Rosinidin Protects Streptozotocin-Induced Memory Impairment-Activated Neurotoxicity by Suppressing Oxidative Stress and Inflammatory Mediators in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Protocol
- Group I—Normal
- Group II—Streptozotocin + Saline
- Group III—Streptozotocin + Rosinidin-10 mg/kg
- Group IV—Streptozotocin + Rosinidin-20 mg/kg
2.4. Acute Toxicological Studies
2.5. Motor Function
2.5.1. Y-Maze Test
2.5.2. Morris Water Maze Test
2.6. Biochemical Parameters
- Sacrifice and Homogenization
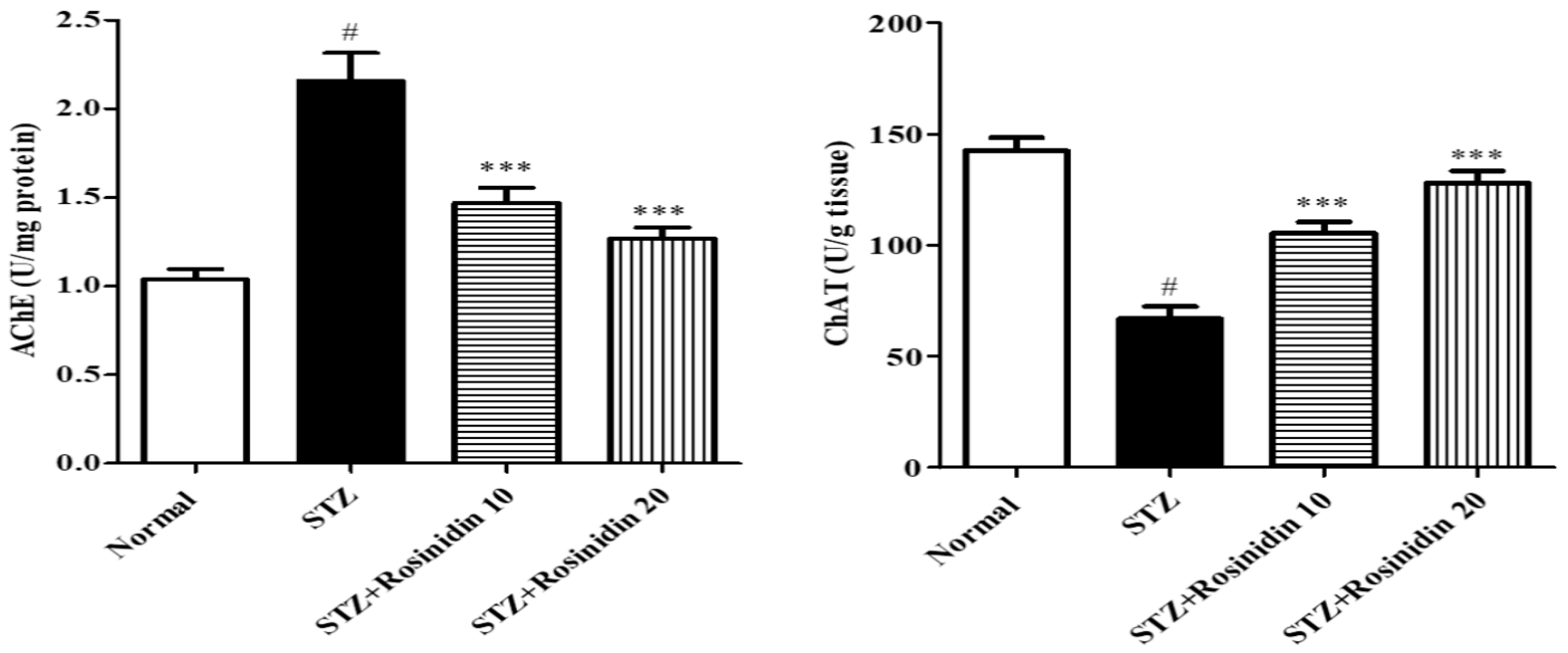
2.6.1. Acetylcholinesterase (AchE) and Choline Acetyltransferase (ChAT)
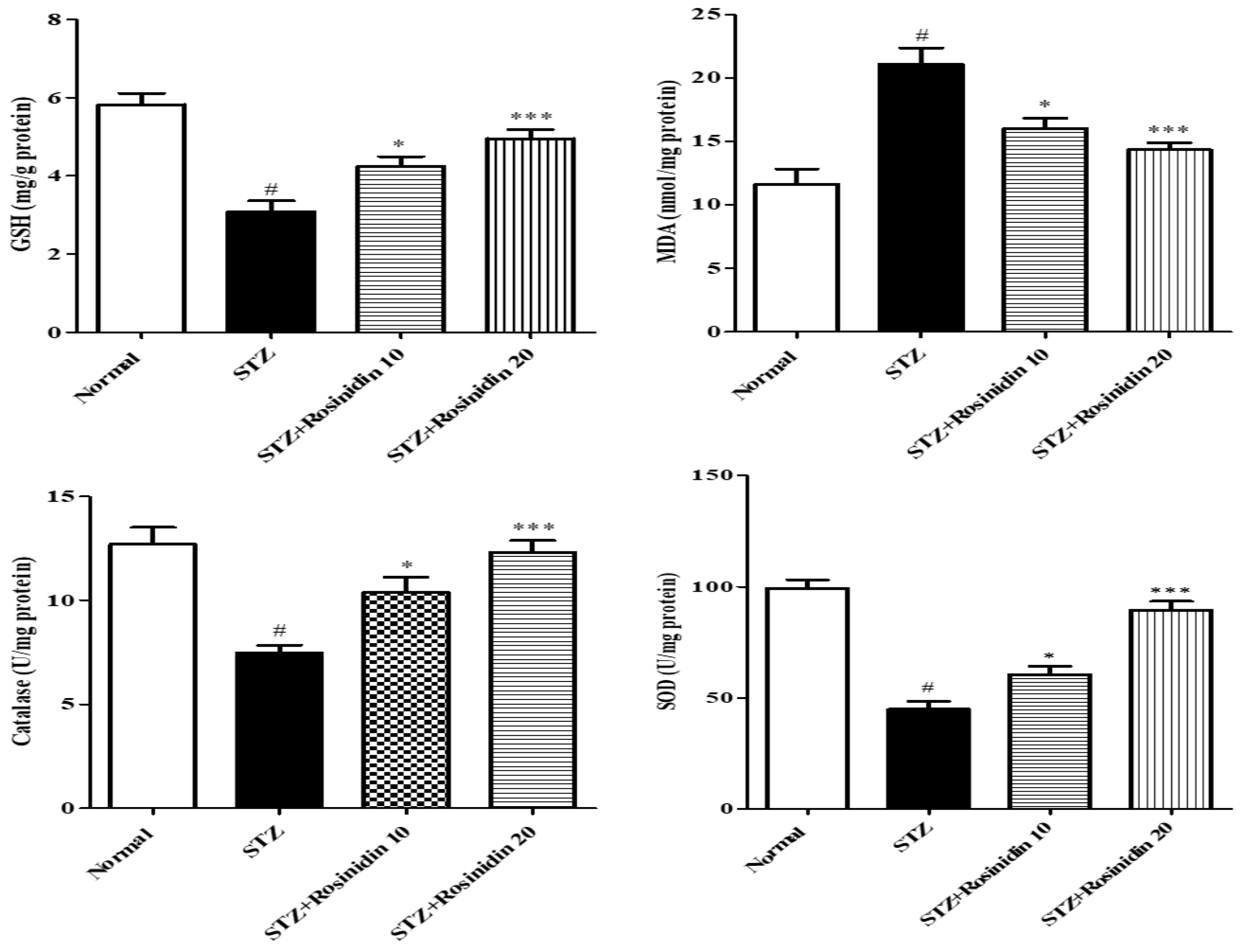
2.6.2. Antioxidant Enzyme Estimation
Malondialdehyde Determination (MDA)
Reduced Glutathione (GSH) Estimation
Superoxide Dismutase (SOD)
Catalase (CAT) Assay
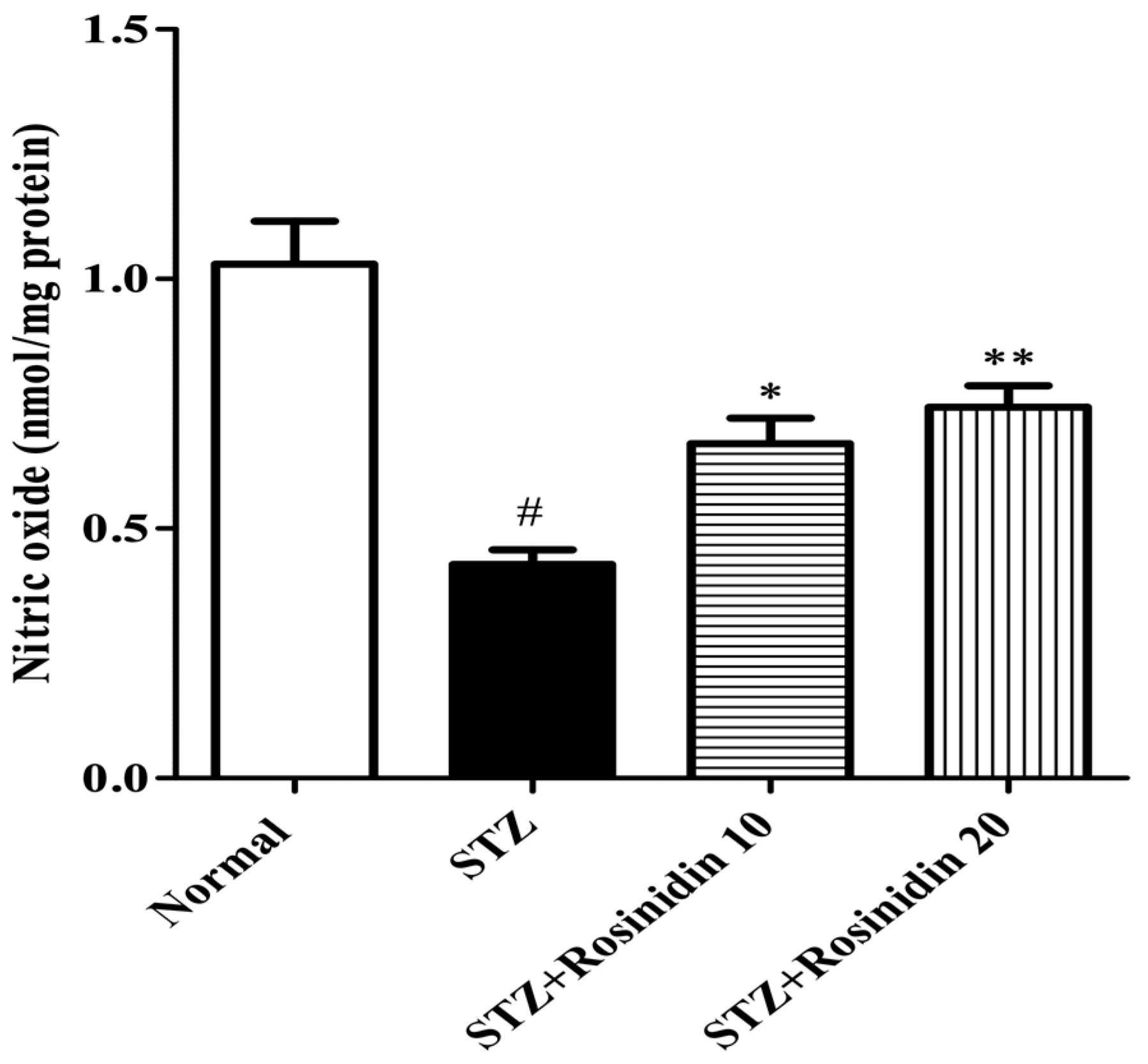
2.6.3. Nitrite Estimation
2.6.4. Neuroinflammatory Parameters
2.7. Statistical Analysis
3. Results
3.1. Acute Toxicity Study
3.2. Behavioural Tests
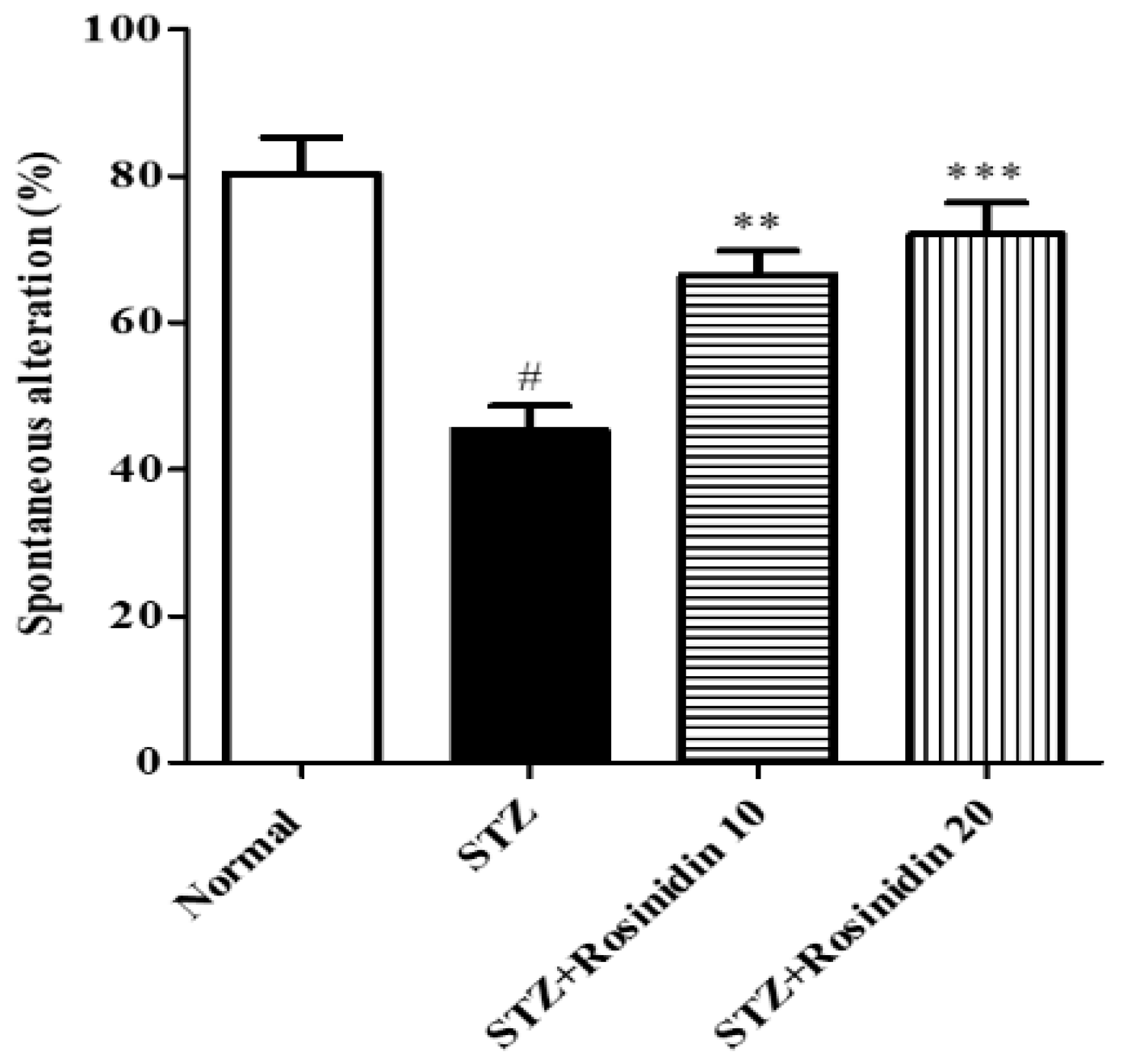
- Y-maze Test
- Morris Water Maze
3.3. Biochemical Parameters
- Acetylcholinesterase and Choline Acetyltransferase
- Antioxidant Parameters
- Nitrite Assay
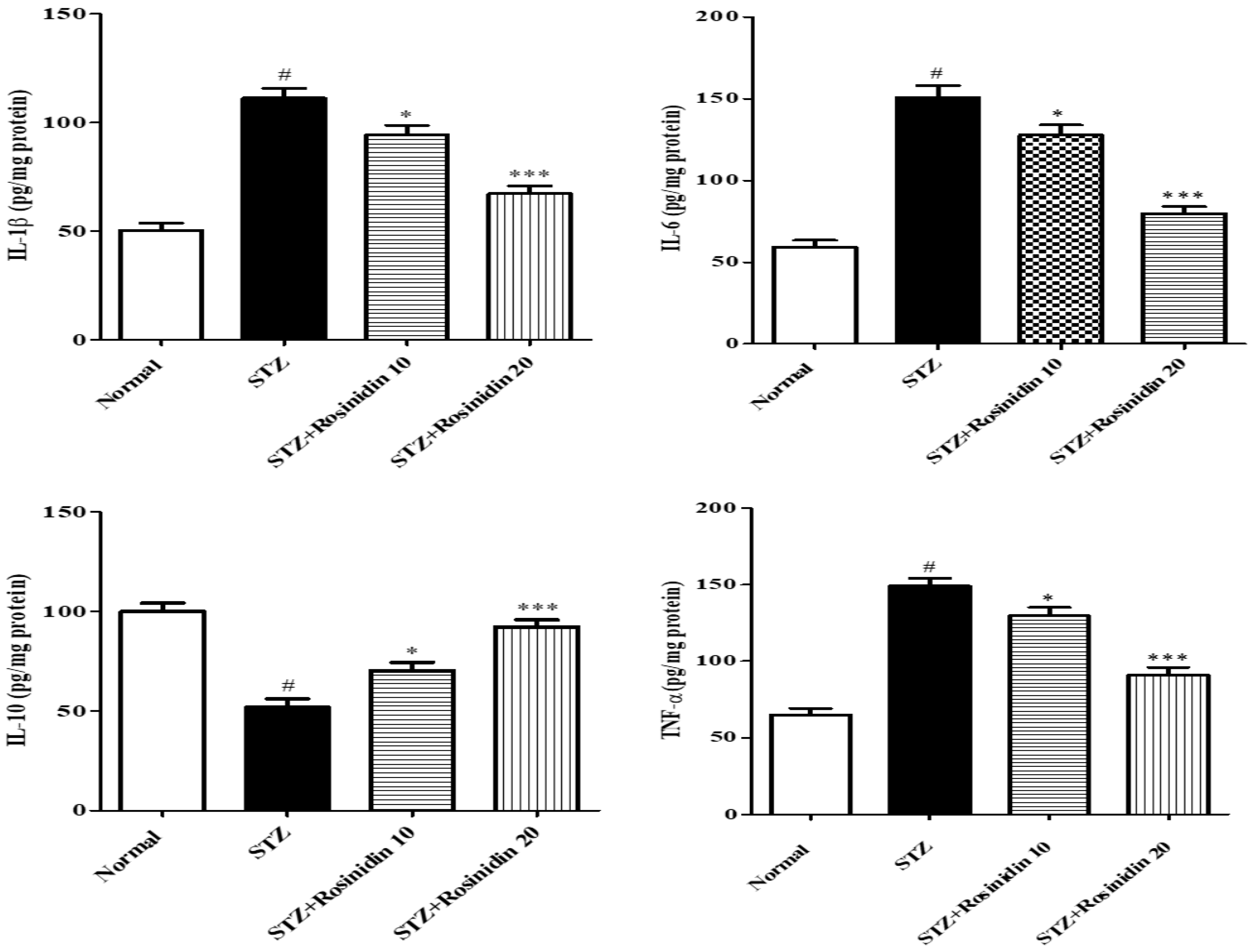
- Neuroinflammatory Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinitha, E.; Singh, H.J.; Kakalij, R.M.; Kshirsagar, R.P.; Kumar, B.H.; Diwan, P. Neuroprotective effect of Prunus avium on streptozotocin induced neurotoxicity in mice. Biomed. Prev. Nutr. 2014, 4, 519–525. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 943–972. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef]
