with DNA methylation of diabetes susceptibility genes. Diabetologia 2016, 59, 998–1006. [Google Scholar] [CrossRef]
- Gottlieb, P.A.; Alkanani, A.K.; Michels, A.W.; Lewis, E.C.; Shapiro, L.; Dinarello, C.A.; Zipris, D. alpha1-Antitrypsin therapy downregulates toll-like receptor-induced IL-1beta responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E1418–E1426. [Google Scholar] [CrossRef]
- Weir, G.C.; Ehlers, M.R.; Harris, K.M.; Kanaparthi, S.; Long, A.; Phippard, D.; Weiner, L.J.; Jepson, B.; McNamara, J.G.; Koulmanda, M.; et al. Alpha-1 antitrypsin treatment of new-onset type 1 diabetes: An open-label, phase I clinical trial (RETAIN) to assess safety and pharmacokinetics. Pediatr Diabetes 2018, 19, 945–954. [Google Scholar] [CrossRef]
- Lebenthal, Y.; Brener, A.; Hershkovitz, E.; Shehadeh, N.; Shalitin, S.; Lewis, E.C.; Elias, D.; Haim, A.; Barash, G.; Loewenthal, N.; et al. A Phase II, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study Evaluating the Efficacy and Safety of Alpha-1 Antitrypsin (AAT) (Glassia((R))) in the Treatment of Recent-Onset Type 1 Diabetes. Int. J. Mol. Sci. 2019, 20, 6032. [Google Scholar] [CrossRef]
- Lewis, E.C.; Shapiro, L.; Bowers, O.J.; Dinarello, C.A. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc. Natl. Acad Sci. USA 2005, 102, 12153–12158. [Google Scholar] [CrossRef]
- Kalis, M.; Kumar, R.; Janciauskiene, S.; Salehi, A.; Cilio, C.M. alpha 1-antitrypsin enhances insulin secretion and prevents cytokine-mediated apoptosis in pancreatic beta-cells. Islets 2010, 2, 185–189. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, Y.; Campbell-Thompson, M.; Spencer, T.; Wasserfall, C.; Atkinson, M.; Song, S. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes 2007, 56, 1316–1323. [Google Scholar] [CrossRef]
- Rachmiel, M.; Strauss, P.; Dror, N.; Benzaquen, H.; Horesh, O.; Tov, N.; Weintrob, N.; Landau, Z.; Ben-Ami, M.; Haim, A.; et al. Alpha-1 antitrypsin therapy is safe and well tolerated in children and adolescents with recent onset type 1 diabetes mellitus. Pediatr Diabetes 2016, 17, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, C.S.; Ohlsson, B.; Melander, O.; Westin, U.; Mahadeva, R.; Janciauskiene, S. An association between Type 2 diabetes and alpha-antitrypsin deficiency. Diabet Med. 2008, 25, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Brondani, L.A.; Soares, A.A.; Recamonde-Mendoza, M.; Dall’Agnol, A.; Camargo, J.L.; Monteiro, K.M.; Silveiro, S.P. Urinary peptidomics and bioinformatics for the detection of diabetic kidney disease. Sci. Rep. 2020, 10, 1242. [Google Scholar] [CrossRef]
- Park, S.S.; Rodriguez Ortega, R.; Agudelo, C.W.; Perez Perez, J.; Perez Gandara, B.; Garcia-Arcos, I.; McCarthy, C.; Geraghty, P. Therapeutic Potential of Alpha-1 Antitrypsin in Type 1 and Type 2 Diabetes Mellitus. Medicina 2021, 57, 397. [Google Scholar] [CrossRef] [PubMed]
- Di Petta, A.; Greco, K.V.; Castro, E.O.; Lopes, F.D.; Martins, M.A.; Capelozzi, V.L.; Moreira, L.F.; Sannomiya, P. Insulin modulates inflammatory and repair responses to elastase-induced emphysema in diabetic rats. Int. J. Exp. Pathol. 2011, 92, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.R.; Zughaier, S.M.; Guentert, D.E.; Shenep, M.A.; Koval, M.; McCarty, N.A.; Hansen, J.M. Hyperglycemia impedes lung bacterial clearance in a murine model of cystic fibrosis-related diabetes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L43–L49. [Google Scholar] [CrossRef] [PubMed]
- Forgiarini, L.A., Jr.; Kretzmann, N.A.; Porawski, M.; Dias, A.S.; Marroni, N.A. Experimental diabetes mellitus: Oxidative stress and changes in lung structure. J. Bras. Pneumol. 2009, 35, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Popov, D.; Simionescu, M. Alterations of lung structure in experimental diabetes, and diabetes associated with hyperlipidaemia in hamsters. Eur. Respir. J. 1997, 10, 1850–1858. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Z.; Guo, Z.; Zhao, F.; Wang, Y.; Cai, L.; Yang, J. Type 1 diabetes mellitus is an independent risk factor for pulmonary fibrosis. Cell Biochem. Biophys 2014, 70, 1385–1391. [Google Scholar] [CrossRef]
- Talakatta, G.; Sarikhani, M.; Muhamed, J.; Dhanya, K.; Somashekar, B.S.; Mahesh, P.A.; Sundaresan, N.; Ravindra, P.V. Diabetes induces fibrotic changes in the lung through the activation of TGF-beta signaling pathways. Sci. Rep. 2018, 8, 11920. [Google Scholar] [CrossRef]
- Weynand, B.; Jonckheere, A.; Frans, A.; Rahier, J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 1999, 66, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Minette, P.; Buysschaert, M.; Rahier, J.; Veriter, C.; Frans, A. Pulmonary gas exchange in life-long nonsmoking patients with diabetes mellitus. Respiration 1999, 66, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Ofulue, A.F.; Kida, K.; Thurlbeck, W.M. Experimental diabetes and the lung. I. Changes in growth, morphometry, and biochemistry. Am. Rev. Respir. Dis. 1988, 137, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.; Kruger, S.; Merkel, M.; Bramlage, P.; Herth, F.J. Chronic obstructive pulmonary disease and diabetes mellitus: A systematic review of the literature. Respiration 2015, 89, 253–264. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Caramori, G.; Casolari, P.; Papi, A.A.; Edwards, M.; Shamji, B.; Triantaphyllopoulos, K.; Hussain, F.; Pinart, M.; Khan, Y.; et al. Oxidative stress-induced antibodies to carbonyl-modified protein correlate with severity of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 184, 796–802. [Google Scholar] [CrossRef]
