The Effects of COVID-19 on Skeletal Muscles, Muscle Fatigue and Rehabilitation Programs Outcomes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
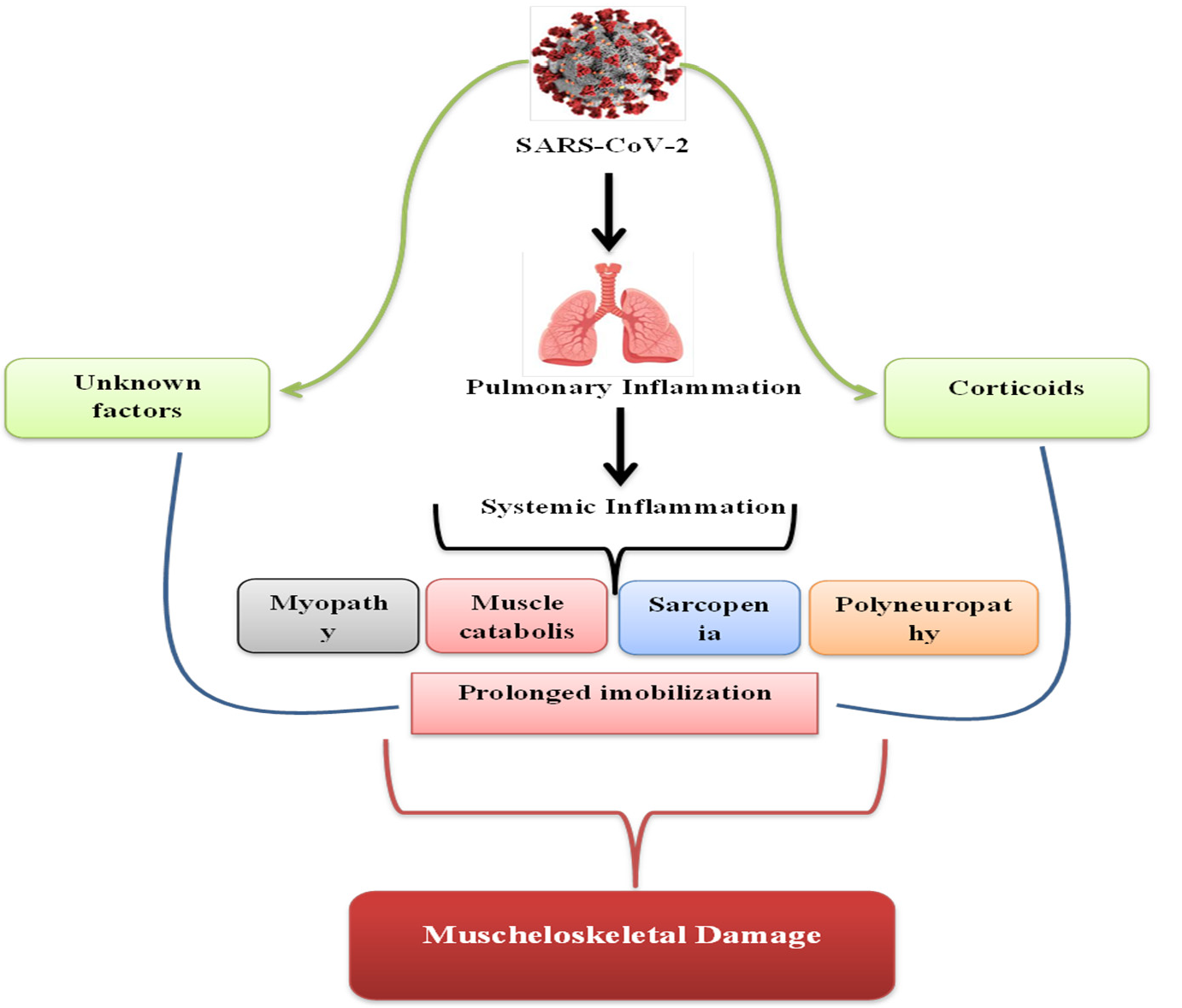
4. Discussion
4.1. Cytokines and Skeletal Muscle
4.2. Proinflammatory Viral Effects
4.3. Sarcopenia
4.4. Muscle Catabolism
4.5. Corticoids as a Therapy
4.6. Muscle Fatigue
4.7. Respiratory Rehabilitation, a Possible Cure?
4.8. Early Mobilization for COVID-19 Patients
4.9. Myopathy and Polyneuropathy, a Possible Complication?
4.10. Psychologycal Disorders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.; Wang, Y.; Wang, G.-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020, 92, 726–730. [Google Scholar] [CrossRef]
- De Micheli, A.J.; Spector, J.A.; Elemento, O.; Cosgrove, B.D. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skelet. Muscle 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Fang, D. SARS: Facts and considerations for the orthopaedic community. J. Orthop. Surg. 2003, 11, 3–5. [Google Scholar] [CrossRef] [PubMed]
- McCray, P.B.J.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.W.; Wong, K.S.; Hui, A.C.; To, K.F.; Lai, S.T.; Ng, W.F.; Ng, H.K. Myopathic changes associated with severe acute respiratory syndrome: A postmortem case series. Arch. Neurol. 2005, 62, 1113–1117. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Del Gaudio, A.; Vittori, A.; Bimonte, S.; Del Prete, P.; Forte, C.A.; Cuomo, A.; De Blasio, E. COVID-Pain: Acute and Late-Onset Painful Clinical Manifestations in COVID-19—Molecular Mechanisms and Research Perspectives. J. Pain Res. 2021, 14, 2403–2412. [Google Scholar] [CrossRef]
- Al-Ramadan, A.; Rabab’h, O.; Shah, J.; Gharaibeh, A. Acute and Post-Acute Neurological Complications of COVID-19. Neurol. Int. 2021, 13, 102–119. [Google Scholar] [CrossRef]
- Mohamed, A.; Qureshi, A.S.; Mohamed, S.A. Neurological Manifestations of COVID-19 in Absence of Respiratory Symptoms or Fever. Cureus 2021, 13, e13887. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Joint Surg. Am. 2020, 102, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.M.-C.; Lee, E.W.-C.; Wong, C.N.-C.; Ng, G.Y.-F.; Jones, A.Y.-M.; Hui, D.S.-C. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch. Phys. Med. Rehabil. 2005, 86, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, H.; Hu, J.; Lian, J.; Gu, J.; Zhang, S.; Ye, C.; Lu, Y.; Jin, C.; Yu, G.; et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020, 94, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Medrinal, C.; Prieur, G.; Bonnevie, T.; Gravier, F.-E.; Mayard, D.; Desmalles, E.; Smondack, P.; Lamia, B.; Combret, Y.; Fossat, G. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol. 2021, 21, 64. [Google Scholar] [CrossRef]
- Langer, D.; Ciavaglia, C.; Faisal, A.; Webb, K.A.; Neder, J.A.; Gosselink, R.; Dacha, S.; Topalovic, M.; Ivanova, A.; O’Donnell, D.E. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J. Appl. Physiol. 2018, 125, 381–392. [Google Scholar] [CrossRef]
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary Rehabilitation in Patients Recovering from COVID-19. Respiration 2021, 100, 416–422. [Google Scholar] [CrossRef]