- Perry, E.K.; Tomlinson, B.E.; Blessed, G.; Bergmann, K.; Gibson, P.H.; Perry, R.H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 1978, 2, 1457–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanwick, G.R.; Kirby, M.; Bruce, I.; Buggy, F.; Coen, R.F.; Coakley, D.; Lawlor, B.A. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer’s disease: Lack of association between longitudinal and cross-sectional findings. Am. J. Psychiatry 1998, 155, 286–289. [Google Scholar] [CrossRef]
- Weerateerangkull, P.; Praputpittaya, C.; Banjerdpongchai, R. Effects of Ascorbic Acid on Streptozotocin-induced Oxidative Stress and Memory Impairment in Rats. Thai J. Physiol. Sci. 2007, 20, 54–61. [Google Scholar]
- Kang, E.B.; Cho, J.Y. Effects of treadmill exercise on brain insulin signaling and β-amyloid in intracerebroventricular streptozotocin induced-memory impairment in rats. J. Exerc. Nutr. Biochem. 2014, 18, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishrat, T.; Hoda, N.; Khan, M.M.; Yousuf, S.; Ahmad, M.; Ahmad, A.; Islam, F. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT). Eur. Neuropsychopharmacol. 2009, 19, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S.; Lannert, H. Long-term effects of corticosterone on behavior, oxidative and energy metabolism of parietotemporal cerebral cortex and hippocampus of rats: Comparison to intracerebroventricular streptozotocin. J. Neural Transm. 2008, 115, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef]