- Schaberg, T.; Klein, U.; Rau, M.; Eller, J.; Lode, H. Subpopulations of alveolar macrophages in smokers and nonsmokers: Relation to the expression of CD11/CD18 molecules and superoxide anion production. Am. J. Respir. Crit. Care Med. 1995, 151, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Ward, S.; Cramer, D.; Barnes, P.J. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000, 162, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Drost, E.M.; Skwarski, K.M.; Sauleda, J.; Soler, N.; Roca, J.; Agusti, A.; MacNee, W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005, 60, 293–300. [Google Scholar] [CrossRef]
- Richens, T.R.; Linderman, D.J.; Horstmann, S.A.; Lambert, C.; Xiao, Y.Q.; Keith, R.L.; Boe, D.M.; Morimoto, K.; Bowler, R.P.; Day, B.J.; et al. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 2009, 179, 1011–1021. [Google Scholar] [CrossRef]
- VanderJagt, D.J.; Harrison, J.M.; Ratliff, D.M.; Hunsaker, L.A.; Vander Jagt, D.L. Oxidative stress indices in IDDM subjects with and without long-term diabetic complications. Clin. Biochem. 2001, 34, 265–270. [Google Scholar] [CrossRef]
- Tang, W.H.W.; McGee, P.; Lachin, J.M.; Li, D.Y.; Hoogwerf, B.; Hazen, S.L.; Grp, D.E.R. Oxidative Stress and Cardiovascular Risk in Type 1 Diabetes Mellitus: Insights From the DCCT/EDIC Study. J. Am. Heart Assoc. 2018, 7, e008368. [Google Scholar] [CrossRef]
- Beyersdorf, N.; Muller, N. Sphingomyelin breakdown in T cells: Role in activation, effector functions and immunoregulation. Biol. Chem. 2015, 396, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.P.; Jacobson, S.; Cruickshank, C.; Hughes, G.J.; Siska, C.; Ory, D.S.; Petrache, I.; Schaffer, J.E.; Reisdorph, N.; Kechris, K. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am. J. Respir. Crit. Care Med. 2015, 191, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Telenga, E.D.; Hoffmann, R.F.; Ruben, T.K.; Hoonhorst, S.J.; Willemse, B.W.; van Oosterhout, A.J.; Heijink, I.H.; van den Berge, M.; Jorge, L.; Sandra, P.; et al. Untargeted lipidomic analysis in chronic obstructive pulmonary disease. Uncovering sphingolipids. Am. J. Respir. Crit. Care Med. 2014, 190, 155–164. [Google Scholar] [CrossRef]
- Petrache, I.; Natarajan, V.; Zhen, L.; Medler, T.R.; Richter, A.T.; Cho, C.; Hubbard, W.C.; Berdyshev, E.V.; Tuder, R.M. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med. 2005, 11, 491–498. [Google Scholar] [CrossRef]
- Agudelo, C.W.; Kumley, B.K.; Area-Gomez, E.; Xu, Y.; Dabo, A.J.; Geraghty, P.; Campos, M.; Foronjy, R.; Garcia-Arcos, I. Decreased surfactant lipids correlate with lung function in chronic obstructive pulmonary disease (COPD). PLoS ONE 2020, 15, e0228279. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Li, C.; Wu, Q.; Yao, Z.; Wu, J.; Huang, P.; Wang, D.; Li, Z. Metabolic profiling of chronic obstructive pulmonary disease model rats and the interventional effects of HuaTanJiangQi decoction using UHPLC-Q-TOF/MS(E). J. Pharm. Biomed. Anal. 2020, 180, 113078. [Google Scholar] [CrossRef]
- Rice, K.L.; Duane, P.G.; Niewoehner, D.E. Lysophosphatidylcholine augments elastase-induced alveolar epithelial permeability and emphysema in the hamster. Am. Rev. Respir. Dis. 1987, 136, 941–946. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Coppieters, K.T.; von Herrath, M.G. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.W.; Samaha, G.; Garcia-Arcos, I. Alveolar lipids in pulmonary disease. A review. Lipids Health Dis. 2020, 19, 122. [Google Scholar] [CrossRef]
- Zipris, D. Epidemiology of type 1 diabetes and what animal models teach us about the role of viruses in disease mechanisms. Clin. Immunol. 2009, 131, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Zimmet, P. The rising tide of childhood type 1 diabetes—What is the elusive environmental trigger? Lancet 2004, 364, 1645–1647. [Google Scholar] [CrossRef]
- Bezemer, G.F.; Sagar, S.; van Bergenhenegouwen, J.; Georgiou, N.A.; Garssen, J.; Kraneveld, A.D.; Folkerts, G. Dual role of Toll-like receptors in asthma and chronic obstructive pulmonary disease. Pharmacol. Rev. 2012, 64, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Foronjy, R.F.; Dabo, A.J.; Taggart, C.C.; Weldon, S.; Geraghty, P. Respiratory syncytial virus infections enhance cigarette smoke induced COPD in mice. PLoS ONE 2014, 9, e90567. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I.; Yun, J.M.; Bremer, A. Demonstration of increased toll-like receptor 2 and toll-like receptor 4 expression in monocytes of type 1 diabetes mellitus patients with microvascular complications. Metabolism 2011, 60, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Dasu, M.R.; Park, S.H.; Jialal, I. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia 2009, 52, 1665–1668. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Rockwood, J.; Winter, W.; Griffen, S.C.; Jialal, I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: Further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008, 93, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Ghanim, H.; Green, K.; Sia, C.L.; Abuaysheh, S.; Kuhadiya, N.; Batra, M.; Dhindsa, S.; Chaudhuri, A. Insulin infusion suppresses while glucose infusion induces Toll-like receptors and high-mobility group-B1 protein expression in mononuclear cells of type 1 diabetes patients. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E810–E818. [Google Scholar] [CrossRef]
