Intermediate-Risk Pulmonary Embolism: A Review of Contemporary Diagnosis, Risk Stratification and Management
Abstract
:1. Introduction
2. Definitions and Classification
3. Approach to Risk Stratification
4. Risk Stratification Tools
4.1. Right Ventricular Dysfunction
4.2. Biomarkers
4.2.1. Cardiac Troponins
4.2.2. Natriuretic Peptides
4.2.3. Others
4.3. Bedside Scoring Systems
5. Management
5.1. Systemic Anticoagulation
5.2. Systemic Thrombolysis
5.3. Reduced Dose Thrombolytics
5.4. Catheter-Directed Therapy
5.5. Thrombolysis versus Systemic Anticoagulation
5.6. Surgical Embolectomy
6. Additional Treatment Options
6.1. Mechanical Support Devices
6.2. Inferior Vena Cava Filter
6.3. Non-Steroidal Anti-Inflammatory Drugs
6.4. Vasodilators
6.5. Newer Treatment Options
7. Pulmonary Embolism Response Team (PERT)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACCP | American College of Chest Physicians |
| AHA | American Heart Association |
| BNP | Brain natriuretic peptide |
| CDT | Catheter directed therapy |
| CT | Computed tomography |
| CTA | Computed tomography angiogram |
| DVT | Deep vein thrombosis |
| ECMO | Extracorporeal membrane oxygenation |
| ESC | European Society of Cardiology |
| FLARE | FlowTriever Pulmonary Embolectomy clinical study |
| FLASH | FlowTriever All-Comer Registry for Patient Safety and Hemodynamics |
| h-FABP | Heart-type fatty acid binding protein |
| ICOPER | International Cooperative Pulmonary Embolism Registry |
| IVC | Inferior vena cava |
| LMWH | Low molecular weight heparin |
| NO | Nitric oxide |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| OPTALYSE | Optimal duration of requested pulse thrombolysis procedure and acute intermediate-risk pulmonary embolism |
| PE | Pulmonary embolism |
| PEITHO | Pulmonary embolism thrombolysis |
| PERT | Pulmonary embolism response team |
| PESI | Pulmonary Embolism Severity Index |
| PREPIC2 | Prevention of recurrent pulmonary embolism by vena cava interruption 2 |
| RCT | Randomized controlled trial |
| RR | Relative risk |
| RV | Right ventricular |
| RVAD | Right ventricular assist device |
| RVD | Right ventricular dysfunction |
| SBP | systolic blood pressure |
| SEATTLE II | EkoSonic endovascular system and Activase for treatment of acute pulmonary embolism |
| sPESI | Simplified Pulmonary Embolism Severity Index |
| SUNSET sPE | Standard vs. Ultrasound-Assisted Catheter thrombolysis for Submassive Pulmonary Embolism |
| TOPCOAT | Tenecteplase or Placebo Cardiopulmonary Outcomes at Three Months |
| TAFI | Thrombin activatable fibrinolysis inhibitor |
| tPA | tissue plasminogen activator |
| ULTIMA | Ultrasound accelerated thrombolysis of pulmonary embolism |
| USAT | Ultrasound-assisted thrombolysis |
References
- Goldhaber, S.; Come, P.; Lee, R.; Braunwald, E.; Parker, J.; Haire, W.; Feldstein, M.; Miller, M.; Toltzis, R.; Smith, J.; et al. Alteplase versus heparin in acute pulmonary embolism: Randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993, 341, 507–511. [Google Scholar] [CrossRef]
- Sharifi, M.; Karandish, K.; Schroeder, B.; Verma, T. Quarter or Safer Dose Thrombolysis in Submassive Pulmonary Embolism. Circulation 2019, 140 (Suppl. 1), A10060. [Google Scholar] [CrossRef]
- Tapson, V.F. Acute Pulmonary Embolism. N. Engl. J. Med. 2008, 358, 1037–1052. [Google Scholar] [CrossRef]
