Is Adnexectomy Mandatory at the Time of Hysterectomy for Uterine Sarcomas? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Method
2.2. Study Selection
2.3. Statistical Analysis
2.4. Quality Assessment
3. Results
3.1. Studies’ Characteristics
3.2. Outcomes
3.2.1. Meta-Analysis
3.2.2. Endometrial Stromal Sarcoma (ESS)
3.2.3. Leiomyosarcoma (LMS)
3.2.4. Adenosarcoma (AS)
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| COHORT | SELECTION | COMPARABILITY | EXPOSURE |
|---|---|---|---|
| Amant 2007 [6] | 3 | 2 | 3 |
| Berchuck 1990 [4] | 2 | 1 | 3 |
| Gadducci 1990 [7] | 2 | 1 | 2 |
| Gadducci 1996 [8] | 2 | 1 | 2 |
| Kapp 2008 [9] | 2 | 1 | 1 |
| Karataşlı 2020 [10] | 4 | 2 | 4 |
| Kim 2008 [11] | 4 | 2 | 3 |
| Lee 2017 [12] | 3 | 2 | 4 |
| Li 2005 [13] | 3 | 3 | 2 |
| Li 2007 [14] | 3 | 2 | 4 |
| Nasioudis 2017 [15] | 4 | 2 | 4 |
| Nasioudis 2020 [16] | 4 | 2 | 3 |
| Sait 2014 [5] | 2 | 1 | 2 |
| Sia 2021 [17] | 3 | 2 | 1 |
| Soh 1999 [18] | 2 | 1 | 1 |
| Stewart 2018 [19] | 2 | 1 | 2 |
Appendix B
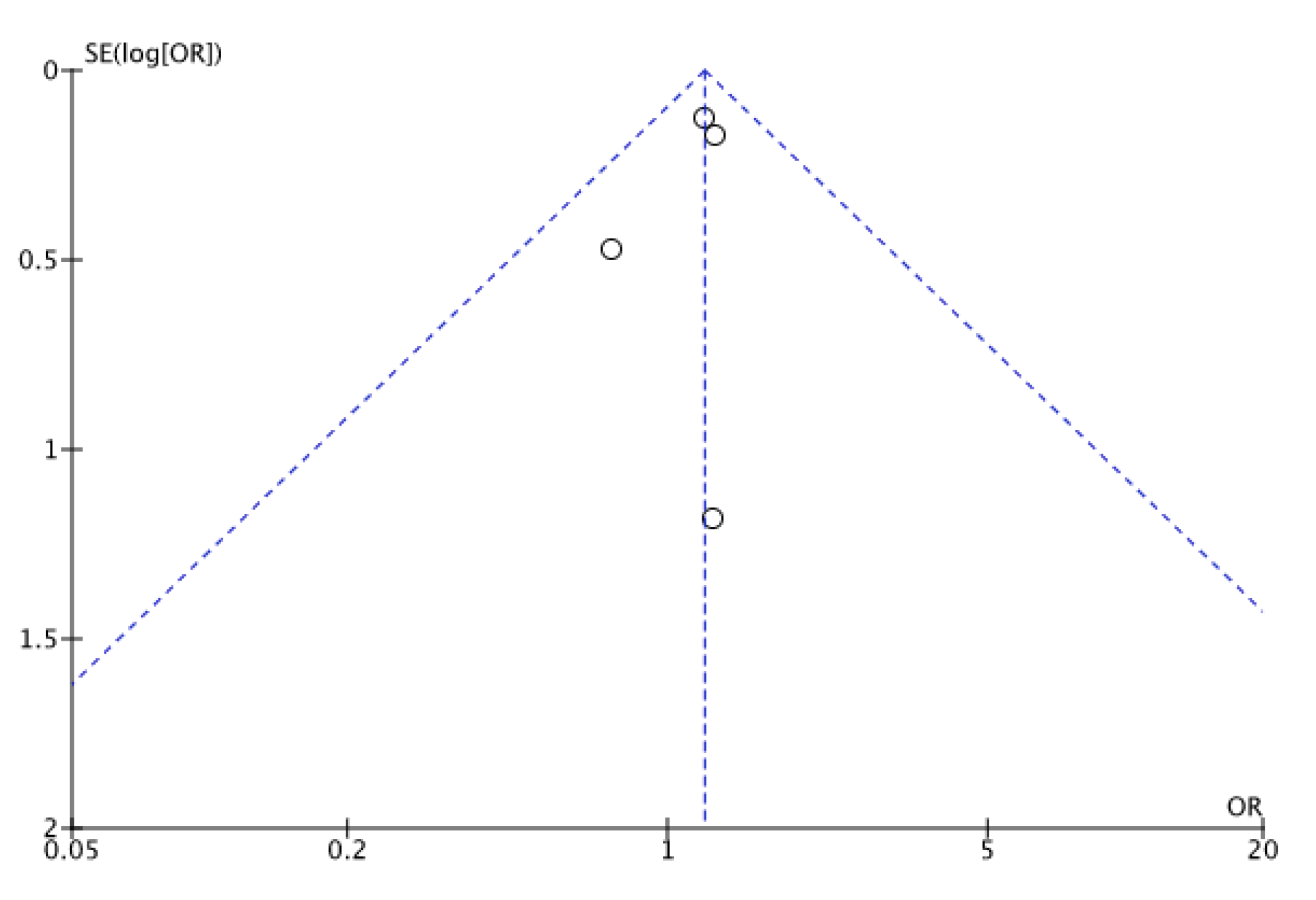
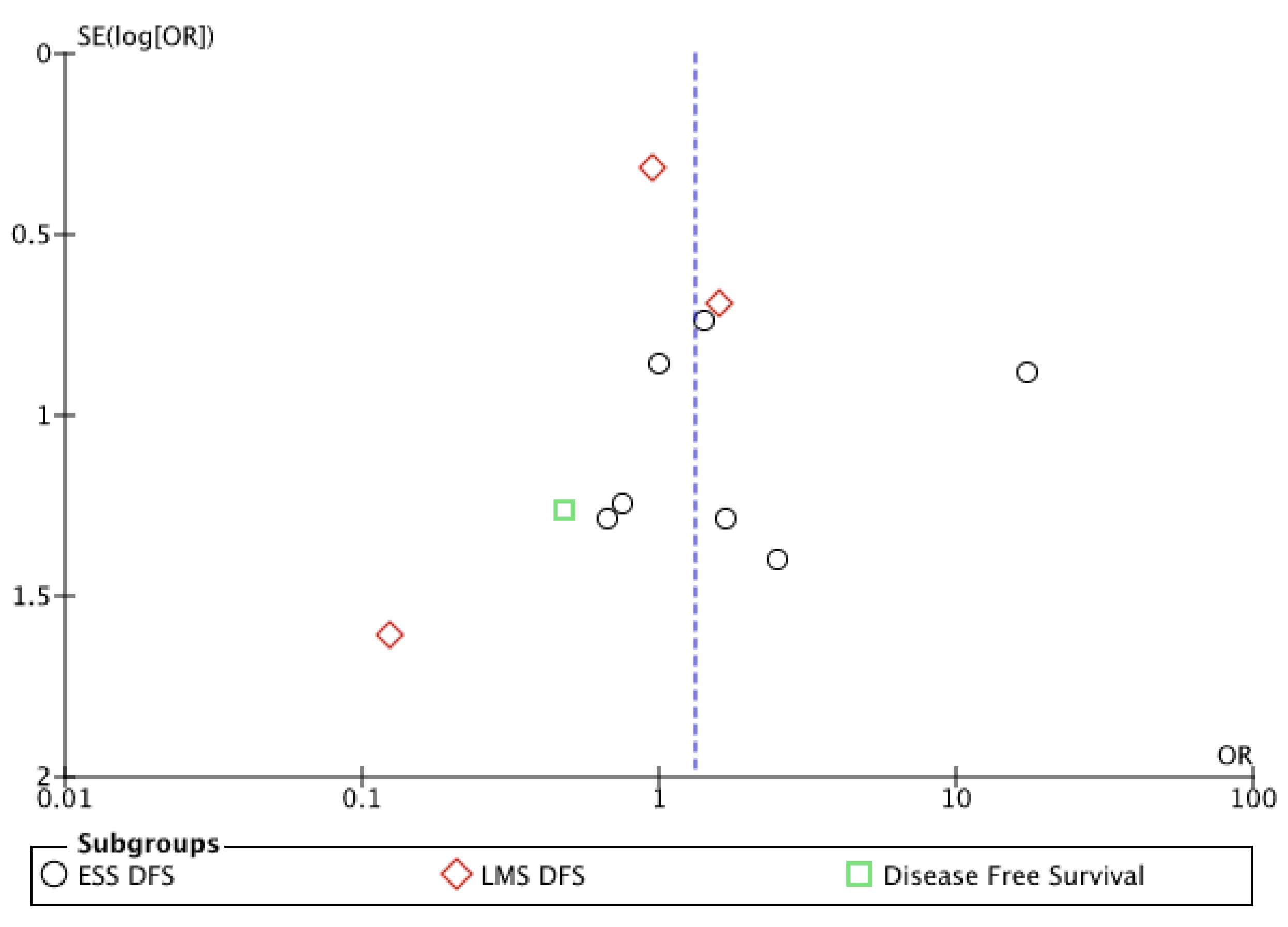
References
- AIOM 2020 Guidelines Upon GIST and Soft Tissues Sarcomas. Available online: https://www.aiom.it/wpcontent/uploads/2020/10/2020_LG_AIOM_Sarcomi.pdf (accessed on 1 March 2020).
- Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [PubMed]
- Kansagara, D.; O’Neil, M.; Nugent, S.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’apuaka, M.; Paynter, R.; et al. Quality Assessment Criteria for Observational Studies, Based on the Newcastle-Ottawa Scale. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK476448/table/appc.t4/ (accessed on 16 August 2021).
- Berchuck, A.; Rubin, S.C.; Hoskins, W.J.; Saigo, P.E.; Pierce, V.K.; Lewis, J.L., Jr. Treatment of endometrial stromal tumors. Gynecol. Oncol. 1990, 36, 60–65. [Google Scholar] [CrossRef]
- Sait, H.K.; Anfinan, N.M.; El Sayed, M.E.; Alkhayyat, S.S.; Ghanem, A.T.; Abayazid, R.M.; Sait, K.H. Uterine sarcoma. Clinico-pathological characteristics and outcome. Saudi Med. J. 2014, 35, 1215–1222. [Google Scholar] [PubMed]
- Amant, F.; De Knijf, A.; Van Calster, B.; Leunen, K.; Neven, P.; Berteloot, P.; Vergote, I.; Van Huffel, S.; Moerman, P. Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Br. J. Cancer. 2007, 97, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Landoni, F.; Sartori, E.; Zola, P.; Maggino, T.; Lissoni, A.; Bazzurini, L.; Arisio, R.; Romagnolo, C.; Cristofani, R. Uterine leiomyosarcoma: Analysis of treatment failures and survival. Gynecol. Oncol. 1996, 62, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Sartori, E.; Landoni, F.; Zola, P.; Maggino, T.; Urgesi, A.; Lissoni, A.; Losa, G.; Fanucchi, A. Endometrial stromal sarcoma: Analysis of treatment failures and survival. Gynecol. Oncol. 1996, 63, 247–253. [Google Scholar] [CrossRef]
- Kapp, D.S.; Shin, J.Y.; Chan, J.K. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: Emphasis on impact of lymphadenectomy and oophorectomy. Cancer 2008, 112, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Karataşlı, V.; Çakır, İ.; Can, B.; Erkılınç, S.; Karadeniz, T.; Kuru, O.; Gökçü, M.; Sancı, M. Does ovarian preservation have an effect on recurrence of early stage low-grade endometrial stromal sarcoma? J. Obstet. Gynaecol. 2021, 41, 797–802. [Google Scholar] [CrossRef]
- Kim, W.Y.; Lee, J.W.; Choi, C.H.; Kang, H.; Kim, T.J.; Kim, B.G.; Lee, J.H.; Bae, D.S. Low-grade endometrial stromal sarcoma: A single center’s experience with 22 cases. Int. J. Gynecol. Cancer 2008, 18, 1084–1089. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Feasibility of uterine preservation in the management of early-stage uterine adenosarcomas: A single institute experience. World J. Surg. Oncol. 2017, 15, 87. [Google Scholar] [CrossRef]
- Li, A.J.; Giuntoli, R.L., 2nd; Drake, R.; Byun, S.Y.; Rojas, F.; Barbuto, D.; Klipfel, N.; Edmonds, P.; Miller, D.S.; Karlan, B.Y. Ovarian preservation in stage I low-grade endometrial stromal sarcomas. Obstet Gynecol. 2005, 106, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, L.Y.; Zhang, H.T.; An, J.S.; Li, X.G.; Ma, S.K. Treatment options in stage I endometrial stromal sarcoma: A retrospective analysis of 53 cases. Gynecol. Oncol. 2008, 108, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Mastroyannis, S.A.; Latif, N.A.; Ko, E.M.; Haggerty, A.F.; Kim, S.H.; Morgan, M.A.; Giuntoli, R.L., 2nd. Effect of bilateral salpingo-oophorectomy on the overall survival of premenopausal patients with stage I low-grade endometrial stromal sarcoma; a National Cancer Database analysis. Gynecol. Oncol. 2020, 157, 634–638. [Google Scholar] [CrossRef]