- Lea, S.R.; Reynolds, S.L.; Kaur, M.; Simpson, K.D.; Hall, S.R.; Hessel, E.M.; Singh, D. The effects of repeated Toll-like receptors 2 and 4 stimulation in COPD alveolar macrophages. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 771–780. [Google Scholar] [CrossRef]
- Haw, T.J.; Starkey, M.R.; Pavlidis, S.; Fricker, M.; Arthurs, A.L.; Nair, P.M.; Liu, G.; Hanish, I.; Kim, R.Y.; Foster, P.S.; et al. Toll-like receptor 2 and 4 have opposing roles in the pathogenesis of cigarette smoke-induced chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L298–L317. [Google Scholar] [CrossRef]
- Zipris, D.; Lien, E.; Xie, J.X.; Greiner, D.L.; Mordes, J.P.; Rossini, A.A. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J. Immunol. 2005, 174, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Krishnamurthy, B.; Mollah, Z.U.; Kay, T.W.; Thomas, H.E. NF-kappaB in type 1 diabetes. Inflamm. Allergy Drug Targets 2011, 10, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bednar, K.J.; Tsukamoto, H.; Kachapati, K.; Ohta, S.; Wu, Y.; Katz, J.D.; Ascherman, D.P.; Ridgway, W.M. Reversal of New-Onset Type 1 Diabetes With an Agonistic TLR4/MD-2 Monoclonal Antibody. Diabetes 2015, 64, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Sauleda, J.; Regueiro, V.; Santos, C.; Lopez, M.; Ferrer, J.; Agusti, A.G.; Bengoechea, J.A. Expression of Toll-like receptor 2 is up-regulated in monocytes from patients with chronic obstructive pulmonary disease. Respir. Res. 2006, 7, 64. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 199–208. [Google Scholar] [CrossRef]
- Devaraj, S.; Cheung, A.T.; Jialal, I.; Griffen, S.C.; Nguyen, D.; Glaser, N.; Aoki, T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 2007, 56, 2790–2796. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzza, A.E.; Morelli, M.; Rizzi, M.; Borgonovo, S.; De Palma, A.; Mameli, C.; Giani, E.; Beretta, S.; Zuccotti, G.V. Impaired diffusing capacity for carbon monoxide in children with type 1 diabetes: Is this the first sign of long-term complications? Acta Diabetol. 2012, 49, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Strojek, K.; Ziora, D.; Sroczynski, J.W.; Oklek, K. Pulmonary complications of type 1 (insulin-dependent) diabetic patients. Diabetologia 1992, 35, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, H.J.; Dorn, J.; Grant, B.J.; Winkelstein, W., Jr.; Trevisan, M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 2000, 118, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Seemungal, T.A.; MacCallum, P.K.; Paul, E.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Meade, T.W. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb. Haemost. 2000, 84, 210–215. [Google Scholar] [PubMed]
- Floyel, T.; Brorsson, C.; Nielsen, L.B.; Miani, M.; Bang-Berthelsen, C.H.; Friedrichsen, M.; Overgaard, A.J.; Berchtold, L.A.; Wiberg, A.; Poulsen, P.; et al. CTSH regulates beta-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc. Natl. Acad Sci. USA 2014, 111, 10305–10310. [Google Scholar] [CrossRef] [PubMed]
- Perotin, J.M.; Adam, D.; Vella-Boucaud, J.; Delepine, G.; Sandu, S.; Jonvel, A.C.; Prevost, A.; Berthiot, G.; Pison, C.; Lebargy, F.; et al. Delay of airway epithelial wound repair in COPD is associated with airflow obstruction severity. Respir. Res. 2014, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H.; Tanaka, M.; Takami, K.; Ohtoshi, T.; Ito, K.; Satoh, M.; Okada, Y.; Yamasawa, F.; Nakahara, K.; Umeda, A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am. J. Respir. Crit. Care Med. 2001, 163, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Onizawa, S.; Nagai, A.; Aoshiba, K. Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir. Res. 2011, 12, 78. [Google Scholar] [CrossRef]
- Togo, S.; Holz, O.; Liu, X.; Sugiura, H.; Kamio, K.; Wang, X.; Kawasaki, S.; Ahn, Y.; Fredriksson, K.; Skold, C.M.; et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am. J. Respir. Crit. Care Med. 2008, 178, 248–260. [Google Scholar] [CrossRef]
- Van Winkle, L.S.; Brown, C.D.; Shimizu, J.A.; Gunderson, A.D.; Evans, M.J.; Plopper, C.G. Impaired recovery from naphthalene-induced bronchiolar epithelial injury in mice exposed to aged and diluted sidestream cigarette smoke. Toxicol. Lett. 2004, 154, 1–9. [Google Scholar] [CrossRef]
- Ganesan, S.; Sajjan, U.S. Repair and Remodeling of airway epithelium after injury in Chronic Obstructive Pulmonary Disease. Curr. Respir. Care Rep. 2013, 2, 145–154. [Google Scholar] [CrossRef]
- Black, E.; Vibe-Petersen, J.; Jorgensen, L.N.; Madsen, S.M.; Agren, M.S.; Holstein, P.E.; Perrild, H.; Gottrup, F. Decrease of collagen deposition in wound repair in type 1 diabetes independent of glycemic control. Arch. Surg. 2003, 138, 34–40. [Google Scholar] [CrossRef]
- Schaffer, M.R.; Tantry, U.; Efron, P.A.; Ahrendt, G.M.; Thornton, F.J.; Barbul, A. Diabetes-impaired healing and reduced wound nitric oxide synthesis: A possible pathophysiologic correlation. Surgery 1997, 121, 513–519. [Google Scholar] [CrossRef]
- Hehenberger, K.; Kratz, G.; Hansson, A.; Brismar, K. Fibroblasts derived from human chronic diabetic wounds have a decreased proliferation rate, which is recovered by the addition of heparin. J. Dermatol. Sci. 1998, 16, 144–151. [Google Scholar] [CrossRef]
- Bitar, M.S.; Farook, T.; Wahid, S.; Francis, I.M. Glucocorticoid-dependent impairment of wound healing in experimental diabetes: Amelioration by adrenalectomy and RU 486. J. Surg. Res. 1999, 82, 234–243. [Google Scholar] [CrossRef]