- Pollack, C.V.; Schreiber, D.; Goldhaber, S.Z.; Slattery, D.; Fanikos, J.; O’Neil, B.J.; Thompson, J.R.; Hiestand, B.; Briese, B.A.; Pendleton, R.C.; et al. Clinical Characteristics, Management, and Outcomes of Patients Diagnosed With Acute Pulmonary Embolism in the Emergency Department: Initial Report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J. Am. Coll. Cardiol. 2011, 57, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Visani, L.; De Rosa, M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999, 353, 1386–1389. [Google Scholar] [CrossRef]
- Jiménez, D.; de Miguel-Díez, J.; Guijarro, R.; Trujillo-Santos, J.; Otero, R.; Barba, R.; Muriel, A.; Meyer, G.; Yusen, R.D.; Monreal, M. Trends in the Management and Outcomes of Acute Pulmonary Embolism: Analysis from the RIETE Registry. J. Am. Coll. Cardiol. 2016, 67, 162–170. [Google Scholar] [CrossRef]
- Konstantinides, S.; Geibel, A.; Olschewski, M.; Heinrich, F.; Grosser, K.; Rauber, K.; Iversen, S.; Redecker, M.; Kienast, J.; Just, H.; et al. Association Between Thrombolytic Treatment and the Prognosis of Hemodynamically Stable Patients with Major Pulmonary Embolism. Circulation 1997, 96, 882–888. [Google Scholar] [CrossRef]
- Goldhaber, S.Z. PEITHO Long-Term Outcomes Study. J. Am. Coll. Cardiol. 2017, 69, 1545–1548. [Google Scholar] [CrossRef]
- Barco, S.; Konstantinides, S.V. Risk-adapted management of pulmonary embolism. Thromb. Res. 2017, 151, S92–S96. [Google Scholar] [CrossRef]
- Stevens, S.M.; Woller, S.C.; Kreuziger, L.B.; Bounameaux, H.; Doerschug, K.; Geersing, G.-J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Antithrombotic Therapy for VTE Disease. Chest 2021, 160, e545–e608. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Piazza, G.; Goldhaber, S.Z. Management of Submassive Pulmonary Embolism. Circulation 2010, 122, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Prognostic Value of Troponins in Acute Pulmonary Embolism. Circulation 2007, 116, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Meinel, F.G.; Nance, J.W.; Schoepf, U.J.; Hoffmann, V.S.; Thierfelder, K.M.; Costello, P.; Goldhaber, S.Z.; Bamberg, F. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am. J. Med. 2015, 128, 747–759.e2. [Google Scholar] [CrossRef]
- Frémont, B.; Pacouret, G.; Jacobi, D.; Puglisi, R.; Charbonnier, B.; de Labriolle, A. Prognostic Value of Echocardiographic Right/Left Ventricular End-Diastolic Diameter Ratio in Patients With Acute Pulmonary Embolism. Chest 2008, 133, 358–362. [Google Scholar] [CrossRef]
- Khemasuwan, D.; Yingchoncharoen, T.; Tunsupon, P.; Kusunose, K.; Moghekar, A.; Klein, A.; Tonelli, A. Right Ventricular Echocardiographic Parameters Are Associated with Mortality after Acute Pulmonary Embolism. J. Am. Soc. Echocardiogr. 2015, 28, 355–362. [Google Scholar] [CrossRef]
- Etesamifard, N.; Shirani, S.; Jenab, Y.; Lotfi-Tokaldany, M.; Pourjafari, M.; Jalali, A. Role of clinical and pulmonary computed tomography angiographic parameters in the prediction of short- and long-term mortality in patients with pulmonary embolism. Intern. Emerg. Med. 2015, 11, 405–413. [Google Scholar] [CrossRef]
- Côté, B.; Jiménez, D.; Planquette, B.; Roche, A.; Marey, J.; Pastré, J.; Meyer, G.; Sanchez, O. Prognostic value of right ventricular dilatation in patients with low-risk pulmonary embolism. Eur. Respir. J. 2017, 50, 1701611. [Google Scholar] [CrossRef]
- Becattini, C.; Agnelli, G.; Vedovati, M.C.; Pruszczyk, P.; Casazza, F.; Grifoni, S.; Salvi, A.; Bianchi, M.; Douma, R.; Konstantinides, S.; et al. Multidetector computed tomography for acute pulmonary embolism: Diagnosis and risk stratification in a single test. Eur. Hear. J. 2011, 32, 1657–1663. [Google Scholar] [CrossRef]
