The Different Clinical Courses of Legionnaires’ Disease in Newborns from the Same Maternity Hospital
Abstract
1. Introduction
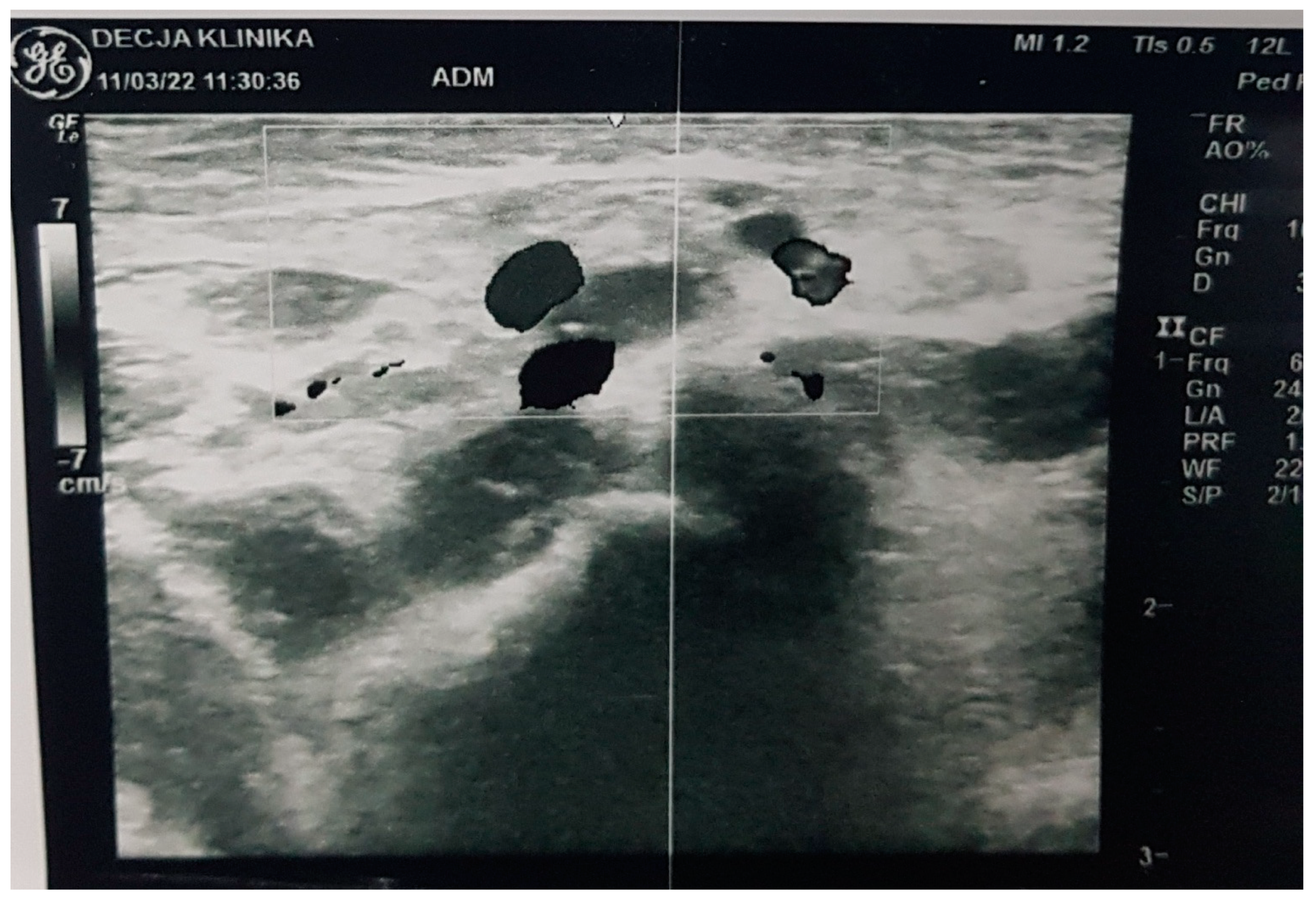
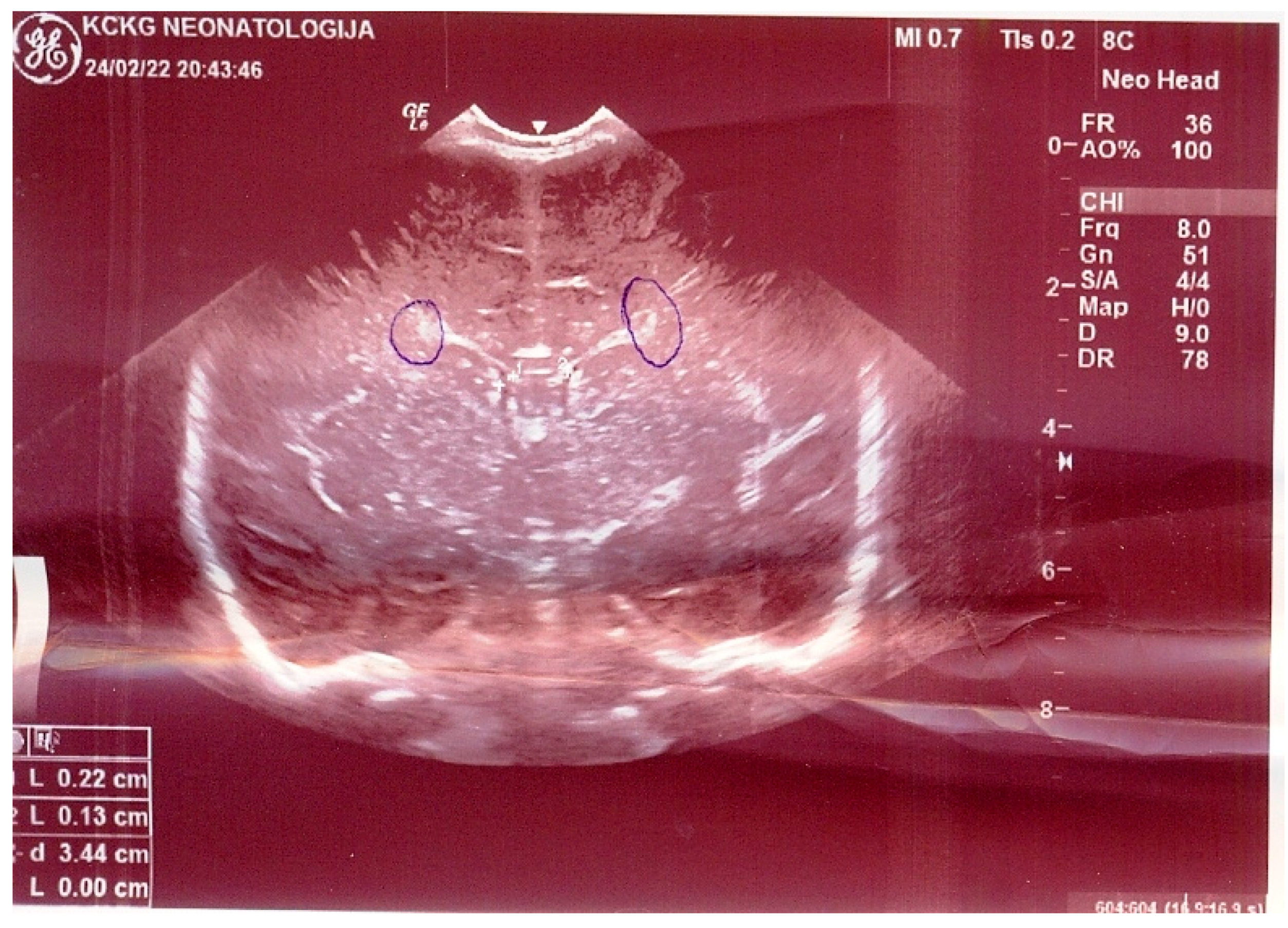
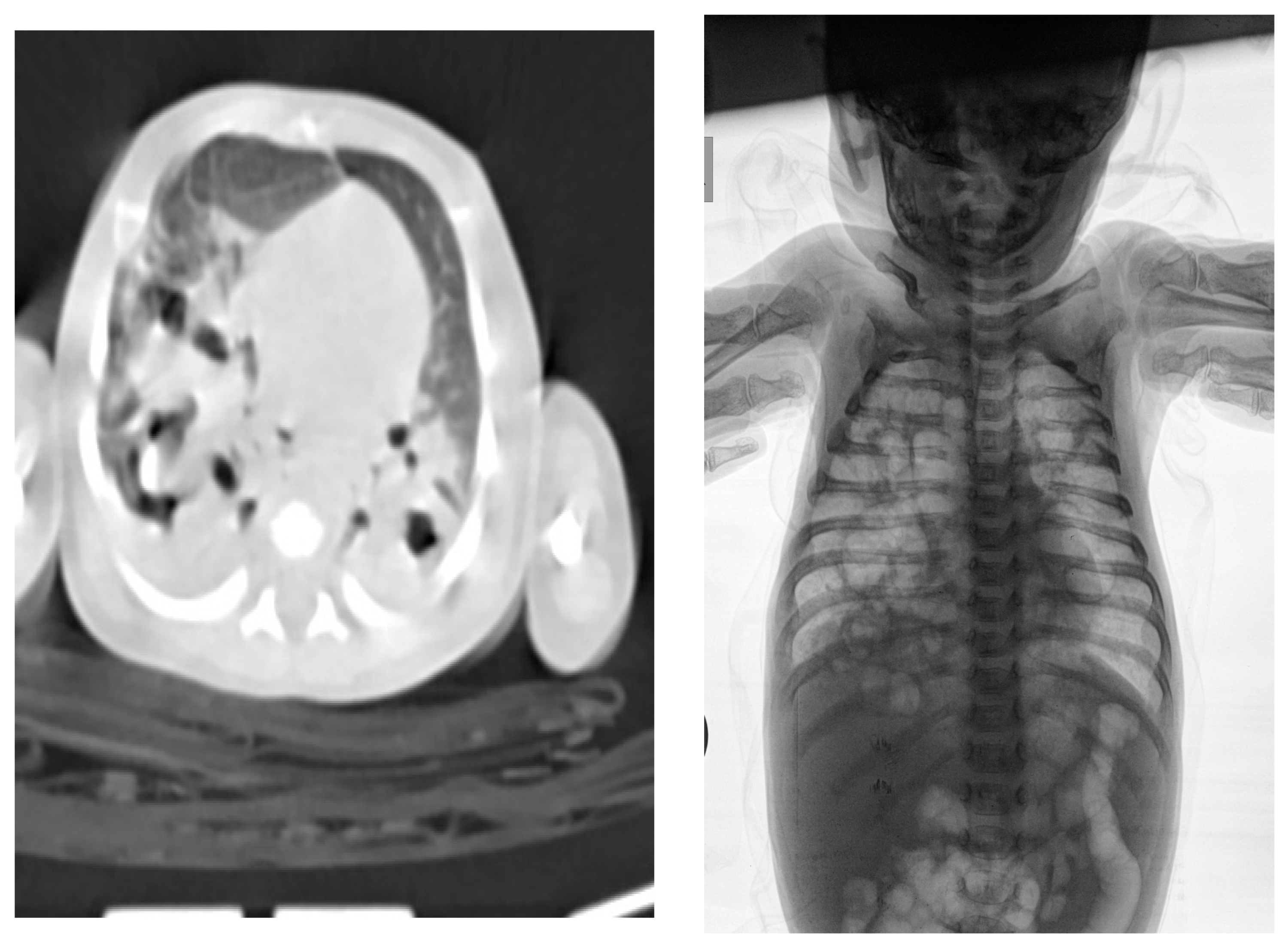
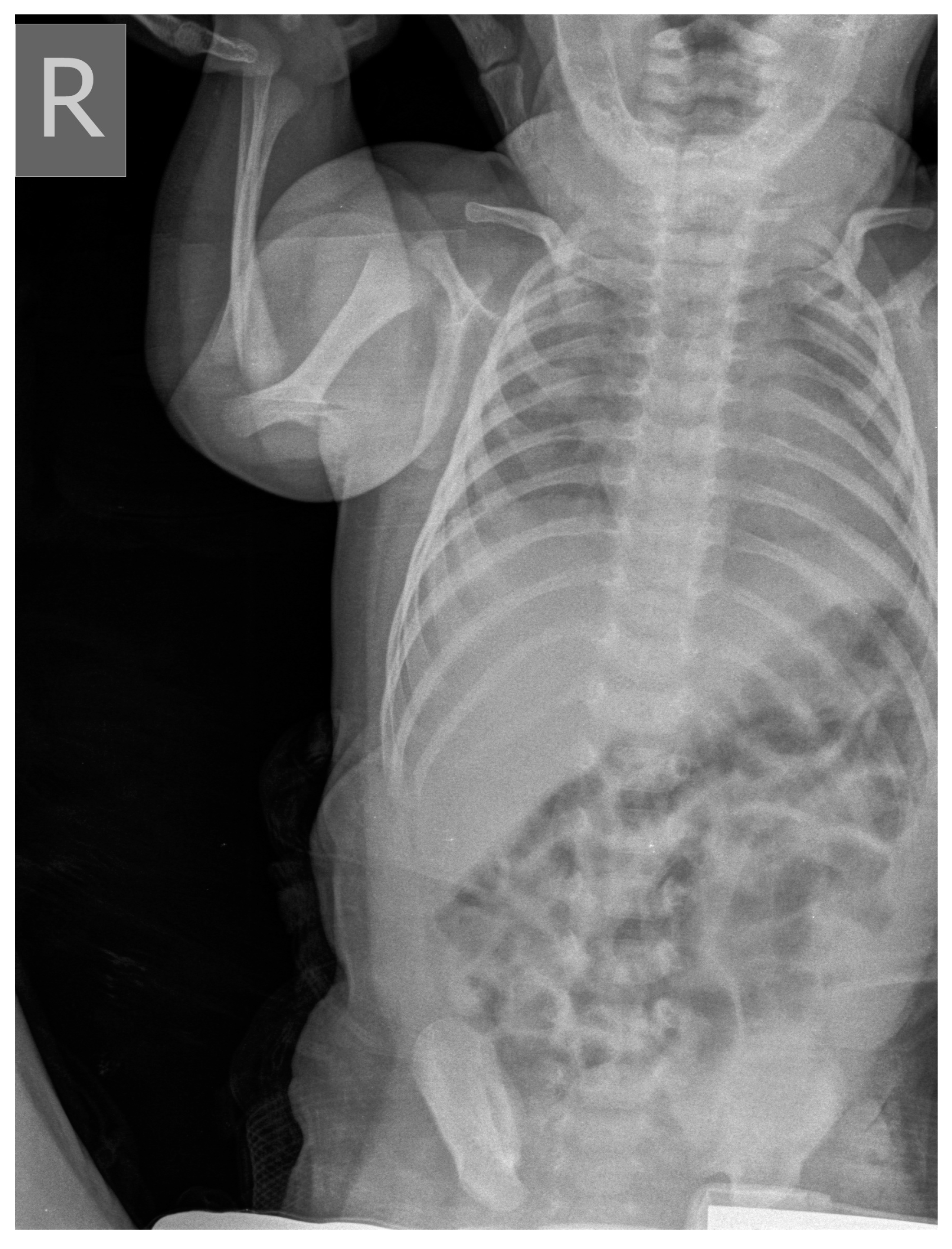
2. Commentary
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Facts about Legionnaires’ Disease. European Centre for Disease Prevention and Control. An Agency of the European Union. Available online: https://www.ecdc.europa.eu/en/legionnaires-disease/facts (accessed on 26 June 2017).
