Effect of Direct-Acting Antiviral Agents on Gastroesophageal Varices in Patients with Hepatitis C Virus-Related Cirrhosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DAA Therapy
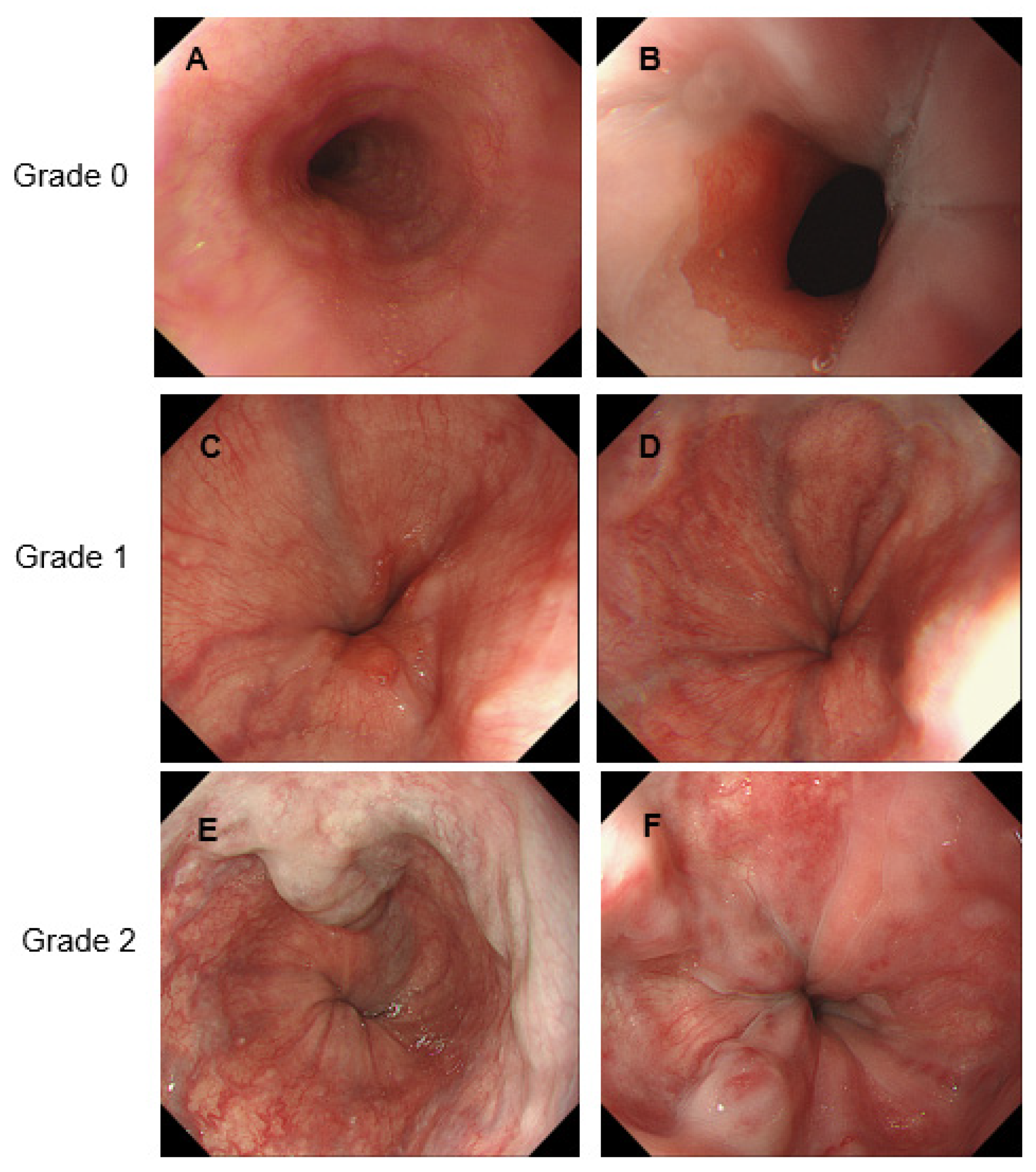
2.3. Evaluation of GEVs
2.4. Statistical Analysis
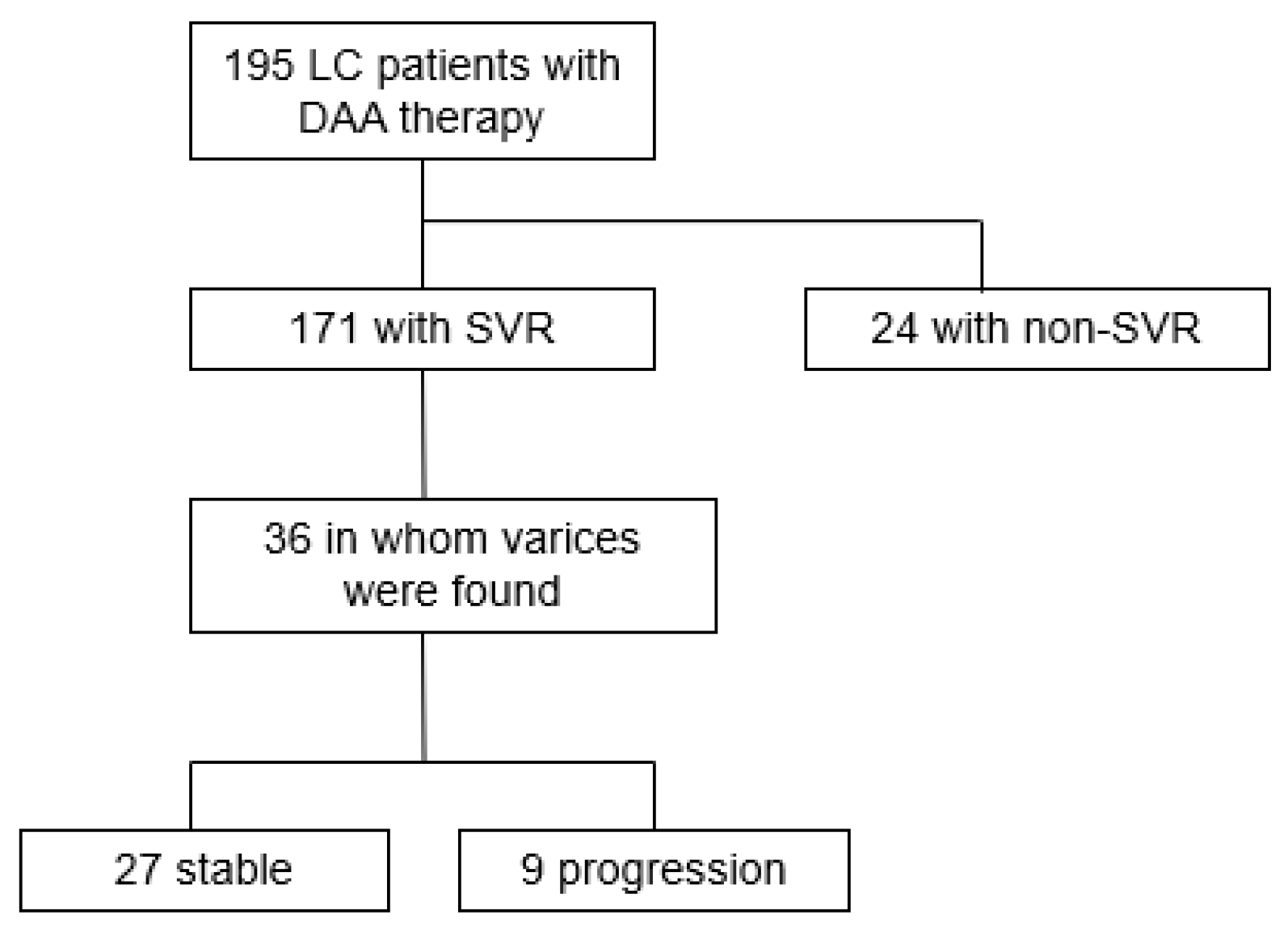
3. Results
3.1. Characteristics of Patients
3.2. Efficacy of DAA Therapy with Respect to Liver Function and GEVs
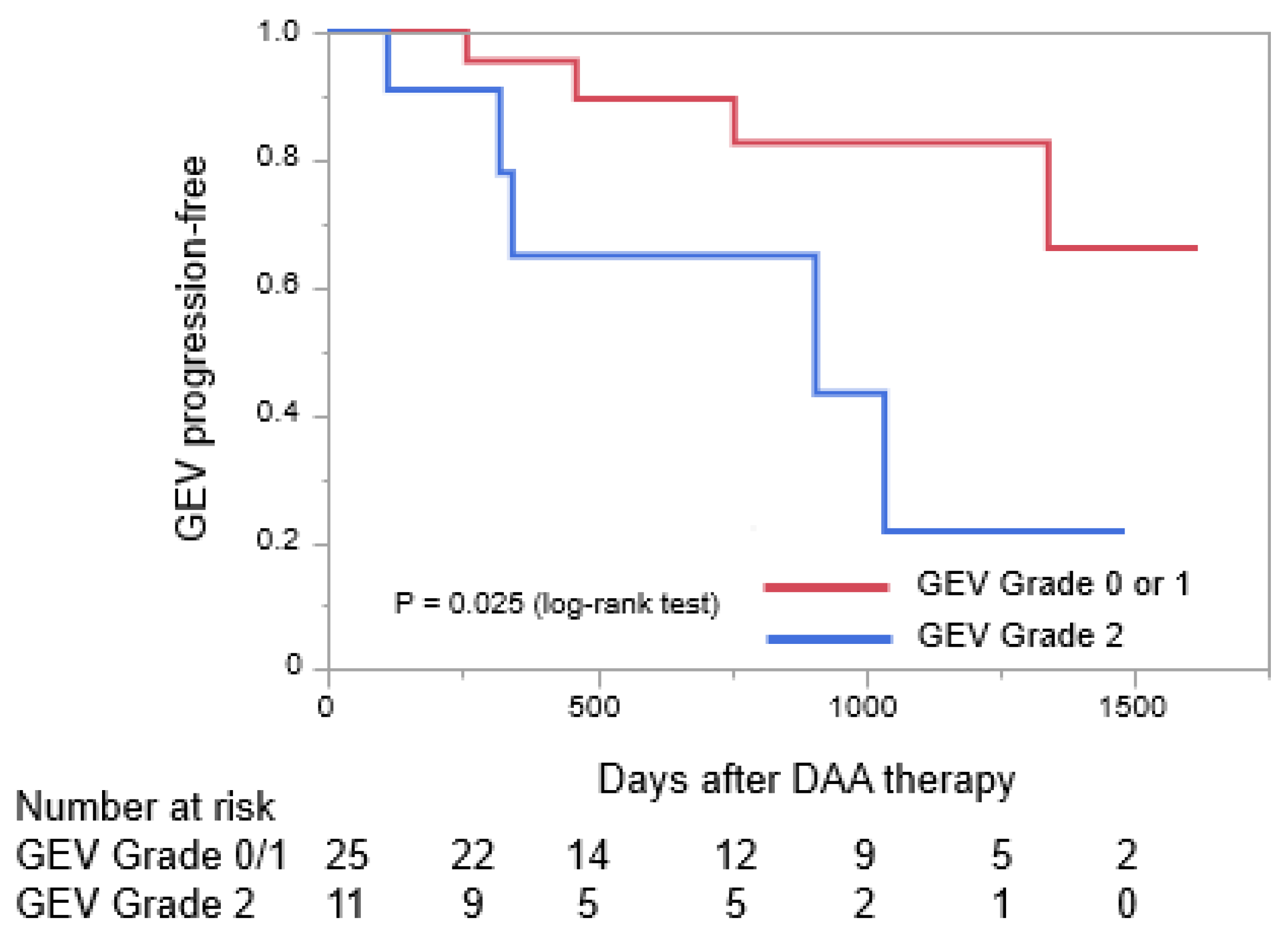
3.3. Differences in Pre-Treatment Characteristics of Patients Who Had Stable GEVs or Progressive GEVs after Receiving DAA Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emmanuel, B.; Wilson, E.M.; O’Brien, T.R.; Kottilil, S.; Lau, G. Shortening the duration of therapy for chronic hepatitis C infection. Lancet Gastroenterol. Hepatol. 2017, 2, 832–836. [Google Scholar] [CrossRef] [Green Version]
- Webster, D.P.; Klenerman, P.; Dusheiko, G.M. Hepatitis C. Lancet 2015, 385, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, A.J.; Bosch, J.; Blei, A.; Arroyo, V. Portal hypertension and its complications. Gastroenterology 2008, 134, 1715–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverter, E.; Tandon, P.; Augustin, S.; Turon, F.; Casu, S.; Bastiampillai, R.; Keough, A.; Llop, E.; González, A.; Seijo, S.; et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology 2014, 146, 412–419.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortune, B.E.; Garcia-Tsao, G.; Ciarleglio, M.; Deng, Y.; Fallon, M.B.; Sigal, S.; Chalasani, N.P.; Lim, J.K.; Reuben, A.; Vargas, H.E.; et al. Child-Turcotte-Pugh class is best at stratifying risk in variceal hemorrhage: Analysis of a US multicenter prospective study. J. Clin. Gastroenterol. 2017, 51, 446–453. [Google Scholar] [CrossRef]