- Sista, A.K.; Kuo, W.T.; Schiebler, M.; Madoff, D.C. Stratification, Imaging, and Management of Acute Massive and Submassive Pulmonary Embolism. Radiology 2017, 284, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.; Trinquart, L.; Colombet, I.; Durieux, P.; Huisman, M.V.; Chatellier, G.; Meyer, G. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: A systematic review. Eur. Hear. J. 2008, 29, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Lankeit, M.; Friesen, D.; Aschoff, J.; Dellas, C.; Hasenfuss, G.; Katus, H.; Konstantinides, S.; Giannitsis, E. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur. Hear. J. 2010, 31, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Kucher, N.; Printzen, G.; Doernhoefer, T.; Windecker, S.; Meier, B.; Hess, O.M. Low Pro-Brain Natriuretic Peptide Levels Predict Benign Clinical Outcome in Acute Pulmonary Embolism. Circulation 2003, 107, 1576–1578. [Google Scholar] [CrossRef]
- Klok, F.A.; Mos, I.C.M.; Huisman, M.V. Brain-Type Natriuretic Peptide Levels in the Prediction of Adverse Outcome in Patients with Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2008, 178, 425–430. [Google Scholar] [CrossRef]
- Kostrubiec, M.; Pruszczyk, P.; Kaczynska, A.; Kucher, N. Persistent NT-proBNP elevation in acute pulmonary embolism predicts early death. Clin. Chim. Acta 2007, 382, 124–128. [Google Scholar] [CrossRef]
- Glatz, J.F.; Kleine, A.H.; Van Nieuwenhoven, F.A.; Hermens, W.T.; Van Dieijen-Visser, M.P.; Van Der Vusse, G.J. Fatty-acid-binding protein as a plasma marker for the estimation of myocardial infarct size in humans. Heart 1994, 71, 135–140. [Google Scholar] [CrossRef]
- Dellas, C.; Puls, M.; Lankeit, M.; Schäfer, K.; Cuny, M.; Berner, M.; Hasenfuss, G.; Konstantinides, S. Elevated Heart-Type Fatty Acid-Binding Protein Levels on Admission Predict an Adverse Outcome in Normotensive Patients With Acute Pulmonary Embolism. J. Am. Coll. Cardiol. 2010, 55, 2150–2157. [Google Scholar] [CrossRef]
- Lauque, D.; Maupas-Schwalm, F.; Bounes, V.; Juchet, H.; Bongard, V.; Roshdy, A.; Botella, J.M.; Charpentier, S. Predictive Value of the Heart-type Fatty Acid-binding Protein and the Pulmonary Embolism Severity Index in Patients With Acute Pulmonary Embolism in the Emergency Department. Acad. Emerg. Med. 2014, 21, 1143–1150. [Google Scholar] [CrossRef]
- Kostrubiec, M.; Łabyk, A.; Pedowska-Włoszek, J.; Dzikowska-Diduch, O.; Wojciechowski, A.; Garlińska, M.; Ciurzyński, M.; Pruszczyk, P. Neutrophil gelatinase-associated lipocalin, cystatin C and eGFR indicate acute kidney injury and predict prognosis of patients with acute pulmonary embolism. Heart 2012, 98, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Lankeit, M.; Kempf, T.; Dellas, C.; Cuny, M.; Tapken, H.; Peter, T.; Olschewski, M.; Konstantinides, S.; Wollert, K.C. Growth Differentiation Factor-15 for Prognostic Assessment of Patients with Acute Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2008, 177, 1018–1025. [Google Scholar] [CrossRef]
- Meyer, G.; Vicaut, E.; Danays, T.; Agnelli, G.; Becattini, C.; Beyer-Westendorf, J.; Bluhmki, E.; Bouvaist, H.; Brenner, B.; Couturaud, F.; et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. N. Engl. J. Med. 2014, 370, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Mallett, S.; Daoud-Elias, M.; Poggi, J.-N.; Clarke, M. Prognostic models in acute pulmonary embolism: A systematic review and meta-analysis. BMJ Open 2016, 6, e010324. [Google Scholar] [CrossRef] [PubMed]