- Nasioudis, D.; Chapman-Davis, E.; Frey, M.; Holcomb, K. Safety of ovarian preservation in premenopausal women with stage I uterine sarcoma. J. Gynecol. Oncol. 2017, 28, e46. [Google Scholar] [CrossRef] [PubMed]
- Sia, T.Y.; Huang, Y.; Gockley, A.; Melamed, A.; Khoury-Collado, F.; St Clair, C.; Hou, J.Y.; Tergas, A.I.; Hershman, D.L.; Wright, J.D. Trends in ovarian conservation and association with survival in premenopausal patients with stage I leiomyosarcoma. Gynecol. Oncol. 2021, 161, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Soh, L.T.; Chew, S.H.; Ang, L. Uterine leiomyosarcoma—A Singapore experience. Aust. N. Z. J. Obstet. Gynaecol. 1999, 39, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.E.; Beck, T.L.; Giannakopoulos, N.V.; Rendi, M.H.; Isacson, C.; Goff, B.A. Impact of oophorectomy and hormone suppression in low grade endometrial stromal sarcoma: A multicenter review. Gynecol. Oncol. 2018, 149, 297–300. [Google Scholar] [CrossRef]
- Giuntoli, R.L., 2nd; Metzinger, D.S.; DiMarco, C.S.; Cha, S.S.; Sloan, J.A.; Keeney, G.L.; Gostout, B.S. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol. Oncol. 2003, 89, 460–469. [Google Scholar] [CrossRef]
- Parker, W.H.; Broder, M.S.; Liu, Z.; Shoupe, D.; Farquhar, C.; Berek, J.S. Ovarian conservation at the time of hysterectomy for benign disease. Obstet. Gynecol. 2005, 106, 219–226. [Google Scholar] [CrossRef]
- Gueli Alletti, S.; Restaino, S.; Finelli, A.; Ronsini, C.; Lucidi, A.; Scambia, G.; Fanfani, F. Step by Step Total Laparoscopic Hysterectomy with Uterine Arteries Ligation at the Origin. J. Minim. Invasive Gynecol. 2020, 27, 22–23. [Google Scholar] [CrossRef]
- Davidson, B.; Kjæreng, M.L.; Førsund, M.; Danielsen, H.E.; Kristensen, G.B.; Abeler, V.M. Progesterone Receptor Expression Is an Independent Prognosticator in FIGO Stage I Uterine Leiomyosarcoma. Am. J. Clin. Pathol. 2016, 145, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Aristei, C.; Biondetti, P.R.; Cananzi, F.C.M.; Casali, P.; Ciccarone, F.; Colombo, N.; Comandone, A.; Corvo’, R.; De Iaco, P.; et al. Italian consensus conference on management of uterine sarcomas on behalf of S.I.G.O. (Societa’ italiana di Ginecologia E Ostetricia). Eur. J. Cancer 2020, 139, 149–168, Erratum in Eur. J. Cancer 2021, 144, 397–398. [Google Scholar] [CrossRef]
- Leitao, M.M.; Sonoda, Y.; Brennan, M.F.; Barakat, R.R.; Chi, D.S. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol. Oncol. 2003, 91, 209–212. [Google Scholar] [CrossRef]
- Chan, J.K.; Kawar, N.M.; Shin, J.Y.; Osann, K.; Chen, L.-M.; Powell, C.B.; Kapp, D.S. Endometrial stromal sarcoma: A population-based analysis. Br. J. Cancer 2008, 99, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Tsuyoshi, H.; Yoshida, Y. Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci. 2018, 109, 1743–1752. [Google Scholar] [CrossRef]