- Van Aerde, N.; Van den Berghe, G.; Wilmer, A.; Gosselink, R.; Hermans, G. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med. 2020, 46, 2083–2085. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Joyner, M.; Lucia, A. Early mobilization in hospitalized patients with COVID-19. Ann. Phys. Rehabil. Med. 2020, 63, 384–385. [Google Scholar] [CrossRef]
- Cabañes-Martínez, L.; Villadóniga, M.; González-Rodríguez, L.; Araque, L.; Díaz-Cid, A.; Ruz-Caracuel, I.; Pian, H.; Sánchez-Alonso, S.; Fanjul, S.; Del Álamo, M.; et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin. Neurophysiol. 2020, 131, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Tankisi, H.; de Carvalho, M.; Z’Graggen, W.J. Critical Illness Neuropathy. J. Clin. Neurophysiol. 2020, 37, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Charmchi, Z.; Seidman, R.J.; Anziska, Y.; Velayudhan, V.; Perk, J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020, 62, E57–E60. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Gąsowski, J.; Michel, J.-P.; Veronese, N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A major cause of cachexia and sarcopenia? J. Cachexia. Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients Without Previous Disabilities Recovering From COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef]
- Kirwan, R.; McCullough, D.; Butler, T.; Perez de Heredia, F.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. GeroScience 2020, 42, 1547–1578. [Google Scholar] [CrossRef]
- Martinez-Ferran, M.; de la Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic Due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549. [Google Scholar] [CrossRef]
- Mayer, K.P.; Thompson Bastin, M.L.; Montgomery-Yates, A.A.; Pastva, A.M.; Dupont-Versteegden, E.E.; Parry, S.M.; Morris, P.E. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit. Care 2020, 24, 637. [Google Scholar] [CrossRef]
- Toussirot, E.; Marotte, H.; Mulleman, D.; Cormier, G.; Coury, F.; Gaudin, P.; Dernis, E.; Bonnet, C.; Damade, R.; Grauer, J.-L.; et al. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: A 12-month multicentre study. Arthritis Res. Ther. 2020, 22, 224. [Google Scholar] [CrossRef]
- Stam, H.J.; Stucki, G.; Bickenbach, J. Covid-19 and Post Intensive Care Syndrome: A Call for Action. J. Rehabil. Med. 2020, 52, jrm00044. [Google Scholar] [CrossRef]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Sagarra-Romero, L.; Viñas-Barros, A. COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly. Int. J. Environ. Res. Public Health 2020, 17, 8715. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Chen, M.; Chen, X.; Yu, W. Diaphragm thickness on computed tomography for nutritional assessment and hospital stay prediction in critical COVID-19. Asia Pac. J. Clin. Nutr. 2022, 31, 33–40. [Google Scholar] [CrossRef] [PubMed]