- Aujesky, D.; Obrosky, D.S.; Stone, R.A.; Auble, T.E.; Perrier, A.; Cornuz, J.; Roy, P.-M.; Fine, M.J. A Prediction Rule to Identify Low-Risk Patients With Pulmonary Embolism. Arch. Intern. Med. 2006, 166, 169–175. [Google Scholar] [CrossRef]
- Jiménez, D.; Aujesky, D.; Moores, L.; Gómez, V.; Lobo, J.L.; Uresandi, F.; Otero, R.; Monreal, M.; Muriel, A.; Yusen, R.D. Simplification of the Pulmonary Embolism Severity Index for Prognostication in Patients With Acute Symptomatic Pulmonary Embolism. Arch. Intern. Med. 2010, 170, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Lankeit, M.; Friesen, D.; Schäfer, K.; Hasenfuß, G.; Konstantinides, S.; Dellas, C. A simple score for rapid risk assessment of non-high-risk pulmonary embolism. Clin. Res. Cardiol. 2012, 102, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bova, C.; Sanchez, O.; Prandoni, P.; Lankeit, M.; Konstantinides, S.; Vanni, S.; Jimenez, D. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur. Respir. J. 2014, 44, 694–703. [Google Scholar] [CrossRef]
- Marcus, J.T.; Gan, C.T.-J.; Zwanenburg, J.J.; Boonstra, A.; Allaart, C.P.; Götte, M.J.; Vonk-Noordegraaf, A. Interventricular Mechanical Asynchrony in Pulmonary Arterial Hypertension: Left-to-Right Delay in Peak Shortening Is Related to Right Ventricular Overload and Left Ventricular Underfilling. J. Am. Coll. Cardiol. 2008, 51, 750–757. [Google Scholar] [CrossRef]
- Jiménez, D.; Yusen, R.D.; Otero, R.; Uresandi, F.; Nauffal, D.; Laserna, E.; Conget, F.; Oribe, M.; Cabezudo, M.A.; Díaz, G. Prognostic Models for Selecting Patients With Acute Pulmonary Embolism for Initial Outpatient Therapy. Chest 2007, 132, 24–30. [Google Scholar] [CrossRef]
- Fernández, C.; Bova, C.; Sanchez, O.; Prandoni, P.; Lankeit, M.; Konstantinides, S.; Vanni, S.; Fernández-Golfín, C.; Yusen, R.D.; Jiménez, D. Validation of a Model for Identification of Patients at Intermediate to High Risk for Complications Associated With Acute Symptomatic Pulmonary Embolism. Chest 2015, 148, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shao, X.; Zhang, Y.; Zhang, Z.; Tao, X.; Zhai, Z.; Wang, C. Assessment of the Bova score for risk stratification of acute normotensive pulmonary embolism: A systematic review and meta-analysis. Thromb. Res. 2020, 193, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Siragusa, S.; Girolami, B.; Fabris, F.; for the BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: A prospective cohort study. Blood 2005, 106, 3049–3054. [Google Scholar] [CrossRef]
- Tapson, V.F.; Weinberg, A.S. Overview of Management of Intermediate- and High-Risk Pulmonary Embolism. Crit. Care Clin. 2020, 36, 449–463. [Google Scholar] [CrossRef]
- Konstantinides, S.; Geibel, A.; Heusel, G.; Heinrich, F.; Kasper, W. Heparin plus Alteplase Compared with Heparin Alone in Patients with Submassive Pulmonary Embolism. N. Engl. J. Med. 2002, 347, 1143–1150. [Google Scholar] [CrossRef]
- Kline, J.A.; Nordenholz, K.E.; Courtney, D.M.; Kabrhel, C.; Jones, A.E.; Rondina, M.T.; Diercks, D.B.; Klinger, J.R.; Hernandez, J. Treatment of submassive pulmonary embolism with tenecteplase or placebo: Cardiopulmonary outcomes at 3 months: Multicenter double-blind, placebo-controlled randomized trial. J. Thromb. Haemost. 2014, 12, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Vicaut, E.; Danays, T.; Becattini, C.; Bertoletti, L.; Beyer-Westendorf, J.; Bouvaist, H.; Couturaud, F.; Dellas, C.; Duerschmied, D.; et al. Impact of Thrombolytic Therapy on the Long-Term Outcome of Intermediate-Risk Pulmonary Embolism. J. Am. Coll. Cardiol. 2017, 69, 1536–1544. [Google Scholar] [CrossRef]