- Bruno, S.; Zuin, M.; Crosignani, A.; Rossi, S.; Zadra, F.; Roffi, L.; Borzio, M.; Redaelli, A.; Chiesa, A.; Silini, E.M.; et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: A long-term prospective study. Am. J. Gastroenterol. 2009, 104, 1147–1158. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W.; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef]
- de Franchis, R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Chhatwal, J.; Wang, X.; Ayer, T.; Kabiri, M.; Chung, R.T.; Hur, C.; Donohue, J.M.; Roberts, M.S.; Kanwal, F. Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals. Hepatology 2016, 64, 1442–1450. [Google Scholar] [CrossRef]
- Jakobsen, J.C.; Nielsen, E.E.; Feinberg, J.; Katakam, K.K.; Fobian, K.; Hauser, G.; Poropat, G.; Djurisic, S.; Weiss, K.H.; Bjelakovic, M.; et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst. Rev. 2017, 9, CD012143. [Google Scholar]
- Afdhal, N.; Everson, G.T.; Calleja, J.L.; McCaughan, G.W.; Bosch, J.; Brainard, D.M.; McHutchison, J.G.; De-Oertel, S.; An, D.; Charlton, M.; et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J. Viral Hepat. 2017, 24, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, A.F.; Reiberger, T.; Beinhardt, S.; et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 2016, 65, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.M.; Green, P.K.; Rockey, D.C.; Berry, K.; Ioannou, G.N. Hepatitis C eradication with direct-acting anti-virals reduces the risk of variceal bleeding. Aliment. Pharmacol. Ther. 2020, 51, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Thabut, D.; Bureau, C.; Layese, R.; Bourcier, V.; Hammouche, M.