- Correia, A.M.; Ferreira, J.S.; Borges, V.; Nunes, A.; Gomes, B.; Capucho, R.; Gonçalves, J.; Antunes, D.M.; Almeida, S.; Mendes, A.; et al. Probable Person-to-Person Transmission of Legionnaires’ Disease. N. Engl. J. Med. 2016, 374, 497–498. [Google Scholar] [CrossRef] [PubMed]
- De Jong, B.; Hallstrom, L.P. European Surveillance of Legionnaires’ Disease. Curr. Issues Mol. Biol. 2021, 42, 81–96. [Google Scholar] [CrossRef]
- Leaflet for Managers of Tourist Accommodation on How to Reduce the Risk of Legionnaires’ disease. ECDC Health Information. European Centre for Disease Prevention and Control. An Agency of the European Union. Available online: https://www.ecdc.europa.eu/en/publications-data/leaflet-managers-tourist-accommodation-how-reduce-risk-legionnaires-disease (accessed on 17 May 2017).
- Ngwaga, T.; Chauhan, D.; Shames, S.R. Mechanisms of Effector-Mediated Immunity Revealed by the Accidental Human Pathogen Legionella pneumophila. Front. Cell. Infect. Microbiol. 2021, 10, 593823. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.; Engels, I.; Daschner, D.F.; Frank, U. The effect of azithromycin on intracellular Legionella pneumophila in the Mono Mac 6 cell line at serum concentrations attainable in vivo. J. Antimicrob. Chemother. 2000, 46, 385–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albin, O.; Mills, J.P.; Saint, S.; Swanson, M.; Deng, J.C. A “Fluid” Diagnosis. N. Engl. J. Med. 2021, 385, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, P.; Guan, H.; Huang, D.; Song, L.; Ouyang, S.; Luo, Z.-Q. Legionella pneumophila temporally regulates the activity of ADP/ATP translocases by reversible ADP-ribosylation. mLife 2022, 1, 51–65. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Management of Legionella in Water Systems; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Beauté, J. Legionnaires’ disease in Europe, 2011 to 2015. Eurosurveillance 2017, 22, 30566. [Google Scholar] [CrossRef] [PubMed]
- Barskey, A.E.; Derado, G.; Edens, C. Rising Incidence of Legionnaires’ Disease and Associated Epidemiologic Patterns, United States, 1992–2018. Emerg. Infect. Dis. 2022, 28, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, L.S.; Robilotti, V.E. Legionella. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R., St. Geme, J.W., Blum, N., Shah, S., Tasker, R., Wilson, K., Behrman, R., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2020; pp. 1421–1423. [Google Scholar]
- Sanden, G.N.; Fields, B.S.; Barbaree, J.M.; Feeley, J.C. Viability of Legionella pneumophila in choline-free water at elevated temperatures. Curr. Microbiol. 1989, 18, 61–65. [Google Scholar] [CrossRef]
- Paediatric Formulary Committee. BNF for Children 2019–2020; Pharmaceutical Press: London, UK, 2019. [Google Scholar]
