Correlation between Maternal Weight Gain in Each Trimester and Fetal Growth According to Pre-Pregnancy Maternal Body Mass Index in Twin Pregnancies
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
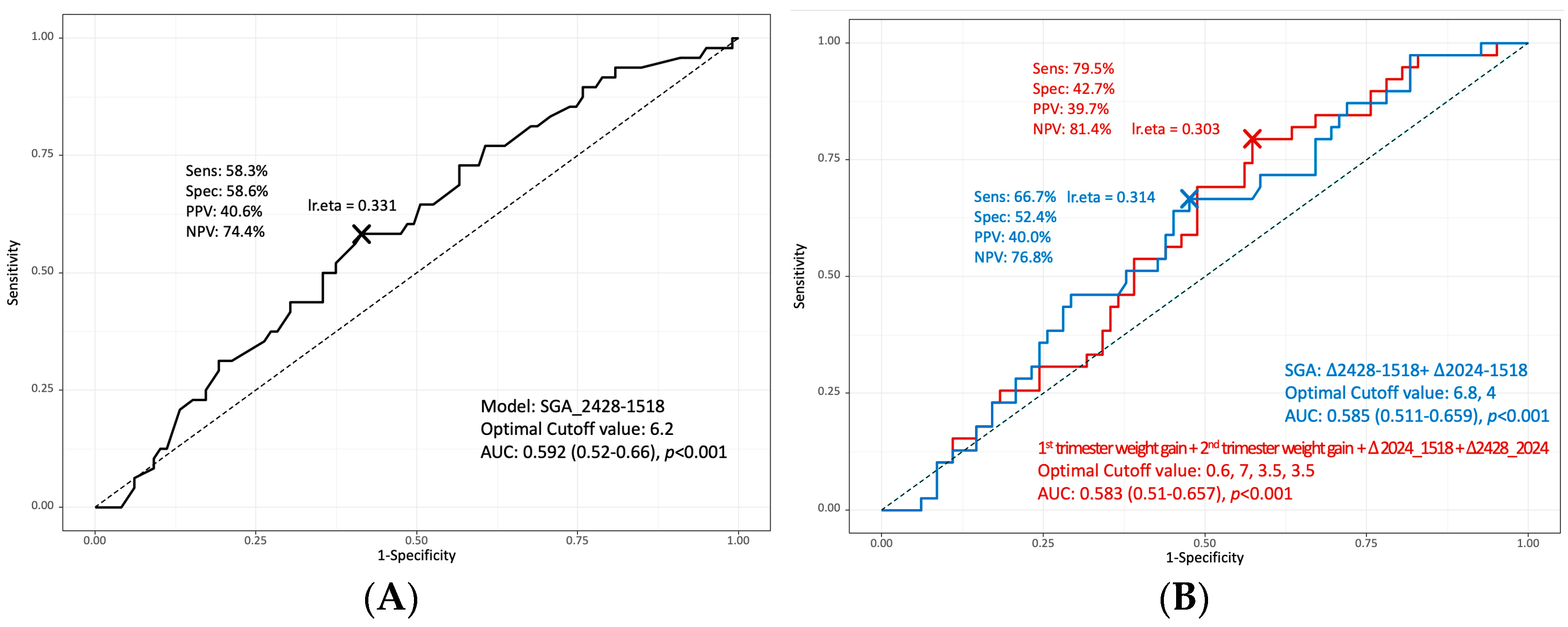
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 144: Multifetal gestations: Twin, triplet, and higher-order multifetal pregnancies. Obstet. Gynecol. 2014, 123, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, Y.N.; Im, D.H.; Kim, D.H.; Byun, J.M.; Jeong, D.H.; Lee, K.B.; Sung, M.S. Neonatal outcomes between discordant monochorionic and dichorionic twins. J. Matern. Fetal Neonatal Med. 2019, 34, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Brown, M.B. Contemporary risks of maternal morbidity and adverse outcomes with increasing maternal age and plurality. Fertil. Steril. 2007, 88, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.N.; Nakagawa, S.; Gonzalez, J.M. Hypertension in dichorionic twin gestations: How is birthweight affected? J. Matern. Fetal Neonatal Med. 2017, 30, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K.; Drake, P. Births: Final data for 2017. Natl. Vital Stat. Rep. 2018, 67, 1–50. [Google Scholar]
- MacDorman, M.F.; Gregory, E.C. Fetal and perinatal mortality: United States, 2013. Natl. Vital Stat. Rep. 2015, 64, 1–24. [Google Scholar] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #56: Multiple gestation: Complicated twin, triplet, and high-order multifetal pregnancy. Obstet. Gynecol. 2004, 104, 869–883. [Google Scholar]
- Pugh, S.J.; Albert, P.S.; Kim, S.; Grobman, W.; Hinkle, S.N.; Newman, R.B.; Wing, D.A.; Grantz, K.L. Patterns of gestational weight gain and birthweight outcomes in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies-Singletons: A prospective study. Am. J. Obstet. Gynecol. 2017, 217, 346.e1–346.e11. [Google Scholar] [CrossRef]
- Sridhar, S.B.; Xu, F.; Hedderson, M.M. Trimester-specific gestational weight gain and infant size for gestational age. PLoS ONE 2016, 11, e0159500. [Google Scholar] [CrossRef]
- Luke, B.; Gillespie, B.; Min, S.J.; Avni, M.; Witter, F.R.; O’Sullivan, M.J. Critical periods of maternal weight gain: Effect on twin birth weight. Am. J. Obstet. Gynecol. 1997, 177, 1055–1062. [Google Scholar] [CrossRef]
- Lantz, M.E.; Chez, R.A.; Rodriguez, A.; Porter, K.B. Maternal weight gain patterns and birth weight outcome in twin gestation. Obstet. Gynecol. 1996, 87, 551–556. [Google Scholar] [CrossRef]
- Luke, B.; Minogue, J.; Witter, F.R.; Keith, L.G.; Johnson, T.R. The ideal twin pregnancy: Patterns of weight gain, discordancy, and length of gestation. Am. J. Obstet. Gynecol. 1993, 169, 588–597. [Google Scholar] [CrossRef]
- Institute of Medicine, National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: Reports funded by National Institutes of Health. In Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; Copyright ©. National Academy of Sciences; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Oken, E.; Kleinman, K.P.; Rich-Edwards, J.; Gillman, M.W. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Rodgers, M.; Baschat, A.; Bhide, A.; Gratacos, E.; Hecher, K.; Kilby, M.D.; Lewi, L.; Nicolaides, K.H.; Oepkes, D.; et al. ISUOG practice guidelines: Role of ultrasound in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef]