- De Andrade-Junior, M.C.; de Salles, I.C.D.; de Brito, C.M.M.; Pastore-Junior, L.; Righetti, R.F.; Yamaguti, W.P. Skeletal Muscle Wasting and Function Impairment in Intensive Care Patients With Severe COVID-19. Front. Physiol. 2021, 12, 640973. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Yoon, J.S.; Kim, E.J.; Hong, H.-L.; Kwon, H.H.; Jung, C.Y.; Kim, K.C.; Sung, Y.S.; Park, S.-H.; Kim, S.-K.; et al. Prognostic Implication of Baseline Sarcopenia for Length of Hospital Stay and Survival in Patients With Coronavirus Disease 2019. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e110–e116. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Jiang, L.; Wang, Y.; Xi, X. Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol. Scand. 2018, 138, 104–114. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Kiekens, C.; Boldrini, P.; Andreoli, A.; Avesani, R.; Gamna, F.; Grandi, M.; Lombardi, F.; Lusuardi, M.; Molteni, F.; Perboni, A.; et al. Rehabilitation and respiratory management in the acute and early post-acute phase. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020, 56, 323–326. [Google Scholar] [CrossRef]
- Bonorino, K.C.; Cani, K.C. Early mobilization in the time of COVID-19. Rev. Bras. Ter. intensiva 2020, 32, 484–486. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, P.H.; Mercatelli, D.; Ceraolo, C.; Giorgi, F.M. Master Regulator Analysis of the SARS-CoV-2/Human Interactome. J. Clin. Med. 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Fu, S.; Fu, X.; Song, Y.; Li, M.; Pan, P.; Tang, T.; Zhang, C.; Jiang, T.; Tan, D.; Fan, X.; et al. Virologic and clinical characteristics for prognosis of severe COVID-19: A retrospective observational study in Wuhan, China. medRxiv 2020. [Google Scholar] [CrossRef]
- Forcina, L.; Miano, C.; Scicchitano, B.M.; Rizzuto, E.; Berardinelli, M.G.; De Benedetti, F.; Pelosi, L.; Musarò, A. Increased Circulating Levels of Interleukin-6 Affect the Redox Balance in Skeletal Muscle. Oxid. Med. Cell. Longev. 2019, 2019, 3018584. [Google Scholar] [CrossRef]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Li, Y.; Wang, Q. Sarcopenia: An underlying treatment target during the COVID-19 pandemic. Nutrition 2021, 84, 111104. [Google Scholar] [CrossRef]
- Kortebein, P.; Symons, T.B.; Ferrando, A.; Paddon-Jones, D.; Ronsen, O.; Protas, E.; Conger, S.; Lombeida, J.; Wolfe, R.; Evans, W.J. Functional impact of 10 days of bed rest in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1076–1081. [Google Scholar] [CrossRef] [Green Version]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Castillero, E.; Alamdari, N.; Aversa, Z.; Gurav, A.; Hasselgren, P.-O. PPARβ/δ regulates glucocorticoid- and sepsis-induced FOXO1 activation and muscle wasting. PLoS ONE 2013, 8, e59726. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Pereira, B.; Dutheil, F.; Giraud, C.; Courteix, D.; Sapin, V.; Frayssac, T.; Mathieu, S.; Malochet-Guinamand, S.; Soubrier, M. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J. Cachexia. Sarcopenia Muscle 2017, 8, 639–646. [Google Scholar] [CrossRef]
- Schakman, O.; Gilson, H.; Thissen, J.P. Mechanisms of glucocorticoid-induced myopathy. J. Endocrinol. 2008, 197, 1–10. [Google Scholar] [CrossRef]