- Primhak, S.; Myttaraki, E.; Whittaker, E. Congenital infections of the respiratory tract. In Respiratory Diseases of the Newborn Infant (ERS Monograph); Sinha, I.P., Bhatt, J.M., Cleator, A., Wallace, H., Eds.; European Respiratory Society: Sheffield, UK, 2021; Volume 92, pp. 245–258. [Google Scholar] [CrossRef]
- Kumar, P.; Chauhan, A.; Bhardwaj, P.; Chauhan, L.; Karol, M. D-dimer: A useful marker in neonatal sepsis. J. Clin. Neonatol. 2015, 4, 101–103. [Google Scholar] [CrossRef]
- Stojkovic, A.; Dajic, K.; Milovanovic, J.; Jankovic, S.M.; Markovic, N.V.; Kostic, A. Effects of Supplementation in Vitamin D3 Deficient or Insufficient Children with Allergic Diseases. Medicina 2021, 57, 1052. [Google Scholar] [CrossRef] [PubMed]
- Simović, A.M.; Knezević, J.; Igrutinović, Z.; Stojanović, N.; Kocić, S. Cardiac troponin as a biochemical marker of perinatal asphyxia and hypoxic myocardial injury. Vojnosanit. Pregl. 2009, 66, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, S.; Kabata, T.; Maeda, K.; Ito, Y.; Sakaeda, T. Pharmacokinetics of Macrolide Antibiotics and Transport into the Interstitial Fluid: Comparison among Erythromycin, Clarithromycin, and Azithromycin. Antibiotics 2020, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Woodland, C.; Murphy, G.; Sinha, I.P. Management of BPD: Strategies to prevent short- and long-term complications following discharge from the NICU. In Respiratory Diseases of the Newborn Infant (ERS Monograph); Sinha, I.P., Bhatt, J.M., Cleator, A., Wallace, H., Eds.; European Respiratory Society: Sheffield, UK, 2021; pp. 79–88. [Google Scholar] [CrossRef]
- Puia-Dumitrescu, M.; Wood, T.R.; Comstock, B.A.; Law, J.B.; German, K.; Perez, K.M.; Gogcu, S.; Mayock, D.E.; Heagerty, P.J.; Juul, S.E.; et al. Dexamethasone, Prednisolone, and Methylprednisolone Use and 2-Year Neurodevelopmental Outcomes in Extremely Preterm Infants. JAMA Netw Open 2022, 5, e221947. [Google Scholar] [CrossRef] [PubMed]
- Perez Ortiz, A.; Hahn, C.; Schaible, T.; Rafat, N.; Lange, B. Severe Pneumonia in Neonates Associated with Legionella pneumophila: Case Report and Review of the Literature. Pathogens 2021, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
| NN1, Boy | NN2, Boy | |
|---|---|---|
| Apgar score at 1st/5th minute | 10/10 | 9/10 |
| Weight at birth (g) | 4050 | 3850 |
| Birth naturally way | yes | yes |
| Breastfeeding, exclusively | yes | yes |
| Family history | the older boy died of congenital intestinal atresia | - |
| Age of hospitalization (day) | 8 | 6 |
| Duration of 1st hospitalization (day) | 49 | 21 |