| Name | Country | Study Design | FIGO Stage I/Population | No. of Participants | Mean FUP Months |
|---|---|---|---|---|---|
| Berchuck 1990 [4] | USA | Observational Monocentric Retrospective Cohort study | 6/6 ESS (H + BSO a). 2/6 (33%) relapse | 12 | 81 |
| Sait 2014 [5] | Saudi Arabia | Observational Monocentric Retrospective Cohort study | 2/15 (13.3%) uterine sarcoma relapse (H + BSO a) | 15 | 24 |
| Comparative Studies | |||||
| Name | Country | Study Design | FIGO Stage I/Population | No. of Participants | Mean FUP Months |
| Amant 2007 [6] | Belgium | Observational Multicenter Retrospective Cohort study | 18/34 ESS (12 H + BSO a, 6 OP b). RR c: 25% vs. 17% respectively | 18 | 62 and 64 (women with or without recurrence, respectively) |
| Gadducci 1990 [7] | Italy | Observational Multicenter Retrospective Cohort study | 2/6 (33%) LG-ESS d relapse (H + BSO a) 1/6 (16.67%) LG-ESS d relapse (OP b) | 12 | 92 |
| Gadducci 1996 [8] | Italy | Observational Multicentric Retrospective Cohort study | 88/126 leiomyosarcoma RR c 33.3% (H + BSO a) 23.8% (OP b) | 88 | 80 |
| Kapp 2008 [9] | USA | Observational Multicentric Retrospective Cohort study | 240/341 leiomyosarcoma (H + BSO a) 5 yr DSS e 83.2% 101/341 leiomyosarcoma (OP b) 5 yr DSS e 83.2% | 341 | Not known |
| Karataşlı 2020 [10] | Turkey | Observational Monocentric Retrospective Cohort study | 1/7 LG-ESS d relapse (H + BSO a) 3/27 LG-ESS d relapse (OP b) | 34 | 109 |
| Kim 2008 [11] | Korea | Observational Monocentric Retrospective Cohort study | 5/11 (45.4%) LG-ESS d relapse (H + BSO a); DFS f 110mo 5/11(45.4%) LG-ESS d relapse (OP b); DFS f 120mo | 22 | 77 |
| Lee 2017 [12] | Korea | Observational Monocentric Retrospective Cohort study | 1/13 uterine adenosarcoma relapse (H + BSO a) 2/18 uterine adenosarcoma relapse (OP b) | 31 | 32 |
| Li 2005 [13] | USA | Observational Multicentric Retrospective Cohort study | 10/24 (42%) LG-ESS d (H + BSO a) PFS g 91.3 mo 4/12 (33%) relapse (OP b) PFS g 68.6 mo | 36 | 33 (case) 60 (control) |
| Li 2007 [14] | China | Observational Monocentric Retrospective Cohort study | 10/44 ESS d relapse (H + BSO a) (RR c 22.7%) 3/44 deaths (H + BSO a) 9/9 LG-ESS d relapse (OP b) (RR c 100%) 2/9 deaths (OP b) | 53 | 66 |
| Nasioudis 2017 [15] | USA | Observational Multicentric Retrospective Cohort study | LMS h: 238 (OP b), 562 (H + BSO a) 5 yr OS l (72.8% vs. 68.9%) LG-ESS d: 151 (OP b), 369 (H + BSO a) comparable OS l (p = 0.410) AS i: 30 (OP b), 132 (H + BSO a) no difference in OS l (p = 0.350) | 1482 patients; 800 (54.0%) cases of LMS; 520 (35.1%) cases of LG-ESS; 162 (10.9%) cases of AS | 94 (OP) 123 (BSO) |
| Nasioudis 2020 [16] | USA | Observational Multicentric Retrospective Cohort study | LG-ESS d: 6/202 deaths (OP b), 5 yr OS l 97.1% 20/541 deaths (H + BSO a) 5 yr OS 96.2% | 743 | 79.97 (OP) 64.99 (BSO |
| Sia 2021 [17] | USA | Observational Multicentric Retrospective Cohort study | 151/225 alive (OP b), 5 yr OS l 67.1% 410/568 alive (H + BSO a) 5 yr OS l 72.2% | 793 | Not known |