- El Sayed, N.S.; Ghoneum, M.H. Antia, a natural antioxidant product, attenuates cognitive dysfunction in streptozotocin-induced mouse model of sporadic Alzheimer’s disease by targeting the amyloidogenic, inflammatory, autophagy, and oxidative stress pathways. Oxidative Med. Cell. Longev. 2020, 2020, 4386562. [Google Scholar] [CrossRef]
- Hindam, M.O.; Sayed, R.H.; Skalicka-Woźniak, K.; Budzyńska, B.; El Sayed, N.S. Xanthotoxin and umbelliferone attenuate cognitive dysfunction in a streptozotocin-induced rat model of sporadic Alzheimer’s disease: The role of JAK2 / STAT3 and Nrf2/ HO -1 signalling pathway modulation. Phytother. Res. 2020, 34, 2351–2365. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.F.M.; Viana, J.D.O.; Nayarisseri, A.; Zondegoumba, E.N.; Junior, F.J.B.M.; Scotti, M.T.; Scotti, L. Computational Studies Applied to Flavonoids against Alzheimer’s and Parkinson’s Diseases. Oxidative Med. Cell. Longev. 2018, 2018, e7912765. [Google Scholar] [CrossRef]
- Singh, D.; Hembrom, S. Neuroprotective Effect of Flavonoids: A Systematic Review. Int. J. Aging Res. 2019, 2, 26. [Google Scholar] [CrossRef]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.-L.; Luo, Y.; Jin, S.-H.; Yuan, K.; Guo, Y. Ameliorative effect of anthocyanin on depression mice by increasing monoamine neurotransmitter and up-regulating BDNF expression. J. Funct. Foods 2020, 66, 103757. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S. Rosinidin Attenuates Lipopolysaccharide-Induced Memory Impairment in Rats: Possible Mechanisms of Action Include Antioxidant and Anti-Inflammatory Effects. Biomolecules 2021, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, M.; Baydas, G. Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur. J. Pharmacol. 2006, 537, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Moghadamnia, A.A.; Hakiminia, S.; Baradaran, M.; Kazemi, S.; Ashrafpour, M. Vitamin D Improves Learning and Memory Impairment in Streptozotocin-Induced Diabetic Mice. Arch. Iran. Med. 2015, 18, 362–366. [Google Scholar] [PubMed]