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; et al. Validation of Baveno VI criteria for screening and surveillance of esophageal varices in patients with compensated cirrhosis and a sustained response to antiviral therapy. Gastroenterology 2019, 156, 997–1009.e5. [Google Scholar] [CrossRef]
- Lens, S.; Alvarado-Tapias, E.; Mariño, Z.; Londoño, M.C.; LLop, E.; Martinez, J.; Fortea, J.I.; Ibañez, L.; Ariza, X.; Baiges, A.; et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology 2017, 153, 1273–1283.e1. [Google Scholar] [CrossRef] [Green Version]
- Yuri, Y.; Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; et al. Impact of sustained virological response for gastroesophageal varices in hepatitis-C-virus-related liver cirrhosis. J. Clin. Med. 2019, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- Puigvehí, M.; Londoño, M.C.; Torras, X.; Lorente, S.; Vergara, M.; Morillas, R.M.; Masnou, H.; Serrano, T.; Miquel, M.; Gallego, A.; et al. Impact of sustained virological response with DAAs on gastroesophageal varices and Baveno criteria in HCV-cirrhotic patients. J. Gastroenterol. 2020, 55, 205–216. [Google Scholar] [CrossRef]
- Miyaaki, H.; Ichikawa, T.; Taura, N.; Miuma, S.; Isomoto, H.; Nakao, K. Endoscopic management of esophagogastric varices in Japan. Ann. Transl. Med. 2014, 2, 42. [Google Scholar] [CrossRef]
- Wan, S.; Wei, Y.; Zhang, X.; Liu, X.; Zhang, W.; He, Y.; Yuan, F.; Yao, S.; Yue, Y.; Song, B. Multiparametric radiomics nomogram may be used for predicting the severity of esophageal varices in cirrhotic patients. Ann. Transl. Med. 2020, 8, 186. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Feld, J.J. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis C virus infection? Gastroenterology 2019, 156, 446–460.e2. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Toyoda, H.; Mizuno, K.; Sone, Y.; Kataoka, S.; Kataoka, S.; Hashinokuchi, S. Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J. Gastroenterol. Hepatol. 2017, 32, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.L.; Villesen, I.F.; Leeming, D.J.; Karsdal, M.A.; Sølund, C.; Tarp, B.; Kristensen, L.H.; Holmboe, C.H.; Leutscher, P.; Laursen, A.L.; et al. Altered balance between collagen formation and degradation after successful direct-acting antiviral therapy of chronic hepatitis C. J. Viral Hepat. 2021, 28, 236–244. [Google Scholar] [CrossRef]
- Persico, M.; Rosato, V.; Aglitti, A.; Precone, D.; Corrado, M.; De Luna, A.; Morisco, F.; Camera, S.; Federico, A.; Dallio, M.; et al. Sustained virological response by direct antiviral agents in HCV leads to an early and significant improvement of liver fibrosis. Antivir. Ther. 2018, 23, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belli, L.S.; Perricone, G.; Adam, R.; Cortesi, P.A.; Strazzabosco, M.; Facchetti, R.; Karam, V.; Salizzoni, M.; Andujar, R.L.; Fondevila, C.; et al. Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J. Hepatol. 2018, 69, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Lim, J.K.; Kuo, A.; Di Bisceglie, A.M.; Galati, J.S.; Morelli, G.; Everson, G.T.; Kwo, P.Y.; Brown, R.S., Jr.; Sulkowski, M.S.; et al. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: Observations through HCV-TARGET database. Aliment. Pharmacol. Ther. 2017, 45, 115–126. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2017, 68, 25–32. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, R.; Aghemo, A.; Rumi, M.G.; Ronchi, G.; Donato, M.F.; Paradis, V.; Colombo, M.; Bedossa, P. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 2012, 56, 532–543. [Google Scholar] [CrossRef]