- Alanis, M.C.; Goodnight, W.H.; Hill, E.G.; Robinson, C.J.; Villers, M.S.; Johnson, D.D. Maternal super-obesity (body mass index > or = 50) and adverse pregnancy outcomes. Acta Obstet. Gynecol. Scand. 2010, 89, 924–930. [Google Scholar] [CrossRef]
- Torloni, M.R.; Betrán, A.P.; Horta, B.L.; Nakamura, M.U.; Atallah, A.N.; Moron, A.F.; Valente, O. Prepregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes. Rev. 2009, 10, 194–203. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zafman, K.B.; Fox, N.S. Weight gain and pregnancy outcomes in overweight or obese women with twin gestations. J. Matern. Fetal Neonatal. Med. 2021, 34, 1774–1779. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hong, S.Y.; Kim, Y.; Kwon, D.Y.; Park, H.; Sung, J.H.; Choi, S.J.; Oh, S.Y.; Roh, C.R. Maternal pre-pregnancy body mass index and the risk for gestational diabetes mellitus in women with twin pregnancy in South Korea. Taiwan J. Obstet. Gynecol. 2021, 60, 863–868. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zafman, K.B.; Fox, N.S. The association between gestational weight gain in each trimester and pregnancy outcomes in twin pregnancies. Am. J. Perinatol. 2021, 38, 567–574. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zafman, K.B.; Fox, N.S. Weight gain and pregnancy outcomes in underweight women with twin gestations. J. Matern. Fetal Neonatal Med. 2020, 33, 2877–2881. [Google Scholar] [CrossRef]
- Lucovnik, M.; Blickstein, I.; Verdenik, I.; Steblovnik, L.; Trojner Bregar, A.; Tul, N. Impact of pre-gravid body mass index and body mass index change on preeclampsia and gestational diabetes in singleton and twin pregnancies. J. Matern. Fetal Neonatal Med. 2014, 27, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.S.; Saltzman, D.H.; Kurtz, H.; Rebarber, A. Excessive weight gain in term twin pregnancies: Examining the 2009 Institute of Medicine definitions. Obstet. Gynecol. 2011, 118, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- González-Quintero, V.H.; Kathiresan, A.S.; Tudela, F.J.; Rhea, D.; Desch, C.; Istwan, N. The association of gestational weight gain per institute of medicine guidelines and prepregnancy body mass index on outcomes of twin pregnancies. Am. J. Perinatol. 2012, 29, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Shamshirsaz, A.A.; Haeri, S.; Ravangard, S.F.; Sangi-Haghpeykar, H.; Gandhi, M.; Ozhand, A.; Spiel, M.; Trout, S.; Sadowski, A.; Hussain, N.; et al. Perinatal outcomes based on the institute of medicine guidelines for weight gain in twin pregnancies. J. Matern. Fetal Neonatal Med. 2014, 27, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, T.; Bacak, S.J.; Zozzaro-Smith, P.; Li, D.; Sagcan, S.; Seligman, N.; Glantz, C.J. Assessing weight gain by the 2009 Institute of Medicine guidelines and perinatal outcomes in twin pregnancy. Matern. Child Health J. 2017, 21, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Himes, K.P.; Abrams, B.; Lash, T.L.; Parisi, S.M.; Eckhardt, C.L.; Braxter, B.J.; Minion, S.; Hutcheon, J.A. Gestational weight gain and adverse birth outcomes in twin pregnancies. Obstet. Gynecol. 2019, 134, 1075–1086. [Google Scholar] [CrossRef]