- Goodson, W.H., 3rd; Hung, T.K. Studies of wound healing in experimental diabetes mellitus. J. Surg. Res. 1977, 22, 221–227. [Google Scholar] [CrossRef]
- Bitar, M.S.; Labbad, Z.N. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J. Surg. Res. 1996, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fahey, T.J., 3rd; Sadaty, A.; Jones, W.G., 2nd; Barber, A.; Smoller, B.; Shires, G.T. Diabetes impairs the late inflammatory response to wound healing. J. Surg. Res. 1991, 50, 308–313. [Google Scholar] [CrossRef]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef]
- Morishige, W.K.; Uetake, C.A.; Greenwood, F.C.; Akaka, J. Pulmonary insulin responsivitiy: In vivo effects of insulin on the diabetic rat lung and specific insulin binding to lung receptors in normal rats. Endocrinology 1977, 100, 1710–1722. [Google Scholar] [CrossRef]
- Moxley, M.A.; Longmore, W.J. Studies on the effects of alloxan and streptozotocin induced diabetes on lipid metabolism in the isolated perfused rat lung. Life Sci. 1975, 17, 921–925. [Google Scholar] [CrossRef]
- Sugahara, K.; Ushijima, K.; Morioka, T.; Usuku, G. Studies of the lung in diabetes mellitus. I. Ultrastructural studies of the lungs in alloxan-induced diabetic rats. Virchows. Arch. A Pathol. Anat. Histol. 1981, 390, 313–324. [Google Scholar] [CrossRef]
- De Prost, N.; Saumon, G. Glucose transport in the lung and its role in liquid movement. Respir. Physiol. Neurobiol. 2007, 159, 331–337. [Google Scholar] [CrossRef]
- Dieterle, C.D.; Schmauss, S.; Arbogast, H.; Domsch, C.; Huber, R.M.; Landgraf, R. Pulmonary function in patients with type 1 diabetes before and after simultaneous pancreas and kidney transplantation. Transplantation 2007, 83, 566–569. [Google Scholar] [CrossRef]
- Lee, W.H.; Wu, D.W.; Chen, Y.C.; Liu, Y.H.; Liao, W.S.; Chen, S.C.; Hung, C.H.; Kuo, C.H.; Su, H.M. Association of Pulmonary Function Decline over Time with Longitudinal Change of Glycated Hemoglobin in Participants without Diabetes Mellitus. J. Pers. Med. 2021, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Ducharme-Smith, K.; Mora-Garcia, G.; de Castro Mendes, F.; Ruiz-Diaz, M.S.; Moreira, A.; Villegas, R.; Garcia-Larsen, V. Lung function, COPD and Alternative Healthy Eating Index in US adults. ERJ Open Res. 2021, 7, 927–2020. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef]
- Tong, X.; Chaudhry, Z.; Lee, C.C.; Bone, R.N.; Kanojia, S.; Maddatu, J.; Sohn, P.; Weaver, S.A.; Robertson, M.A.; Petrache, I.; et al. Cigarette smoke exposure impairs beta-cell function through activation of oxidative stress and ceramide accumulation. Mol. Metab. 2020, 37, 100975. [Google Scholar] [CrossRef]
- Van den Borst, B.; Gosker, H.R.; Koster, A.; Yu, B.; Kritchevsky, S.B.; Liu, Y.; Meibohm, B.; Rice, T.B.; Shlipak, M.; Yende, S.; et al. The influence of abdominal visceral fat on inflammatory pathways and mortality risk in obstructive lung disease. Am. J. Clin. Nutr. 2012, 96, 516–526. [Google Scholar] [CrossRef]
- Vozoris, N.T.; O’Donnell, D.E. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can. Respir. J. 2012, 19, 732618. [Google Scholar] [CrossRef] [PubMed]
- Van den Borst, B.; Gosker, H.R.; Wesseling, G.; de Jager, W.; Hellwig, V.A.; Snepvangers, F.J.; Schols, A.M. Low-grade adipose tissue inflammation in patients with mild-to-moderate chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2011, 94, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.I.; Li, Y.; Man, S.F.P.; Tashkin, D.; Wise, R.A.; Connett, J.E.; Anthonisen, N.A.; Churg, A.; Wright, J.L.; Sin, D.D. The complex relationship of serum adiponectin to COPD outcomes COPD and adiponectin. Chest 2012, 142, 893–899. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, Y.; Shi, H.; Liu, C.L.; Panganiban, R.A.; Chung, W.; O’Connor, L.J.; Himes, B.E.; Gazal, S.; Hasegawa, K.; et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J. Allergy Clin. Immunol. 2020, 145, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, J.; Si, J.; Ma, B.; Shi, H.; Lv, J.; Cao, W.; Guo, Y.; Millwood, I.Y.; Walters, R.G.; et al. A large-scale genome-wide association analysis of lung function in the Chinese population identifies novel loci and highlights shared genetic aetiology with obesity. Eur. Respir. J. 2021, 58, 2100199. [Google Scholar] [CrossRef] [PubMed]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 1. Strategies for Improving Care. Diabetes Care 2016, 39 (Suppl. S1), S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mohanty, P.; Chaudhuri, A.; Garg, R.; Aljada, A. Insulin infusion in acute illness. J. Clin. Investig. 2005, 115, 2069–2072. [Google Scholar] [CrossRef]
- Tessaro, F.H.G.; Ayala, T.S.; Nolasco, E.L.; Bella, L.M.; Martins, J.O. Insulin Influences LPS-Induced TNF-alpha and IL-6 Release Through Distinct Pathways in Mouse Macrophages from Different Compartments. Cell Physiol. Biochem. 2017, 42, 2093–2104. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Capuani, B.; Facciolo, F.; Cazzola, M.; Lauro, D.; Matera, M.G. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am. J. Respir. Cell. Mol. Biol. 2016, 55, 804–814. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Linderholm, A.; Haczku, A.; Kenyon, N. Glucagon-like peptide 1: A potential anti-inflammatory pathway in obesity-related asthma. Pharm. Ther. 2017, 180, 139–143. [Google Scholar] [CrossRef]