- Afzal, M.; Sayyed, N.; Alharbi, K.S.; Alzarea, S.I.; Alshammari, M.S.; Alomar, F.A.; Alenezi, S.K.; Quazi, A.M.; Alzarea, A.I.; Kazmi, I. Anti-Huntington’s Effect of Rosiridin via Oxidative Stress/AchE Inhibition and Modulation of Succinate Dehydrogenase, Nitrite, and BDNF Levels against 3-Nitropropionic Acid in Rodents. Biomolecules 2022, 12, 1023. [Google Scholar] [CrossRef]
- Gilani, S.J.; Bin-Jumah, M.N.; Al-Abbasi, F.A.; Nadeem, M.S.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Afzal, M.; Alzarea, S.I.; Sayyed, N.; et al. Rosinidin Flavonoid Ameliorates Hyperglycemia, Lipid Pathways and Proinflammatory Cytokines in Streptozotocin-Induced Diabetic Rats. Pharmaceutics 2022, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Yuan, J.; Tang, Z.-J.; Wei, H.-J.; Zhu, W.-W.; Zhang, P.; Gu, H.-F.; Wang, C.-Y.; Tang, X.-Q. Hydrogen sulfide ameliorates cognitive dysfunction in streptozotocin-induced diabetic rats: Involving suppression in hippocampal endoplasmic reticulum stress. Oncotarget 2017, 8, 64203–64216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassani, T.B.; Turnes, J.M.; Moura, E.L.; Bonato, J.M.; Cóppola-Segovia, V.; Zanata, S.M.; Oliveira, R.M.M.W.; Vital, M.A.B.F. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017, 335, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Sharma, V.; Mehan, S.; Sharma, N.; Bedi, K. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine—A PDE1 inhibitor. Eur. J. Pharmacol. 