| Soh 1999 [18] | Singapore | Observational Monocentric Retrospective Cohort study | 6/13 (46%) leiomyosarcoma relapse (H + BSO a) 3/3 (100%) leiomyosarcoma relapse (OP b) | 16 | Not known |
| Study | Population | BSO RR | OP RR | BSO OS | OP OS | BSO DFS | OP DFS |
|---|---|---|---|---|---|---|---|
| Amant 2007 [6] | 18 patients with ESS | 25% | 17% | / | / | / | / |
| Berchuck 1990 [4] | 6 patients with ESS | 33.3% | / | / | / | / | / |
| Gadducci 1990 [7] | 12 patients with ESS | 33.3% | 16.7% | / | / | / | / |
| Gadducci 1996 [8] | 42 patients with LMS | 33.3% | 23.8% | / | / | / | / |
| Giuntoli 1998 [20] | 180 patients with LMS | / | / | / | / | 88% | 92% |
| Kapp 2008 [9] | 341 patients with LMS | / | / | / | / | 83.2% | 83.2% |
| Kim 2008 [11] | 22 patients with ESS | 45.4% | / | / | / | / | / |
| Karataşlı 2020 [10] | 34 patients with ESS | 11.1% | 14.2% | / | / | / | / |
| Lee 2017 [12] | 24 patients with AS | 7.7% | 18.1% | / | / | / | / |
| Li 2005 [13] | 36 patients with ESS | 42% | 33% | / | / | / | / |
| Li 2007 [14] | 62 patients with ESS | 100% | 22.7% | 91.5% | 85.9% | / | / |
| Nasioudis 2017 [15] | 1482 patients with ESS, LMS, AS | / | / | 68.9% | 72.8% | / | / |
| Nasioudis 2020 [16] | 681 patients with ESS | / | / | 96.2% | 97.1% | / | / |
| Sait 2014 [5] | 15 patients with CS, LMS, US | 13.3% | / | / | / | / | / |
| Sia 2021 [17] | 793 patients with LMS | / | / | 72.2% | 67.1% | / | / |
| Soh 1999 [18] | 16 patients with LMS | 46.1% | / | / | / | / | / |
| Stewart 2018 [19] | 47 patients with ESS | 35% | 66% | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronsini, C.; Foresta, A.; Giudice, M.; Reino, A.; La Verde, M.; della Corte, L.; Bifulco, G.; Franciscis, P.d.; Cianci, S.; Capozzi, V.A. Is Adnexectomy Mandatory at the Time of Hysterectomy for Uterine Sarcomas? A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1140. https://doi.org/10.3390/medicina58091140
Ronsini C, Foresta A, Giudice M, Reino A, La Verde M, della Corte L, Bifulco G, Franciscis Pd, Cianci S, Capozzi VA. Is Adnexectomy Mandatory at the Time of Hysterectomy for Uterine Sarcomas? A Systematic Review and Meta-Analysis. Medicina. 2022; 58(9):1140. https://doi.org/10.3390/medicina58091140
Chicago/Turabian StyleRonsini, Carlo, Aniello Foresta, Matteo Giudice, Antonella Reino, Marco La Verde, Luigi della Corte, Giuseppe Bifulco, Pasquale de Franciscis, Stefano Cianci, and Vito Andrea Capozzi. 2022. "Is Adnexectomy Mandatory at the Time of Hysterectomy for Uterine Sarcomas? A Systematic Review and Meta-Analysis" Medicina 58, no. 9: 1140. https://doi.org/10.3390/medicina58091140
APA StyleRonsini, C., Foresta, A., Giudice, M., Reino, A., La Verde, M., della Corte, L., Bifulco, G., Franciscis, P. d., Cianci, S., & Capozzi, V. A. (2022). Is Adnexectomy Mandatory at the Time of Hysterectomy for Uterine Sarcomas? A Systematic Review and Meta-Analysis. Medicina, 58(9), 1140. https://doi.org/10.3390/medicina58091140