| Clinical picture at admission in Pediatric Clinic UCC | NN1 had a preserved sensorium, was high febrile 39.4 °C (rectal), with tachy-dyspnea, sobs, and moans, indents jugulum, dissatisfied cries, TM 4050 g, subclinical jaundice of the skin, and visible mucosa. Auscultation revealed attenuated respiratory sound, diffusely fine crackles, SaO2 82%, R 56/min, F 196/min. The umbilical stump persisted, the surrounding skin became red and swollen, there was hypotonia of the body axis, large fontanelle within the bony borders, greatness 20 × 30 mm. The other physical findings were normal. | NN2 had the preserved sensorium, was afebrile 37.7 °C (rectal), TM 3920 g, eupnoeic, presented sobs and moans, plethoric and icteric skin, nasal vestibules filled with seromucous secretion, and hyperemic throat. Auscultatory revealed a normal breathing sound is heard with transmitted wheezes from the upper parts of the airways and systolic murmur of 1-2/6 according to Levin, SaO2 97%, R 32/min, F 168/min. The umbilical stump persisted, thin, and the borders developed a serous–hemorrhagic discharge. There was mild hypotonia of the shoulder girdle and trunk axis, primitive reflexes were slowly elicited, large fontanelle was below the plane of the bony borders, and slightly spaced sutures, greatness 40 × 40 mm. The other physical findings were normal. |
| NN1, Boy | NN2, Boy | ||
|---|---|---|---|
| Infection related biomarkers | CRP (mg/L) | 219, 223, 255, 209, 127, 14, 62, 7 | 26, 69, 5.6, 0.5, 0.2 |
| PCT (ng/mL) | 83, 40, 76, 92, 1.0, 0.8, 0.7, 0.2, 0.1, 0.1 | 0.67, 1.14, 0.08, 0.13 | |
| IL6 (pg/mL) | 3335, 94.4 | 760 | |
| WBC (×10/−9 L) | 11.7, 3.0, 2.5, 4.7, 44, 20.2, 19.1, 21, 17 | 31.6, 24.4, 19.2, 24.1, 12.8, 11.7, 12.7 | |
| n (%) | 72, 58, 37, 18, 19, 66, 70, 65, 58, 45 | 56, 58, 63, 33, 36, 44 | |
| RBC (×10/−12 L) | 3.7, 3.5, 3.7, 3.0, 3.5, 3.9, 4.3, 3.9, 4.9, 3.3, 4.1, 2.8, 3.1 | 4.0, 4.2, 4.1, 3.7, 3.4, 3.7 | |
| hemoglobin (g/L) | 126, 114, 100, 119, 125, 125, 157,103, 86, 91 | 143, 114, 136, 143, 117, 107, 110 | |
| Tr (×10/−9 L) | 435, 441, 99, 53, 40, 199, 211, 616, 600, 591 | 374, 437, 266, 705, 367, 611, 465 | |
| PT (s) | 21.9 | 15.2, 13.3, 14.8 | |
| PT(INR) | 1.7 | 1.13, 1.03, 0.99, 1.11 | |
| APTT (s) | 41.2 | 34.3, 27.5, 28.8, 35 | |
| d-dimer (μg/mL FEU) | 6.3, 8.8, 7.0, 1.9, 3.3, 2.1 | 1.99, 12.6, 2.9, 1.2, 0.8, 0.8 | |
| Fibrinogen (g/L) | 7.4, 4.1, 4.4 | 7.3, 3.3, 2.4 | |
| Anti Xa 12 h (IU/mL) | 0.35, 0.41 | - | |
| Feritin (μg/L) | 920 | 791 | |
| Liver function test | AST (IU/L) | 36, 42, 43 | 33, 39, 42 |
| ALT (IU/L) | 28, 18, 22 | 28, 97, 53 | |
| ALP (IU/L) | 79 | - | |
| Gamma GT (IU/L) | 35, 242, 102, 293 | 33 | |
| Serum bilirubin total/direct (μmol/L) | 142/18 | 127/11, 26/8 | |
| Serum total protein/albumin(g/L) | 64/25, 63/35 | 57/33 | |
| Urea (mmol/L) | 3.7, 4.3 | 3.6, 4.0, 4.9 | |
| Creatinine (μmol/L) | 56, 48 | 54, 32, 30 | |
| Troponin (mcg/L) | 0.013 | 0.015, 0.07, 0.06, 0.13 | |
| Gas analysis -capillary | pH | 7.3, 7.2, 7.5, 7.6, 7.4, 7.4 | 7.4, 7.4, 7.4, 7.4, 7.5 |
| pCO2 (kPa) | 6.8, 10, 4.1, 3.6, 5.3, 5.3 | 4.1, 3.6, 5.5, 5.1, 4.8 | |
| pO2 (kPa) | 9.5, 6.4, 5.8, 6.0, 9.5, 7.7 | 18.0, 12.1, 4.9, 3.6, 8.9 | |