2009, 620, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Storozheva, Z.I.; Zakharova, E.I.; Proshin, A.T. Evaluation of the Activity of Choline Acetyltransferase From Different Synaptosomal Fractions at the Distinct Stages of Spatial Learning in the Morris Water Maze. Front. Behav. Neurosci. 2021, 15, e755373. [Google Scholar] [CrossRef] [PubMed]
- Wills, E.D. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966, 99, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.W.I.; Belcher, R.V. A colorimetric micro-method for the determination of glutathione. Biochem. J. 1965, 94, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Masayasu, M.; Hiroshi, Y. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar] [CrossRef]
- Hadwan, M. New Method for Assessment of Serum Catalase Activity. Indian J. Sci. Technol. 2016, 9, e80499. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pinton, S.; da Rocha, J.T.; Gai, B.M.; Nogueira, C.W. Sporadic dementia of Alzheimer’s type induced by streptozotocin promotes anxiogenic behavior in mice. Behav. Brain Res. 2011, 223, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S.; Lannert, H. Inhibition of the Neuronal Insulin Receptor Causes Alzheimer-like Disturbances in Oxidative/Energy Brain Metabolism and in Behavior in Adult Rats. Ann. N. Y. Acad. Sci. 1999, 893, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, N.O.A.; El Sayed, N.S.; El-Khatib, A.S. Targeting central β2 receptors ameliorates streptozotocin-induced neuroinflammation via inhibition of glycogen synthase kinase3 pathway in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 65–75. [Google Scholar] [CrossRef]