- Furfaro, D.; Stephens, R.S.; Streiff, M.B.; Brower, R. Catheter-directed Thrombolysis for Intermediate-Risk Pulmonary Embolism. Ann. Am. Thorac. Soc. 2018, 15, 134–144. [Google Scholar] [CrossRef]
- Sharifi, M.; Bay, C.; Skrocki, L.; Rahimi, F.; Mehdipour, M. Moderate Pulmonary Embolism Treated With Thrombolysis (from the “MOPETT” Trial). Am. J. Cardiol. 2013, 111, 273–277. [Google Scholar] [CrossRef]
- Yilmaz, E.S.; Uzun, O. Low-dose thrombolysis for submassive pulmonary embolism. J. Investig. Med. 2021, 69, 1439–1446. [Google Scholar] [CrossRef]
- Kiser, T.H.; Burnham, E.L.; Clark, B.; Ho, P.M.; Allen, R.R.; Moss, M.; Vandivier, R.W. Half-Dose Versus Full-Dose Alteplase for Treatment of Pulmonary Embolism*. Crit. Care Med. 2018, 46, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Banerjee, A.; Kim, P.S.; DeMarco, F.J., Jr.; Levy, J.R.; Facchini, F.R.; Unver, K.; Bertini, M.J.; Sista, A.; Hall, M.J.; et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT)Initial Results From a Prospective Multicenter Registry. Chest 2015, 148, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, E.D.; Jaber, W.; Lacomis, J.; Markel, K.; McDaniel, M.; Rivera-Lebron, B.N.; Ross, C.B.; Sechrist, J.; Toma, C.; Chaer, R.; et al. Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism. JACC: Cardiovasc. Interv. 2021, 14, 1364–1373. [Google Scholar] [CrossRef]
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; Tebbe, U.; Horstkotte, J.; Müller, R.; Blessing, E.; et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J.; Gurley, J.C.; Bhatheja, R.; Kennedy, R.J.; et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Tapson, V.F.; Sterling, K.; Jones, N.; Elder, M.; Tripathy, U.; Brower, J.; Maholic, R.L.; Ross, C.B.; Natarajan, K.; Fong, P.; et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism. JACC: Cardiovasc. Interv. 2018, 11, 1401–1410. [Google Scholar] [CrossRef]
- Weinstein, T.; Deshwal, H.; Brosnahan, S.B. Advanced management of intermediate-high risk pulmonary embolism. Crit. Care 2021, 25, 1–8. [Google Scholar] [CrossRef]
- Al-Hakim, R.; Li, N.; Nonas, S.; Zakhary, B.; Maughan, B.; Schenning, R.; Farsad, K.; Kaufman, J.A. Evaluation and Management of Intermediate and High-Risk Pulmonary Embolism. Am. J. Roentgenol. 2020, 214, 671–678. [Google Scholar] [CrossRef]
- Piazza, G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism. J. Am. Coll. Cardiol. 2020, 76, 2117–2127. [Google Scholar] [CrossRef]
- Kuo, W.T.; Gould, M.K.; Louie, J.D.; Rosenberg, J.K.; Sze, D.Y.; Hofmann, L.V. Catheter-directed Therapy for the Treatment of Massive Pulmonary Embolism: Systematic Review and Meta-analysis of Modern Techniques. J. Vasc. Interv. Radiol. 2009, 20, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; De Luca, A.; Pasceri, V.; Tanzilli, G.; Speciale, G.; Gaudio, C. Safety and Outcome of Rheolytic Thrombectomy for the Treatment of Acute Massive Pulmonary Embolism. J. Invasive Cardiol. 2020, 32, 412–416. [Google Scholar] [PubMed]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism. JACC: Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef]