- Silva, D.F.O.; Lima, S.C.V.C.; Sena-Evangelista, K.C.M.; Marchioni, D.M.L.; Cobucci, R.N.O.; de Andrade, F.B. Nutritional Risk Screening Tools for Older Adults with COVID-19: A Systematic Review. Nutrients 2020, 12, 2956. [Google Scholar] [CrossRef]
- Lau, H.M.-C.; Ng, G.Y.-F.; Jones, A.Y.-M.; Lee, E.W.-C.; Siu, E.H.-K.; Hui, D.S.-C. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust. J. Physiother. 2005, 51, 213–219. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Bloomfield, S.A. Changes in musculoskeletal structure and function with prolonged bed rest. Med. Sci. Sports Exerc. 1997, 29, 197–206. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Michel, J.-P.; Maggi, S.; Ecarnot, F. Raising awareness of the needs of older COVID patients after hospital discharge. Aging Clin. Exp. Res. 2020, 32, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
| Authors | Type of Study | Pathology | Results |
|---|---|---|---|
| Disser NP et al., 2020 [11] | Prospective | COVID-19 | Muscle, cortical and synovial tissues are direct locus of SARS-CoV-2 infection. |
| Lau HMC et al., 2021 [12] | Randomized Controlled Trial | Severe Acute Respiratory Syndrome And COVID-19 | The pulmonary rehabilitation programs reduced the fatigue and increased the cardiopulmonary health. |
| Zhang et al., 2020 [13] | Cohort and Case Control | COVID-19 | Moderate/severe lung damage on CT is linked to muscle pain and general weakness. |
| Mao L. et al., 2019 [3] | Observational Study | COVID-19 | Lung damage CT imaging is a negative predictor factor for the patient’s recovery. |
| Ofori-Asenso R et al., 2019 [14] | Systematic Review and Meta-analysis | Frailty | The negative impact of prolonged bed rest is more evident among the elderly. |
| Huang et al., 2021 [15] | Cohort Study | COVID-19 | The main symptomatology post-illness is fatigue or muscle weakness. |
| Medrinal et al., 2021 [16] | Observational Study | COVID-19 | One month after extubation, the main symptoms were muscle weakness of the limbs and respiratory muscle weakness. |
| Langer et al., 2018 [17] | Clinical Trial | Chronic Obstructive Pulmonary Disease (COPD) | A 2-month respiratory exercise program reduced the dyspnea, strengthened the inspiratory muscles and increased the capacity of physical effort. |
| Zamponga et al., 2021 [18] | Retrospective Data Analysis | COVID-19 | Pulmonary rehabilitation is possible and effective in patients recovering from COVID-19. |
| Van Aerde et al., 2020 [19] | Observational Study | COVID-19 | Post-COVID-19 recovery programs post-ICU speed up the healing process and reduce the long-term impact of the disease. |
| Valenzuela et al., 2020 [20] | Meta-analysis | COVID-19 | Early initiation of mobilization programs initiated since the period of hospitalization in COVID-19 patients hastens their healing and recovery. |
| Martinez et al., 2020 [21] | Retrospective Study | COVID-19 | The myopathy and polyneuropathy induced by the COVID-19 disease does not have any distinctive features compared to those induced by other pathologies. |