- Zhang, C.T.; Lin, J.R.; Wu, F.; Ghosh, A.; Tang, S.S.; Hu, M.; Long, Y.; Bin Sun, H.; Hong, H. Montelukast ameliorates streptozotocin-induced cognitive impairment and neurotoxicity in mice. NeuroToxicology 2016, 57, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-C.; Xie, H.; Chen, F.; Hu, M.; Long, Y.; Sun, H.-B.; Kong, L.-Y.; Hong, H.; Tang, S.-S. Simvastatin ameliorates memory impairment and neurotoxicity in streptozotocin-induced diabetic mice. Neuroscience 2017, 355, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Zendehbad, B.; Zabihi, H.; Hosseini, M.; Hadjzadeh, M.A.R.; Hayatdavoudi, P. Beneficial Effect of Leptin on Spatial Learning and Memory in Streptozotocin-Induced Diabetic Rats. Balk. Med. J. 2016, 33, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Baydas, G.; Nedzvetskii, V.S.; Nerush, P.A.; Kirichenko, S.V.; Yoldas, T. Altered expression of NCAM in hippocampus and cortex may underlie memory and learning deficits in rats with streptozotocin-induced diabetes mellitus. Life Sci. 2003, 73, 1907–1916. [Google Scholar] [CrossRef]
- Deshmukh, R.; Kaundal, M.; Bansal, V. Samardeep Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats. Biomed. Pharmacother. 2016, 81, 56–62. [Google Scholar] [CrossRef]
- Saxena, G.; Singh, S.P.; Agrawal, R.; Nath, C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur. J. Pharmacol. 2008, 581, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.R.; Warburton, E.C. Critical role of the cholinergic system for object-in-place associative recognition memory. Learn. Mem. 2008, 16, 8–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonkusare, S.; Srinivasan, K.; Kaul, C.; Ramarao, P. Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci. 2005, 77, 1–14. [Google Scholar] [CrossRef]