- Yang, W.; Ni, H.; Wang, H.; Gu, H. NLRP3 inflammasome is essential for the development of chronic obstructive pulmonary disease. Int. J. Clin. Exp. Pathol. 2015, 8, 13209–13216. [Google Scholar] [PubMed]
- Nachmias, N.; Langier, S.; Brzezinski, R.Y.; Siterman, M.; Stark, M.; Etkin, S.; Avriel, A.; Schwarz, Y.; Shenhar-Tsarfaty, S.; Bar-Shai, A. NLRP3 inflammasome activity is upregulated in an in-vitro model of COPD exacerbation. PLoS ONE 2019, 14, e0214622. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, T.J.; Morris, R.T.; House, L.M.; Barnes, T.M.; Otero, Y.F.; Barham, W.J.; Hunt, R.P.; Zaynagetdinov, R.; Yull, F.E.; Blackwell, T.S.; et al. NF-kappaB-dependent airway inflammation triggers systemic insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1144–R1152. [Google Scholar] [CrossRef] [PubMed]
- Clerk, L.H.; Vincent, M.A.; Jahn, L.A.; Liu, Z.; Lindner, J.R.; Barrett, E.J. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006, 55, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Alberiche, M.; Zenere, M.B.; Bonadonna, R.C.; Muggeo, M.; Bonora, E. Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1997, 82, 3619–3624. [Google Scholar] [CrossRef]
- Widen, E.; Ekstrand, A.; Saloranta, C.; Franssila-Kallunki, A.; Eriksson, J.; Schalin-Jantti, C.; Groop, L. Insulin resistance in type 2 (non-insulin-dependent) diabetic patients with hypertriglyceridaemia. Diabetologia 1992, 35, 1140–1145. [Google Scholar] [CrossRef]
- Yazdani, R.; Gholamrezapour, M.; Hassanaghaei, T. Adiponectin Level in Serum and BAL Sample of Patients with Chronic Obstructive Pulmonary Disease. Tanaffos 2013, 12, 53–57. [Google Scholar]
- Sato, K.; Shibata, Y.; Abe, S.; Inoue, S.; Igarashi, A.; Yamauchi, K.; Aida, Y.; Nunomiya, K.; Nakano, H.; Sato, M.; et al. Association between plasma adiponectin levels and decline in forced expiratory volume in 1 s in a general Japanese population: The Takahata study. Int. J. Med. Sci. 2014, 11, 758–764. [Google Scholar] [CrossRef]
- Kawamoto, R.; Tabara, Y.; Kohara, K.; Miki, T.; Ohtsuka, N.; Kusunoki, T.; Abe, M. Smoking status is associated with serum high molecular adiponectin levels in community-dwelling Japanese men. J. Atheroscler. Thromb. 2010, 17, 423–430. [Google Scholar] [CrossRef]
- Hodge, A.M.; Westerman, R.A.; de Courten, M.P.; Collier, G.R.; Zimmet, P.Z.; Alberti, K.G. Is leptin sensitivity the link between smoking cessation and weight gain? Int. J. Obes. Relat. Metab. Disord. 1997, 21, 50–53. [Google Scholar] [CrossRef]
- Wei, M.; Stern, M.P.; Haffner, S.M. Serum leptin levels in Mexican Americans and non-Hispanic whites: Association with body mass index and cigarette smoking. Ann. Epidemiol. 1997, 7, 81–86. [Google Scholar] [CrossRef]
- Eliasson, B.; Smith, U. Leptin levels in smokers and long-term users of nicotine gum. Eur. J. Clin. Investig. 1999, 29, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.R.; Kadam, S.; Brahme, A.; Agrawal, M.; Apte, K.; Narke, G.; Kekan, K.; Madas, S.; Salvi, S. Systemic Immuno-metabolic alterations in chronic obstructive pulmonary disease (COPD). Respir. Res. 2019, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Czajka, A.; Ajaz, S.; Gnudi, L.; Parsade, C.K.; Jones, P.; Reid, F.; Malik, A.N. Altered Mitochondrial Function, Mitochondrial DNA and Reduced Metabolic Flexibility in Patients With Diabetic Nephropathy. EBioMedicine 2015, 2, 499–512. [Google Scholar] [CrossRef]
- O’Beirne, S.L.; Kikkers, S.A.; Oromendia, C.; Salit, J.; Rostmai, M.R.; Ballman, K.V.; Kaner, R.J.; Crystal, R.G.; Cloonan, S.M. Alveolar Macrophage Immunometabolism and Lung Function Impairment in Smoking and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 735–739. [Google Scholar] [CrossRef]
- Taylor, A.E.; Finney-Hayward, T.K.; Quint, J.K.; Thomas, C.M.; Tudhope, S.J.; Wedzicha, J.A.; Barnes, P.J.; Donnelly, L.E. Defective macrophage phagocytosis of bacteria in COPD. Eur. Respir. J. 2010, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. Obesity and lung inflammation. J. Appl. Physiol. 2010, 108, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Maachi, M.; Pieroni, L.; Bruckert, E.; Jardel, C.; Fellahi, S.; Hainque, B.; Capeau, J.; Bastard, J.P. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Broekhuizen, R.; Vernooy, J.H.; Schols, A.M.; Dentener, M.A.; Wouters, E.F. Leptin as local inflammatory marker in COPD. Respir. Med. 2005, 99, 70–74. [Google Scholar] [CrossRef]
- Bruno, A.; Chanez, P.; Chiappara, G.; Siena, L.; Giammanco, S.; Gjomarkaj, M.; Bonsignore, G.; Bousquet, J.; Vignola, A.M. Does leptin play a cytokine-like role within the airways of COPD patients? Eur. Respir. J. 2005, 26, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Coats, V.; Després, J.P.; Alméras, N.; Martin, M.; Sin, D.D.; Rabasa-Lhoret, R.; Larose, É.; Tan, W.C.; Bourbeau, J.; Maltais, F. Ectopic adiposity and cardiometabolic health in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Hollenbach, J.; Lopez-Rodriguez, E.; Muhlfeld, C.; Schipke, J. Voluntary Activity Modulates Sugar-Induced Elastic Fiber Remodeling in the Alveolar Region of the Mouse Lung. Int. J. Mol. Sci. 2019, 20, 2438. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.M.; Brennan, A.L.; Philips, B.J.; Baker, E.H. Effect of hyperglycaemia on glucose concentration of human nasal secretions. Clin. Sci. 2004, 106, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.J.; Redman, J.; Brennan, A.; Wood, D.; Holliman, R.; Baines, D.; Baker, E.H. Glucose in bronchial aspirates increases the risk of respiratory MRSA in intubated patients. Thorax 2005, 60, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Rogliani, P.; Lauro, D.; Novelli, L.; Page, C.P.; Kanabar, V.; Matera, M.G. High glucose enhances responsiveness of human airways smooth muscle via the Rho/ROCK pathway. Am. J. Respir. Cell. Mol. Biol. 2012, 47, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, J.; Ribeiro, C.T.; da Rosa-Silva, H.T.; Bortolin, R.C.; Rabelo, T.K.; Peixoto, D.O.; Moreira, J.C.F.; Gelain, D.P. Systemic Inflammation Changes the Site of RAGE Expression from Endothelial Cells to Neurons in Different Brain Areas. Mol. Neurobiol. 2019, 56, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Kloting, I.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Crandall, J.; Goldberg, T.; Oberstein, R.; Dardaine, V.; Peppa, M.; Rayfield, E.J. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc. Natl. Acad Sci. USA 2002, 99, 15596–15601. [Google Scholar] [CrossRef]
- Ferhani, N.; Letuve, S.; Kozhich, A.; Thibaudeau, O.; Grandsaigne, M.; Maret, M.; Dombret, M.C.; Sims, G.P.; Kolbeck, R.; Coyle, A.J.; et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Pratte, K.A.; Curtis, J.L.; Kechris, K.; Couper, D.; Cho, M.H.; Silverman, E.K.; DeMeo, D.L.; Sciurba, F.C.; Zhang, Y.; Ortega, V.E.; et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COPD. Respir. Res. 2021, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Beck, K.C.; Dietz, N.M.; Joyner, M.J.; Turner, S.T.; Johnson, B.D. Influence of beta2-adrenergic receptor genotype on airway function during exercise in healthy adults. Chest 2006, 129, 762–770. [Google Scholar] [CrossRef]
- Ahren, B.; Schersten, B. Beta 2-adrenoceptor induced increase of plasma insulin levels in man: Evidence of direct and indirect B-cell stimulation and liver effects. Diabetes Res. 1986, 3, 443–445. [Google Scholar] [PubMed]
- Papatheodorou, A.; Latsi, P.; Vrettou, C.; Dimakou, A.; Chroneou, A.; Makrythanasis, P.; Kaliakatsos, M.; Orfanidou, D.; Roussos, C.; Kanavakis, E.; et al. Development of a novel microarray methodology for the study of SNPs in the promoter region of the TNF-alpha gene: Their association with obstructive pulmonary disease in Greek patients. Clin. Biochem. 2007, 40, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.J.; Goldberg, A.P.; Ryan, A.S. ADRB2 haplotype is associated with glucose tolerance and insulin sensitivity in obese postmenopausal women. Obesity 2011, 19, 396–401. [Google Scholar] [CrossRef]
- Thomsen, M.; Dahl, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. beta2 -adrenergic receptor Thr164IIe polymorphism, blood pressure and ischaemic heart disease in 66 750 individuals. J. Intern. Med. 2012, 271, 305–314. [Google Scholar] [CrossRef]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–1188. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Li, Y.Y.; Huang, C.; Li, J.; Yao, H.W. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget 2017, 8, 22513–22523. [Google Scholar] [CrossRef]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef]
- Hur, K.Y.; Lee, M.S. New mechanisms of metformin action: Focusing on mitochondria and the gut. J. Diabetes Investig. 2015, 6, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Patkee, W.R.; Carr, G.; Baker, E.H.; Baines, D.L.; Garnett, J.P. Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth. J. Cell. Mol. Med. 2016, 20, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, C.; Kusaka, Y.; Kimura, S.; Yamaguchi, T.; Nanjo, Y.; Ishii, Y.; Udono, H.; Standiford, T.J.; Tateda, K. Metformin Mediates Protection against Legionella Pneumonia through Activation of AMPK and Mitochondrial Reactive Oxygen Species. J. Immunol. 2018, 200, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.W.; Huang, C.T.; Tsai, Y.J.; Lien, A.S.; Lai, F.; Yu, C.J. Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir. Res. 2019, 20, 69. [Google Scholar] [CrossRef]
- Kahnert, K.; Andreas, S.; Kellerer, C.; Lutter, J.I.; Lucke, T.; Yildirim, O.; Lehmann, M.; Seissler, J.; Behr, J.; Frankenberger, M.; et al. Reduced decline of lung diffusing capacity in COPD patients with diabetes and metformin treatment. Sci. Rep. 2022, 12, 1435. [Google Scholar] [CrossRef]
- Sexton, P.; Metcalf, P.; Kolbe, J. Respiratory effects of insulin sensitisation with metformin: A prospective observational study. Copd 2014, 11, 133–142. [Google Scholar] [CrossRef]
- Hitchings, A.W.; Lai, D.; Jones, P.W.; Baker, E.H. Metformin in severe exacerbations of chronic obstructive pulmonary disease: A randomised controlled trial. Thorax 2016, 71, 587–593. [Google Scholar] [CrossRef]
- Zhu, A.; Teng, Y.; Ge, D.; Zhang, X.; Hu, M.; Yao, X. Role of metformin in treatment of patients with chronic obstructive pulmonary disease: A systematic review. J. Thorac. Dis. 2019, 11, 4371–4378. [Google Scholar] [CrossRef]