- Toma, C.; Bunte, M.C.; Cho, K.H.; Jaber, W.A.; Chambers, J.; Stegman, B.; Gondi, S.; Leung, D.A.; Savin, M.; Khandhar, S.; et al. Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: Interim results of the FLASH registry. Catheter. Cardiovasc. Interv. 2022, 99, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Quezada, C.A.; Bikdeli, B.; Barrios, D.; Barbero, E.; Chiluiza, D.; Muriel, A.; Casazza, F.; Monreal, M.; Yusen, R.D.; Jiménez, D. Meta-Analysis of Prevalence and Short-Term Prognosis of Hemodynamically Unstable Patients With Symptomatic Acute Pulmonary Embolism. Am. J. Cardiol. 2019, 123, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Jerjes-Sanchez, C.; Ramirez-Rivera, A.; de Lourdes Garcia, M.; Arriaga-Nava, R.; Valencia, S.; Rosado-Buzzo, A.; Pierzo, J.A.; Rosas, E. Streptokinase and heparin versus heparin alone in massive pulmonary embolism: A randomized controlled trial. J. Thromb. Thrombolysis 1995, 2, 227–229. [Google Scholar] [CrossRef]
- Marti, C.; John, G.; Konstantinides, S.; Combescure, C.; Sanchez, O.; Lankeit, M.; Meyer, G.; Perrier, A. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. Eur. Hear. J. 2014, 36, 605–614. [Google Scholar] [CrossRef]
- Becattini, C.; Agnelli, G.; Salvi, A.; Grifoni, S.; Pancaldi, L.G.; Enea, I.; Balsemin, F.; Campanini, M.; Ghirarduzzi, A.; Casazza, F. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb. Res. 2010, 125, e82–e86. [Google Scholar] [CrossRef]
- Erkens, P.M.G.; Prins, M.H. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst. Rev. 2010, CD001100. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, A.; Weinberg, I.; Kadakia, M.B.; Wilensky, R.L.; Sardar, P.; Kumbhani, D.J.; Mukherjee, D.; Jaff, M.R.; Giri, J. Thrombolysis for Pulmonary Embolism and Risk of All-Cause Mortality, Major Bleeding, and Intracranial Hemorrhage. JAMA 2014, 311, 2414–2421. [Google Scholar] [CrossRef]
- Nakamura, S.; Takano, H.; Kubota, Y.; Asai, K.; Shimizu, W. Impact of the efficacy of thrombolytic therapy on the mortality of patients with acute submassive pulmonary embolism: A meta-analysis. J. Thromb. Haemost. 2014, 12, 1086–1095. [Google Scholar] [CrossRef]
- Ismayl, M.; Balakrishna, A.M.; Aboeata, A.; Gupta, T.; Young, M.N.; Altin, S.E.; Aronow, H.D.; Goldsweig, A.M. Meta-Analysis Comparing Catheter-Directed Thrombolysis Versus Systemic Anticoagulation Alone for Submassive Pulmonary Embolism. Am. J. Cardiol. 2022, 178, 154–162. [Google Scholar] [CrossRef]
- Lee, T.; Itagaki, S.; Chiang, Y.P.; Egorova, N.N.; Adams, D.H.; Chikwe, J. Survival and recurrence after acute pulmonary embolism treated with pulmonary embolectomy or thrombolysis in New York State, 1999 to 2013. J. Thorac. Cardiovasc. Surg. 2018, 155, 1084–1090.e12. [Google Scholar] [CrossRef]
- Keeling, W.B.; Sundt, T.; Leacche, M.; Okita, Y.; Binongo, J.; Lasajanak, Y.; Aklog, L.; Lattouf, O.M. Outcomes After Surgical Pulmonary Embolectomy for Acute Pulmonary Embolus: A Multi-Institutional Study. Ann. Thorac. Surg. 2016, 102, 1498–1502. [Google Scholar] [CrossRef]
- Pasrija, C.; Kronfli, A.; Rouse, M.; Raithel, M.; Bittle, G.J.; Pousatis, S.; Ghoreishi, M.; Gammie, J.S.; Griffith, B.P.; Sanchez, P.G.; et al. Outcomes after surgical pulmonary embolectomy for acute submassive and massive pulmonary embolism: A single-center experience. J. Thorac. Cardiovasc. Surg. 2018, 155, 1095–1106.e2. [Google Scholar] [CrossRef]
- Khandhar, S.J.; Mehta, M.; Cilia, L.; Palevsky, H.; Matthai, W.; Rivera-Lebron, B.; Toma, C. Invasive hemodynamic assessment of patients with submassive pulmonary embolism. Catheter. Cardiovasc. Interv. 2019, 95, 13–18. [Google Scholar] [CrossRef]