- Poynard, T.; Moussalli, J.; Munteanu, M.; Thabut, D.; Lebray, P.; Rudler, M.; Ngo, Y.; Thibault, V.; Mkada, H.; Charlotte, F.; et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J. Hepatol. 2013, 59, 675–683. [Google Scholar] [CrossRef]
- Libânio, D.; Marinho, R.T. Impact of hepatitis C oral therapy in portal hypertension. World J. Gastroenterol. 2017, 23, 4669–4674. [Google Scholar] [CrossRef]
| n | 36 |
|---|---|
| Male/female | 16/20 |
| Age (years), median (range) | 72 (56–83) |
| Dacratasvir-asunaprevir/sofosbuvir/ledipasvir-sofosbuvir/ombitasvir-paritaprevir-ritonavir/glecaprevir-pibrentasvir | 16/11/5/1/3 |
| Child–Pugh cirrhosis classification, A/B/C | 30/6/0 |
| Past history of GEV treatment, yes/no | 8/28 |
| Past history of HCC treatment, yes/no | 20/16 |
| Presence of GEVs before DAA, yes/no | 35/1 |
| GEV, EV/GV/EV + GV | 16/2/17 |
| Form, F1/F2/F3 | 31/4/0 |
| RC sign, RC0/RC1/RC2/RC3 | 26/8/1/0 |
| GEV grade, 0/1/2/3 | 1/24/11/0 |
| Before DAAs | After DAAs | p-Value | |
|---|---|---|---|
| AST (IU/L) | 67.4 [46.9] | 30.5 [9.7] | <0.001 |
| ALT (IU/L) | 59.8 [36.3] | 20.6 [9.3] | <0.001 |
| Platelet count (×104/mm3) | 8.2 [3.0] | 9.6 [3.8] | 0.002 |
| Serum albumin (g/dL) | 3.45 [0.37] | 3.99 [0.63] | <0.001 |
| Total bilirubin (mg/dL) | 0.95 [0.37] | 1.04 [0.48] | 0.14 |
| Prothrombin time (%) | 77.5 [16.0] | 86.4 [15.1] | 0.002 |
| ALBI | 2.15 [0.35] | 2.58 [0.53] | <0.001 |
| Type IV collagen | 258 [75.1] | 210 [66.4] | <0.001 |
| FIB-4 index | 9.29 [4.69] | 6.27 [3.00] | <0.001 |
| Child–Pugh, A/B/C | 30/6/0 | 35/1/0 | 0.14 |