- Yen, F.S.; Wei, J.C.; Yang, Y.C.; Hsu, C.C.; Hwu, C.M. Respiratory outcomes of metformin use in patients with type 2 diabetes and chronic obstructive pulmonary disease. Sci. Rep. 2020, 10, 10298. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Craddock, P.R.; Hammerschmidt, D.E.; August, J.T. Corticosteroids block binding of chemotactic peptide to its receptor on granulocytes and cause disaggregation of granulocyte aggregates in vitro. J. Clin. Investig. 1981, 68, 13–20. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Russell, R.; Mares, R.; Burden, A.; Skinner, D.; Mikkelsen, H.; Ding, C.; Brice, R.; Chavannes, N.H.; Kocks, J.W.; et al. Metabolic Effects Associated with ICS in Patients with COPD and Comorbid Type 2 Diabetes: A Historical Matched Cohort Study. PLoS ONE 2016, 11, e0162903. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.E.; Roughead, E.E.; Vitry, A.I.; McDermott, R.A.; Shakib, S.; Gilbert, A.L. Comorbidity in the elderly with diabetes: Identification of areas of potential treatment conflicts. Diabetes Res. Clin. Pract. 2010, 87, 385–393. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Rennard, S.; Gerstein, H.; Radner, F.; Peterson, S.; Lindberg, B.; Carlsson, L.G.; Sin, D.D. Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids. Respir. Med. 2012, 106, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Kezouh, A.; Ernst, P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am. J. Med. 2010, 123, 1001–1006. [Google Scholar] [CrossRef]
- Dendukuri, N.; Blais, L.; LeLorier, J. Inhaled corticosteroids and the risk of diabetes among the elderly. Br. J. Clin. Pharmacol. 2002, 54, 59–64. [Google Scholar] [CrossRef]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef]
- Way, J.M.; Harrington, W.W.; Brown, K.K.; Gottschalk, W.K.; Sundseth, S.S.; Mansfield, T.A.; Ramachandran, R.K.; Willson, T.M.; Kliewer, S.A. Comprehensive messenger ribonucleic acid profiling reveals that peroxisome proliferator-activated receptor gamma activation has coordinate effects on gene expression in multiple insulin-sensitive tissues. Endocrinology 2001, 142, 1269–1277. [Google Scholar] [CrossRef]
- Rinne, S.T.; Liu, C.F.; Feemster, L.C.; Collins, B.F.; Bryson, C.L.; O’Riordan, T.G.; Au, D.H. Thiazolidinediones are associated with a reduced risk of COPD exacerbations. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1591–1597. [Google Scholar] [CrossRef]
- Petrache, I.; Fijalkowska, I.; Zhen, L.; Medler, T.R.; Brown, E.; Cruz, P.; Choe, K.H.; Taraseviciene-Stewart, L.; Scerbavicius, R.; Shapiro, L.; et al. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2006, 173, 1222–1228. [Google Scholar] [CrossRef]
- Chapman, K.R.; Burdon, J.G.; Piitulainen, E.; Sandhaus, R.A.; Seersholm, N.; Stocks, J.M.; Stoel, B.C.; Huang, L.; Yao, Z.; Edelman, J.M.; et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 360–368. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Gopal, R.; Alcorn, J.F.; Forno, E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology 2019, 24, 646–651. [Google Scholar] [CrossRef]
- Isoda, K.; Young, J.L.; Zirlik, A.; MacFarlane, L.A.; Tsuboi, N.; Gerdes, N.; Schonbeck, U.; Libby, P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Antioxidant and anti-AGE therapeutics: Evaluation and perspectives. J. Soc. Biol. 2001, 195, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.J.; Sethi, S. COPD and the microbiome. Respirology 2016, 21, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.P.; Baker, E.H.; Naik, S.; Lindsay, J.A.; Knight, G.M.; Gill, S.; Tregoning, J.S.; Baines, D.L. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax 2013, 68, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Kahnert, K.; Jorres, R.A.; Lucke, T.; Trudzinski, F.C.; Mertsch, P.; Bickert, C.; Ficker, J.H.; Behr, J.; Bals, R.; Watz, H.; et al. Lower Prevalence of Osteoporosis in Patients with COPD Taking Anti-Inflammatory Compounds for the Treatment of Diabetes: Results from COSYCONET. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 3189–3199. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and risk of chronic obstructive pulmonary disease in diabetes patients. Diabetes Metab. 2019, 45, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Wu, T.D.; Rojas-Quintero, J.; Wang, X.; Mayo, J.; Tomchaney, M.; Tram, J.; Packard, S.; Zhang, D.; Cleveland, K.H.; et al. Metformin: Experimental and Clinical Evidence for a Potential Role in Emphysema Treatment. Am. J. Respir. Crit. Care Med. 2021, 204, 651–666. [Google Scholar] [CrossRef]
- Zulkifli, K.K.; Mohamed Shah, F.Z.; Ismail, A.I.; Abdul Rahman, T.H.; Ghani, R.A. Prevalence and associated factors of dysglycemia among patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 2021, 18, 1–8. [Google Scholar] [CrossRef]
- Ceriello, A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab. Res. Rev. 2008, 24, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Birrell, M.A.; Patel, H.J.; McCluskie, K.; Wong, S.; Leonard, T.; Yacoub, M.H.; Belvisi, M.G. PPAR-gamma agonists as therapy for diseases involving airway neutrophilia. Eur. Respir. J. 2004, 24, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Lea, S.; Plumb, J.; Metcalfe, H.; Spicer, D.; Woodman, P.; Fox, J.C.; Singh, D. The effect of peroxisome proliferator-activated receptor-gamma ligands on in vitro and in vivo models of COPD. Eur. Respir. J. 2014, 43, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Rabe, K.F.; Martinez, F.J.; Bredenbroker, D.; Brose, M.; Goehring, U.M.; Calverley, P.M.A. Efficacy of roflumilast in the COPD frequent exacerbator phenotype. Chest 2013, 143, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Laudisio, A.; Costanzo, L.; Di Gioia, C.; Delussu, A.S.; Traballesi, M.; Gemma, A.; Antonelli Incalzi, R. Dietary intake of elderly outpatients with chronic obstructive pulmonary disease. Arch. Gerontol. Geriatr. 2016, 64, 75–81. [Google Scholar] [CrossRef]
- Van de Bool, C.; Mattijssen-Verdonschot, C.; van Melick, P.P.; Spruit, M.A.; Franssen, F.M.; Wouters, E.F.; Schols, A.M.; Rutten, E.P. Quality of dietary intake in relation to body composition in patients with chronic obstructive pulmonary disease eligible for pulmonary rehabilitation. Eur. J. Clin. Nutr. 2014, 68, 159–165. [Google Scholar] [CrossRef]