- Rivera-Lebron, B.; McDaniel, M.; Ahrar, K.; Alrifai, A.; Dudzinski, D.M.; Fanola, C.; Blais, D.; Janicke, D.; Melamed, R.; Mohrien, K.; et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin. Appl. Thromb. 2019, 25. [Google Scholar] [CrossRef]
- Hobohm, L.; Sagoschen, I.; Habertheuer, A.; Barco, S.; Valerio, L.; Wild, J.; Schmidt, F.P.; Gori, T.; Münzel, T.; Konstantinides, S.; et al. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation 2021, 170, 285–292. [Google Scholar] [CrossRef]
- Omar, H.R.; Miller, J.; Mangar, D.; Camporesi, E.M. Experience with extracorporeal membrane oxygenation in massive and submassive pulmonary embolism in a tertiary care center. Am. J. Emerg. Med. 2013, 31, 1616–1617. [Google Scholar] [CrossRef]
- Elder, M.; Blank, N.; Kaki, A.; Alraies, M.C.; Grines, C.L.; Kajy, M.; Hasan, R.; Mohamad, T.; Schreiber, T. Mechanical circulatory support for acute right ventricular failure in the setting of pulmonary embolism. J. Interv. Cardiol. 2018, 31, 518–524. [Google Scholar] [CrossRef]
- Salsano, A.; Sportelli, E.; Olivieri, G.M.; Di Lorenzo, N.; Borile, S.; Santini, F. RVAD Support in the Setting of Submassive Pulmonary Embolism. J. Extra-Corporeal Technol. 2017, 49, 304–306. [Google Scholar]
- Mismetti, P.; Laporte, S.; Pellerin, O.; Ennezat, P.-V.; Couturaud, F.; Elias, A.; Falvo, N.; Meneveau, N.; Quere, I.; Roy, P.-M.; et al. Effect of a Retrievable Inferior Vena Cava Filter Plus Anticoagulation vs Anticoagulation Alone on Risk of Recurrent Pulmonary Embolism. Jama 2015, 313, 1627–1635. [Google Scholar] [CrossRef]
- Kucher, N.; Rossi, E.; De Rosa, M.; Goldhaber, S.Z. Massive Pulmonary Embolism. Circulation 2006, 113, 577–582. [Google Scholar] [CrossRef]
- Stein, P.D.; Matta, F.; Keyes, D.C.; Willyerd, G.L. Impact of Vena Cava Filters on In-hospital Case Fatality Rate from Pulmonary Embolism. Am. J. Med. 2012, 125, 478–484. [Google Scholar] [CrossRef]
- Bikdeli, B.; Chatterjee, S.; Desai, N.R.; Kirtane, A.J.; Desai, M.M.; Bracken, M.B.; Spencer, F.A.; Monreal, M.; Goldhaber, S.Z.; Krumholz, H.M. Inferior Vena Cava Filters to Prevent Pulmonary Embolism. J. Am. Coll. Cardiol. 2017, 70, 1587–1597. [Google Scholar] [CrossRef]
- Jimenez, D.; Nieto, R.; Corres, J.; Fernández-Golfín, C.; Barrios, D.; Morillo, R.; Quezada, C.A.; Huisman, M.; Yusen, R.D.; Kline, J. Diclofenac for reversal of right ventricular dysfunction in acute normotensive pulmonary embolism: A pilot study. Thromb. Res. 2018, 162, 1–6. [Google Scholar] [CrossRef]
- Kline, J.A.; Hall, C.L.; Jones, A.E.; Puskarich, M.A.; Mastouri, R.A.; Lahm, T. Randomized trial of inhaled nitric oxide to treat acute pulmonary embolism: The iNOPE trial. Am. Hear. J. 2017, 186, 100–110. [Google Scholar] [CrossRef]
- Nguyen, P.; Stevens, H.; Peter, K.; McFadyen, J. Submassive Pulmonary Embolism: Current Perspectives and Future Directions. J. Clin. Med. 2021, 10, 3383. [Google Scholar] [CrossRef]
- Sista, A.K.; A Friedman, O.; Dou, E.; Denvir, B.; Askin, G.; Stern, J.; Estes, J.; Salemi, A.; Winokur, R.S.; Horowitz, J.M. A pulmonary embolism response team’s initial 20 month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc. Med. 2017, 23, 65–71. [Google Scholar] [CrossRef] [Green Version]
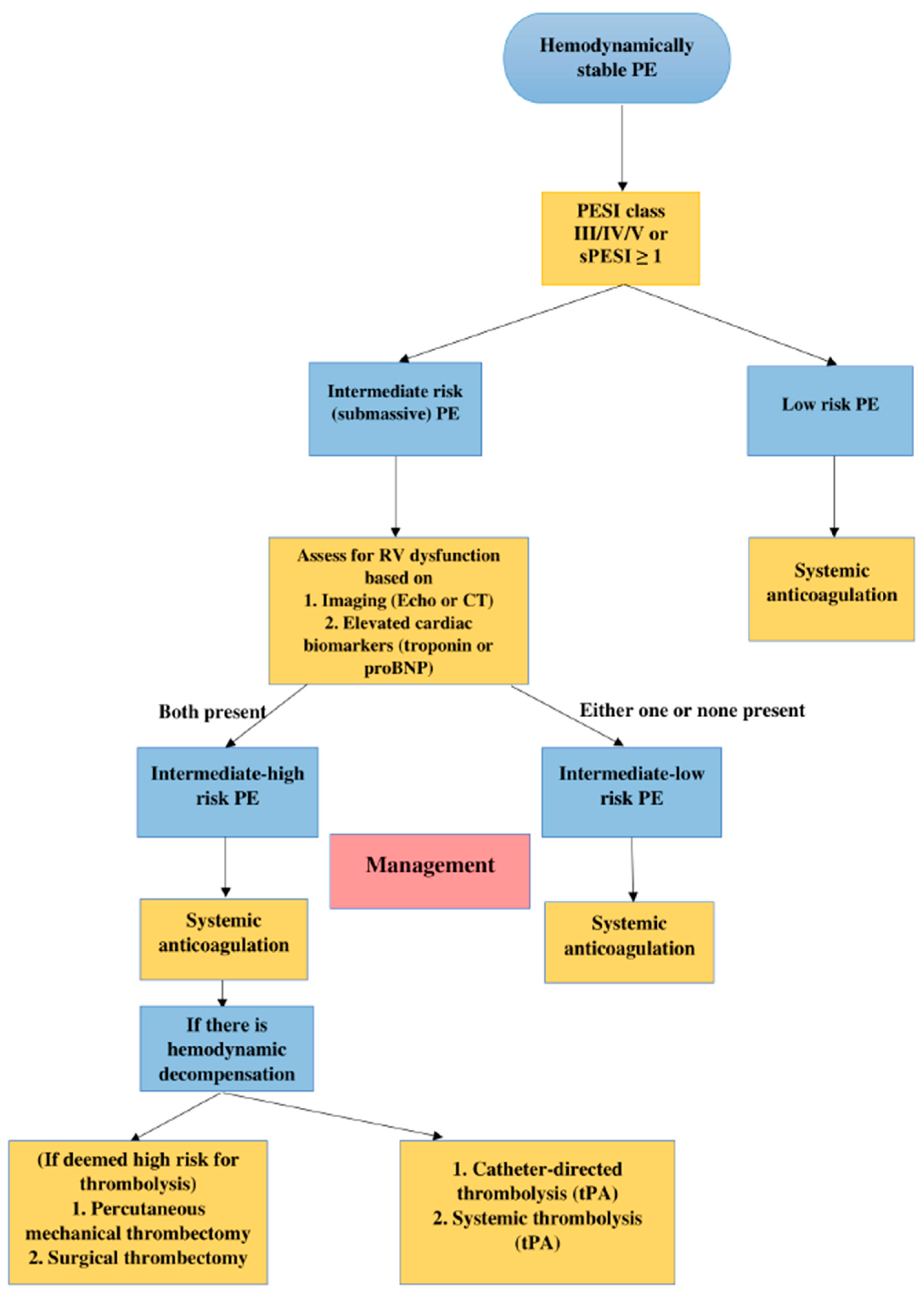
| Hemodynamic Instability | RVD or Biomarker Elevation | PESI Class/sPESI Score | |
|---|---|---|---|
| American College of Chest Physicians | |||
| Low risk | No | No | --- |