| K (mmol/L) | 2.9, 3.8, 2.3, 3.0, 5.1, 5.6 | 4.5, 4.6, 5.7, 4.8, 4.9 | |
| Na (mmol/L) | 135, 132, 129, 136, 134, 136 | 132, 134, 137, 128, 130 | |
| HCO3 std | 21, 25, 26, 31, 23, 23 | 22, 22, 26, 25, 27 | |
| Glu (mmol/L) | 11, 6.5, 9.5, 6.7, 5.0, 5.3 | 5.3, 5.0, 5.1, 4.8, 5.0 | |
| Lac (mmol/L) | 4.1, 4.2, 3.6, 2.4, 1.4, 1.8 | 2.5, 3.6, 1.9, 1.6, 2.3 | |
| BEecf (mmol/L) | −4.2, 0.8, 4.9, 7.3, −2.2, −2.8 | −5.0, −5.9, 2.1, 0.9, 3.3 | |
| Vitamin D (ng/mL) | 11.7 | 16.2 | |
| Serology/microbiology findings | PCR-SARSCoV2 | negative | negative |
| Blood culture I | coagulase-negative staphylococci | coagulase-negative staphylococci | |
| Blood culture II | sterile | sterile | |
| Tracheal aspirate by RT-PCR testing | Legionella pneumophila serogroup 2-15 | Legionella pneumophila serogroup 2-15 | |
| Culture of tracheal aspirate on the GVPC nutrient media | Legionella pneumophila, Pseudomanas aeriginosa | Legionella pneumophila | |
| Culture of navel swab | coagulase-negative Staphylococci | Klebsiella, Enterobacter spp. | |
| Urine by rapid Uni-Gold plus | without pathological flora | without pathological flora | |
| TORCH screen | not detected | not detected | |
| Cells immuno-phenotyping (after RBC lysis) | A neutrophil population of 76% and 14% of lymphocytes stood out. In the lymphocyte population, a lower percentage of T lymphocytes is registered, while 19% are B lymphocytes. | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostic, A.; Cukovic, K.; Stankovic, L.; Raskovic, Z.; Nestorovic, J.; Savic, D.; Simovic, A.; Prodanovic, T.; Zivojinovic, S.; Andrejevic, S.; et al. The Different Clinical Courses of Legionnaires’ Disease in Newborns from the Same Maternity Hospital. Medicina 2022, 58, 1150. https://doi.org/10.3390/medicina58091150
Kostic A, Cukovic K, Stankovic L, Raskovic Z, Nestorovic J, Savic D, Simovic A, Prodanovic T, Zivojinovic S, Andrejevic S, et al. The Different Clinical Courses of Legionnaires’ Disease in Newborns from the Same Maternity Hospital. Medicina. 2022; 58(9):1150. https://doi.org/10.3390/medicina58091150
Chicago/Turabian StyleKostic, Andrijana, Katarina Cukovic, Lidija Stankovic, Zorica Raskovic, Jelena Nestorovic, Dragana Savic, Aleksandra Simovic, Tijana Prodanovic, Suzana Zivojinovic, Sladjana Andrejevic, and et al. 2022. "The Different Clinical Courses of Legionnaires’ Disease in Newborns from the Same Maternity Hospital" Medicina 58, no. 9: 1150. https://doi.org/10.3390/medicina58091150
APA StyleKostic, A., Cukovic, K., Stankovic, L., Raskovic, Z., Nestorovic, J., Savic, D., Simovic, A., Prodanovic, T., Zivojinovic, S., Andrejevic, S., Erovic, I., Djordjevic, Z., Rsovac, S., Sazdanovic, P., & Stojkovic, A. (2022). The Different Clinical Courses of Legionnaires’ Disease in Newborns from the Same Maternity Hospital. Medicina, 58(9), 1150. https://doi.org/10.3390/medicina58091150