| Tankisi H et al., 2020 [22] | Review | Critical Illness Neuropathy | The correct evaluation and diagnosis, through nerve conduction studies and electromyography is crucial in pacients suspicious of CIN. |
| Zhang et al., 2020 [23] | Case Reports | COVID-19-associated myositis | There’s a link between COVID-19 disease and inflammatory mediated myositis. |
| Piotrowicz et al., 2021 [24] | Review | Post-COVID-19 acute sarcopenia | The state of health before infection, the treatment, early mobilization and adequate caloric intake can change the prognosis of the acute sarcopenia COVID-19 induced. |
| Morley et al., 2020 [25] | Editorial | COVID-19 | Elderly patients are at higher risk of acute sarcopenia and malnutrition. |
| Paneroni et al., 2021 [26] | Cross-sectional Study | COVID-19 | The strength of the main muscle groups was significantly affected by the COVID-19 infection. |
| Kirwan R et al., 2020 [27] | Review | COVID-19, Sarcopenia | Physical activity, online and phone-based virtual care and telehealth services can protect vulnerable populations and maintain or improve the health status of the population at large. |
| Martinez-Ferran M. et al., 2020 [28] | Review | COVID-19-related Metabolic Dysfunctions | Adequate control of metabolic disorders could be important to reduce the risk of severe COVID-19. |
| Mayer KP et al., 2020 [29] | Observational Study | ICU-assessed Muscle Alterations | PPARβ/δ regulates FOXO1 activation in glucocorticoid- and sepsis-induced muscle wasting and that treatment with a PPARβ/δ inhibitor may ameliorate loss of muscle mass in these conditions. |
| Toussirot E et al., 2020 [30] | Multicenter Study | Rheumatoid Arthritis (RA) | Tocilizumab may have an anabolic impact on lean mass/skeletal muscle. |
| Michel J-P et al., 2020 [20] | Editorial | COVID-19 | |
| Stam HJ et al., 2020 [31] | Cohort Study | COVID-19 and Post Intensive Care Syndrome | Post Intensive Care Syndrome and other severe conditions will require not only adequate screening but early rehabilitation and other interventions. |
| Ammar et al., 2020 [32] | Electronic Survey | ECLB-COVID19 | While isolation is a necessary measure to protect public health, results indicate that it alters physical activity and eating behaviours in a health compromising direction. |
| Romero-Sagarra L., 2020 [33] | Review | COVID-19 and Muscular Weakness | Older people diagnosed with a COVID-19 infection are at a higher risk of severe muscle weakness and atrophy that may have negative consequences on functional disability. |
| Yong You et al., 2022 [34] | Retrospective Study | COVID-19 and Diaphragm thickness | CT-obtained DT can be used as a dynamic assessment tool for evaluating the nutritional status of patients in isolation wards for COVID-19. |
| Mario Chueire A. et al., 2021 [35] | Prospective study | Skeletal Muscle Wasting and Function Impairment in Intensive Care COVID-19 patients | In intensive care patients with severe COVID-19, muscle wasting and decreased muscle strength occurred early and rapidly during 10 days of ICU stay with improved mobility and respiratory functions, although they remained below normal levels. |