| Intermediate risk | No | Either one present | --- |
| High risk | Yes | --- | --- |
| American Heart Association | |||
| Low risk | No | No | --- |
| Submassive | No | Either one present | --- |
| Massive | Yes | --- | --- |
| European Society of Cardiology | |||
| Low risk | No | No | No |
| Intermediate-low risk | No | Either one positive | PESI Class III-IV or sPESI score ≥ 1 |
| Intermediate-high risk | No | Both positive | PESI Class III-IV or sPESI score ≥ 1 |
| High risk | Yes | --- | PESI Class III-IV or sPESI score ≥ 1 |
| Treatment Option | Advantages | Disadvantages |
|---|---|---|
| Systemic anticoagulation |
|
|
| Systemic thrombolysis |
|
|
| Reduced-dose thrombolysis |
|
|
| Catheter-directed therapy |
|
|
| Surgical embolectomy |
|
|
| Trials | Groups Compared | Outcomes | Summary |
|---|---|---|---|
| MAPPET-3, 2002[45] | Heparin with alteplase vs. heparin with placebo |
| Thrombolytics can prevent clinical decline necessitating the escalation of treatment during the hospital stay |
| TIPES, 2010[69] | Tenecteplase group (Tenecteplase with heparin) vs. placebo group (placebo with heparin) |
| Single dose thrombolytics are associated with reduction of RVD at 24 h |
| MOPETT, 2013[50] | Lower dose thrombolysis along with anticoagulation vs. anticoagulation alone |
| Lower dose thrombolysis is safe and effective in the treatment of moderate PE, with a significant immediate reduction in the pulmonary artery pressure that was maintained at 28 months |
| PEITHO trial, 2014[31] | Systemic thrombolysis with tenecteplase followed by anticoagulation with heparin vs. heparin alone |
| Fibrinolytic therapy prevented hemodynamic decompensation but increased the risk of major hemorrhage and stroke |
| TOPCOAT, 2014[46] | Low molecular weight heparin and tenecteplase vs. low molecular weight heparin |
| Treatment with tenecteplase was associated with an increased probability of a favorable composite outcome |
| ULTIMA, 2014[56] | Heparin alone vs. heparin with catheter-directed thrombolysis (tPA and USAT) |
| Standardized USAT regimen was superior to anticoagulation with heparin alone in reversing RV dilatation at 24 h, without an increase in bleeding complications |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machanahalli Balakrishna, A.; Reddi, V.; Belford, P.M.; Alvarez, M.; Jaber, W.A.; Zhao, D.X.; Vallabhajosyula, S. Intermediate-Risk Pulmonary Embolism: A Review of Contemporary Diagnosis, Risk Stratification and Management. Medicina 2022, 58, 1186. https://doi.org/10.3390/medicina58091186
Machanahalli Balakrishna A, Reddi V, Belford PM, Alvarez M, Jaber WA, Zhao DX, Vallabhajosyula S. Intermediate-Risk Pulmonary Embolism: A Review of Contemporary Diagnosis, Risk Stratification and Management. Medicina. 2022; 58(9):1186. https://doi.org/10.3390/medicina58091186
Chicago/Turabian StyleMachanahalli Balakrishna, Akshay, Vuha Reddi, Peter Matthew Belford, Manrique Alvarez, Wissam A. Jaber, David X. Zhao, and Saraschandra Vallabhajosyula. 2022. "Intermediate-Risk Pulmonary Embolism: A Review of Contemporary Diagnosis, Risk Stratification and Management" Medicina 58, no. 9: 1186. https://doi.org/10.3390/medicina58091186
APA StyleMachanahalli Balakrishna, A., Reddi, V., Belford, P. M., Alvarez, M., Jaber, W. A., Zhao, D. X., & Vallabhajosyula, S. (2022). Intermediate-Risk Pulmonary Embolism: A Review of Contemporary Diagnosis, Risk Stratification and Management. Medicina, 58(9), 1186. https://doi.org/10.3390/medicina58091186