| GEV, none/EV/GV/EV + GV | 1/16/2/17 | 0/17/2/17 | 0.79 |
| Form, none/F1/F2 | 1/31/4 | 0/26/10 | 0.13 |
| RC sign, RC0/RC1/RC2 | 27/8/1 | 25/7/4 | 0.38 |
| GEV grade, 0/1/2/3 | 1/24/11/0 | 0/22/9/5 | 0.098 |
| Stable GEVs | Progressive GEVs | Odds Ratio | p-Value | 95% Confidence Interval | |
|---|---|---|---|---|---|
| n | 27 | 9 | |||
| Male/Female | 11/16 | 5/4 | 1.82 | 0.44 | 0.39–8.33 |
| Age, years (range) | 72 (56–83) | 72 (59–83) | 1.02 | 0.67 | 0.91–1.13 |
| dacratasvir-asunaprevir/sofosbuvir/ledipasvir-sofosbuvir/ombitasvir-paritaprevir-ritonavir/glecaprevir-pibrentasvir | 12/9/2/1/3 | 4/2/3/0/0 | 0.20 | ||
| Child–Pugh; A/B/C | 23/4/0 | 7/2/0 | 1.64 | 0.61 | 0.25–10.95 |
| Past history of GEVs treatment, yes/no | 6/21 | 2/7 | 1.00 | 1.00 | 0.16–6.14 |
| Past history of HCC treatment, yes/no | 15/12 | 5/4 | 1.00 | 1.00 | 0.22–4.56 |
| Presence of GEV varices before DAA, yes/no | 27/0 | 8/1 | 0.00 | 0.08 | 0.00–1.26 |
| AST, mean [SD] | 65.2 [35.3] | 73.8 [74.1] | 2.57 | 0.64 | 0.05–126.2 |
| ALT, mean [SD] | 48.8 [21.2] | 52.4 [50.8] | 1.57 | 0.80 | 0.05–45.7 |
| Platelet count, mean ×104/mm3 [SD] | 8.3 [3.1] | 7.8 [2.6] | 0.51 | 0.68 | 0.02–12.46 |
| Serum albumin, mean g/dL [SD] | 3.44 [0.3] | 3.49 [0.5] | 1.78 | 0.74 | 0.06–51.52 |
| Total bilirubin, mean mg/dL [SD] | 0.91 [0.3] | 1.07 [0.4] | 4.79 | 0.28 | 0.29–80.06 |
| Prothrombin time, mean % [SD] | 78.3 [15.9] | 74.9 [17.0] | 0.40 | 0.57 | 0.02–9.57 |
| ALBI, mean [SD] | −2.2 [0.3] | −2.1 [0.5] | 0.90 | 0.95 | 0.03–22.92 |
| Type IV collagen, mean [SD] | 249.0 [80.6] | 281.5 [55.9] | 5.33 | 0.29 | 0.23–125.14 |
| FIB-4 index, mean [SD] | 8.9 [4.1] | 10.4 [6.2] | 3.87 | 0.41 | 0.15–96.02 |
| GEV; None/EV/GV/EV + GV | 0/14/2/11 | 1/2/0/6 | 0.10 | ||