- Tota, S.; Kamat, P.K.; Shukla, R.; Nath, C. Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment. Behav. Brain Res. 2011, 221, 207–215. [Google Scholar] [CrossRef]
- Kaur, S.; Singla, N.; Dhawan, D.K. Neuro-protective potential of quercetin during chlorpyrifos induced neurotoxicity in rats. Drug Chem. Toxicol. 2019, 42, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Storz, G.; Imlayt, J.A. Oxidative stress. Curr. Opin. Microbiol. 1999, 2, 188–194. [Google Scholar] [CrossRef]
- Metodiewa, D.; Kośka, C. Reactive oxygen species and reactive nitrogen species: Relevance to cyto(neuro)toxic events and neurologic disorders. An overview. Neurotox. Res. 1999, 1, 197–233. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Bala, A.; Deshmukh, R.; Bedi, K.; Sharma, P. Neuroprotective effect of RO-20-1724-a phosphodiesterase4 inhibitor against intracerebroventricular streptozotocin induced cognitive deficit and oxidative stress in rats. Pharmacol. Biochem. Behav. 2012, 101, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.C.H.; Kakalij, R.M.; Kshirsagar, R.P.; Kumar, B.H.; Komakula, S.S.B.; Diwan, P. Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharm. Biol. 2014, 53, 630–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, A.; Kalra, J.K.; Kumar, A. Neuroprotective effect of N-acetyl cysteine against streptozotocin-induced memory dysfunction and oxidative damage in rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 13–23. [Google Scholar] [CrossRef]