| Kim Ji-Won et al., 2021 [36] | Observational study | COVID-19 and Prognostic Sarcopenia for Length of Hospital Stay | Baseline sarcopenia was independently associated with a prolonged hospital stay in patients with COVID-19. Sarcopenia could be a prognostic marker in COVID-19. |
| Tao Yang et al., 2018 [37] | Meta-analysis study | COVID-19 and Risk factors for intensive care unit-acquired weakness | Acute Physiology and Chronic Health Evaluation II score, neuromuscular blocking agents and aminoglycosides were found to be significantly associated with ICUAW. |
| Sartori et al., 2021 [38] | Review | Mechanisms of muscle atrophy and hypertrophy | The lack of any efficient drug that counteracts muscle loss suggests that our view of the mechanistic insights that control atrophying muscle is still limited and needs further exploration. |
| Chaolin Huang et al., 2021 [15] | Cohort study | 6 months consequences of COVID-19 | COVID-19 and Patients who were more severely ill during their hospital stay had more severe impaired pulmonary diffusion capacities and abnormal chest imaging manifestations, and are the main target population for intervention of long-term recovery. |
| Carlotte Kiekens et al., 2020 [39] | Article | Rehabilitation and respiratory management in the acute and early post-acute phase | The first experiences in the field of rehabilitation show clearly the need to prepare for the post acute phase for patients who experienced a severe degree of the disease. |
| Bonorino K. et al., 2020 [40] | Article | Early mobilization in the time of COVID-19 | Early rehabilitation interventions in patients with COVID-19, especially those who develop with severe muscle dysfunction, fatigue and dyspnea, (13) be initiated during hospitalization and continue in specialized rehabilitation programs after discharge. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pescaru, C.C.; Marițescu, A.; Costin, E.O.; Trăilă, D.; Marc, M.S.; Trușculescu, A.A.; Pescaru, A.; Oancea, C.I. The Effects of COVID-19 on Skeletal Muscles, Muscle Fatigue and Rehabilitation Programs Outcomes. Medicina 2022, 58, 1199. https://doi.org/10.3390/medicina58091199
Pescaru CC, Marițescu A, Costin EO, Trăilă D, Marc MS, Trușculescu AA, Pescaru A, Oancea CI. The Effects of COVID-19 on Skeletal Muscles, Muscle Fatigue and Rehabilitation Programs Outcomes. Medicina. 2022; 58(9):1199. https://doi.org/10.3390/medicina58091199
Chicago/Turabian StylePescaru, Camelia Corina, Adelina Marițescu, Emanuela Oana Costin, Daniel Trăilă, Monica Steluța Marc, Ana Adriana Trușculescu, Andrei Pescaru, and Cristian Iulian Oancea. 2022. "The Effects of COVID-19 on Skeletal Muscles, Muscle Fatigue and Rehabilitation Programs Outcomes" Medicina 58, no. 9: 1199. https://doi.org/10.3390/medicina58091199
APA StylePescaru, C. C., Marițescu, A., Costin, E. O., Trăilă, D., Marc, M. S., Trușculescu, A. A., Pescaru, A., & Oancea, C. I. (2022). The Effects of COVID-19 on Skeletal Muscles, Muscle Fatigue and Rehabilitation Programs Outcomes. Medicina, 58(9), 1199. https://doi.org/10.3390/medicina58091199