| EV + GV | 3.82 | 0.14 | 0.64–22.7 | ||
| Form; None/F1/F2 | 0/24/3 | 1/7/1 | 0.10 | ||
| F2 | 1.14 | 0.91 | 0.10–12.8 | ||
| RC sign; RC0/RC1/RC2 | 22/5/0/ | 4/3/1 | 0.13 | ||
| RC1-2 | 2.64 | 0.27 | 0.47–14.9 | ||
| GEV grade; 0/1/2 | 0/21/6 | 1/3/5 | 0.027 | ||
| Grade-2 | 5.83 | 0.04 | 1.07–31.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hisanaga, H.; Takedatsu, H.; Emori, K.; Inoue, H.; Kunitake, Y.; Nakane, T.; Fukunaga, S.; Ide, T.; Mitsuyama, K.; Torimura, T. Effect of Direct-Acting Antiviral Agents on Gastroesophageal Varices in Patients with Hepatitis C Virus-Related Cirrhosis. Medicina 2022, 58, 1077. https://doi.org/10.3390/medicina58081077
Hisanaga H, Takedatsu H, Emori K, Inoue H, Kunitake Y, Nakane T, Fukunaga S, Ide T, Mitsuyama K, Torimura T. Effect of Direct-Acting Antiviral Agents on Gastroesophageal Varices in Patients with Hepatitis C Virus-Related Cirrhosis. Medicina. 2022; 58(8):1077. https://doi.org/10.3390/medicina58081077
Chicago/Turabian StyleHisanaga, Hiroshi, Hidetoshi Takedatsu, Keigo Emori, Hiroto Inoue, Yasuhumi Kunitake, Tomoyuki Nakane, Shuhei Fukunaga, Tatsuya Ide, Keiichi Mitsuyama, and Takuji Torimura. 2022. "Effect of Direct-Acting Antiviral Agents on Gastroesophageal Varices in Patients with Hepatitis C Virus-Related Cirrhosis" Medicina 58, no. 8: 1077. https://doi.org/10.3390/medicina58081077
APA StyleHisanaga, H., Takedatsu, H., Emori, K., Inoue, H., Kunitake, Y., Nakane, T., Fukunaga, S., Ide, T., Mitsuyama, K., & Torimura, T. (2022). Effect of Direct-Acting Antiviral Agents on Gastroesophageal Varices in Patients with Hepatitis C Virus-Related Cirrhosis. Medicina, 58(8), 1077. https://doi.org/10.3390/medicina58081077