- Kaluza, J.; Larsson, S.C.; Orsini, N.; Linden, A.; Wolk, A. Fruit and vegetable consumption and risk of COPD: A prospective cohort study of men. Thorax 2017, 72, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term consumption of fruits and vegetables and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Int. J. Epidemiol. 2018, 47, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Willett, W.C.; Camargo, C.A., Jr. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. Am. J. Epidemiol. 2010, 171, 776–784. [Google Scholar] [CrossRef]
- Ochs-Balcom, H.M.; Grant, B.J.; Muti, P.; Sempos, C.T.; Freudenheim, J.L.; Browne, R.W.; McCann, S.E.; Trevisan, M.; Cassano, P.A.; Iacoviello, L.; et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur. J. Clin. Nutr. 2006, 60, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; de Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Yang, M.D.; Li, P.C.; Fang, H.Y.; Huang, H.Y.; Chan, Y.C.; Bau, D.T. Effect of Oligomeric Proanthocyanidin on the Antioxidant Status and Lung Function of Patients with Chronic Obstructive Pulmonary Disease. In Vivo 2018, 32, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, F.; Lee, A.H.; Oura, A.; Mori, M.; Hiramatsu, N.; Taniguchi, H. Dietary intake of six minerals in relation to the risk of chronic obstructive pulmonary disease. Asia Pac. J. Clin. Nutr. 2010, 19, 572–577. [Google Scholar] [PubMed]
- Scoditti, E.; Massaro, M.; Garbarino, S.; Toraldo, D.M. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients 2019, 11, 1357. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Liu, X.; Zheng, N.; Gu, Y.; Song, Y.; Wang, X. Regulatory roles of external cholesterol in human airway epithelial mitochondrial function through STARD3 signalling. Clin. Transl. Med. 2022, 12, e902. [Google Scholar] [CrossRef] [PubMed]
| Therapy | Mechanism of Action | Outcomes in Patients with T2D | Outcomes in Patients with COPD | Outcomes in Patients with COPD and T2D |
|---|---|---|---|---|
| Metformin | Inhibits proinflammatory NF-kB signaling in human vascular cells [178]. | Reduces the formation of AGEs by improving glycemic control [179]. Reduces CV mortality, all-cause mortality, and CV events in T2D patients with CAD [180]. Changes the composition of gut microbiota, improves insulin resistance, and decrease tissue inflammation [181]. | Reduces inflammation in the elastase-induced emphysema mouse model [179]. The reduction in lung infections modifies glucose flow across the lung tissue [182,183]. Improves survival rates in COPD patients with T2D [184]. Reduces loss of CO diffusing capacity [185]. | Improves health, symptoms, hospitalizations, and mortality in patients with COPD and T2D [186]. Reduces the likelihood of T2D developing COPD [187] T2D with moderate to severe COPD with symptomatic improvement measured in SGRQ and TDI [188]. COPD patients with T2D are at higher risk of pneumonia, COPD exacerbation, and need for mechanical ventilation [189]. |
| Inhaled corticosteroids | Lower the migration of inflammatory cells; reverse capillary permeability and lysosomal stabilization [190]. | Dose-dependent elevation in serum glucose concentration [191] and increase in HbA1C [192]. | Increased risk of new-onset T2D [193,194]. 34% increase in the rate of diabetes and increased rate of diabetes progression [195]. No increased risk of diabetes among current users [196]. | |
| Thiazolidinediones | Increase insulin sensitivity by binding and activating PPARs, altering the transcription of glucose and lipid metabolism-related genes [197]. | Increase glucose utilization in the tissues by increasing insulin sensitivity [198]. | Associated with reduced risk of COPD exacerbations [199] | |
| AAT augmentation | Physiologic AAT inactivates proteolytic enzymes secreted during inflammation and has anti-apoptotic properties [200]. | Safe and well-tolerated in stage 3 T1D [45]. Reduced HbA1c levels in adolescents with recently diagnosed T1D [47]. Children with AAT infusions showed fewer IL-1β producing monocytes and dendritic cells [45]. | AAT augmentation slows the progression of emphysema [201]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.S.; Perez Perez, J.L.; Perez Gandara, B.; Agudelo, C.W.; Rodriguez Ortega, R.; Ahmed, H.; Garcia-Arcos, I.; McCarthy, C.; Geraghty, P. Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD? Medicina 2022, 58, 1030. https://doi.org/10.3390/medicina58081030
Park SS, Perez Perez JL, Perez Gandara B, Agudelo CW, Rodriguez Ortega R, Ahmed H, Garcia-Arcos I, McCarthy C, Geraghty P. Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD? Medicina. 2022; 58(8):1030. https://doi.org/10.3390/medicina58081030
Chicago/Turabian StylePark, Sangmi S., Jessica L. Perez Perez, Brais Perez Gandara, Christina W. Agudelo, Romy Rodriguez Ortega, Huma Ahmed, Itsaso Garcia-Arcos, Cormac McCarthy, and Patrick Geraghty. 2022. "Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD?" Medicina 58, no. 8: 1030. https://doi.org/10.3390/medicina58081030
APA StylePark, S. S., Perez Perez, J. L., Perez Gandara, B., Agudelo, C. W., Rodriguez Ortega, R., Ahmed, H., Garcia-Arcos, I., McCarthy, C., & Geraghty, P. (2022). Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD? Medicina, 58(8), 1030. https://doi.org/10.3390/medicina58081030