- Rajasekar, N.; Nath, C.; Hanif, K.; Shukla, R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STREPTOZOTOCIN (ICV)-induced memory impaired rats. Life Sci. 2017, 173, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Deshmukh, R. Embelin Attenuates Intracerebroventricular Streptozotocin-Induced Behavioral, Biochemical, and Neurochemical Abnormalities in Rats. Mol. Neurobiol. 2016, 54, 6670–6680. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Prashar, A.; Deshmukh, R. Effect of Licofelone—A Dual COX/5-LOX Inhibitor in Intracerebroventricular Streptozotocin-Induced Behavioral and Biochemical Abnormalities in Rats. J. Mol. Neurosci. 2015, 55, 749–759. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, K.S.; Afzal, M.; Alzarea, S.I.; Khan, S.A.; Alomar, F.A.; Kazmi, I. Rosinidin Protects Streptozotocin-Induced Memory Impairment-Activated Neurotoxicity by Suppressing Oxidative Stress and Inflammatory Mediators in Rats. Medicina 2022, 58, 993. https://doi.org/10.3390/medicina58080993
Alharbi KS, Afzal M, Alzarea SI, Khan SA, Alomar FA, Kazmi I. Rosinidin Protects Streptozotocin-Induced Memory Impairment-Activated Neurotoxicity by Suppressing Oxidative Stress and Inflammatory Mediators in Rats. Medicina. 2022; 58(8):993. https://doi.org/10.3390/medicina58080993
Chicago/Turabian StyleAlharbi, Khalid Saad, Muhammad Afzal, Sami I. Alzarea, Shah Alam Khan, Fadhel A. Alomar, and Imran Kazmi. 2022. "Rosinidin Protects Streptozotocin-Induced Memory Impairment-Activated Neurotoxicity by Suppressing Oxidative Stress and Inflammatory Mediators in Rats" Medicina 58, no. 8: 993. https://doi.org/10.3390/medicina58080993