- Wen, L.; Liu, X.; Wang, L.; Zheng, Y.; Li, J.; Tong, C.; Qi, H.; Saffery, R.; Baker, P. Correlation between second trimester weight gain and perinatal outcomes in dichorionic twin pregnancies: The LoTiS cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 64–69. [Google Scholar] [CrossRef]
| Underweight (n = 33) | Normal Weight (n = 244) | Overweight (n = 66) | p Value | p Value α | p Value β | p Value γ | |
|---|---|---|---|---|---|---|---|
| Maternal age, years | 30.24 ± 5.13 | 32.28 ± 4.13 | 33.44 ± 3.95 | <0.001 * | 0.003 * | <0.001 * | 0.004 * |
| Nulliparous, n (%) | 23 (69.70%) | 172 (70.49%) | 41 (62.12%) | 0.018 * | 0.237 | 0.040 * | 0.022 * |
| Height, cm | 161.45 ± 4.58 | 160.97 ± 4.95 | 160.43 ± 5.51 | 0.153 | 0.450 | 0.194 | 0.279 |
| Weight gain during pregnancy, kg | 13.04 ± 5.35 | 14.72 ± 7.13 | 11.53 ± 5.49 | 0.006 * | 0.024 * | 0.068 | <0.001 * |
| ART, n (%) | 15 (45.45%) | 134 (55.14%) | 36 (54.55%) | 0.516 | 0.237 | 0.278 | 0.880 |
| Monochorionic, n (%) | 9 (27.27%) | 59 (24.18%) | 10 (15.15%) | 0.059 | 0.692 | 0.064 | 0.036 * |
| Dichorionic, n (%) | 24 (72.73%) | 185 (75.82%) | 56 (84.85%) | ||||
| Chronic HTN, n (%) | 0 (0.00%) | 0 (0.00%) | 2 (3.03%) | <0.001 * | 0.372 | 0.001 * | |
| Preeclampsia, n (%) | 1 (3.03%) | 32 (13.11%) | 4 (6.06%) | 0.007 * | 0.030 * | 0.566 | 0.037 * |
| Overt DM, n (%) | 1 (3.03%) | 0 (0.00%) | 1 (1.52%) | 0.003 * | 0.006 * | 0.858 | 0.063 |
| Gestational DM, n (%) | 3 (9.09%) | 25 (10.25%) | 18 (27.27%) | <0.001 * | 0.941 | 0.006 * | <0.001 * |
| Placenta previa, n (%) | 2 (6.06%) | 7 (2.87%) | 1 (1.52%) | 0.027 * | 0.014 * | 0.101 | 0.264 |
| Threatened preterm, n (%) | 27 (81.82%) | 142 (58.20%) | 38 (57.58%) | 0.001 * | <0.001 * | 0.001 * | 0.977 |
| PROM, n (%) | 10 (30.30%) | 59 (24.18%) | 17 (25.76%) | 0.549 | 0.354 | 0.612 | 0.795 |
| Short cervix, n (%) | 17 (51.52%) | 118 (48.36%) | 26 (39.39%) | 0.138 | 0.726 | 0.142 | 0.083 |
| Cause of delivery, n (%) | 0.081 | 0.035 * | 0.038 * | 0.531 | |||
| Elective | 7 (21.21%) | 89 (36.63%) | 26 (39.39%) | ||||
| Spontaneous | 20 (60.61%) | 111 (45.68%) | 31 (46.97%) | ||||
| Iatrogenic | 6 (18.18%) | 83 (17.70%) | 9 (13.64%) |
| Under Weight (n = 66) | Normal Weight (n = 488) | Over Weight (n = 132) | p Value | p Value α | p Value β | p Value γ | |
|---|---|---|---|---|---|---|---|
| Gestational age at delivery, weeks | 34.08 ± 2.90 | 34.93 ± 2.89 | 34.82 ± 2.66 | 0.220 | 0.025 * | 0.075 | 0.696 |
| Sex (male), n (%) | 30 (45.45%) | 259 (53.07%) | 62 (46.97%) | 0.286 | 0.302 | 0.960 | 0.251 |
| Birthweight, gram | 1939.02 ± 228.46 | 2193.02 ± 521.92 | 2244.05 ± 549.78 | 0.001 * | <0.001 * | <0.001 * | 0.325 |
| Birthweight percentile | 27.36 ± 20.95 | 30.14 ± 21.23 | 36.61 ± 22.46 | 0.001 * | 0.319 | 0.006 * | 0.002 * |
| Placental weight, gram | 958.79 ± 228.46 | 1029.94 ± 206.78 | 1107.42 ± 252.54 | <0.001 * | 0.010 * | <0.001 * | 0.001 * |
| SGA, n (%) | 13 (19.70%) | 93 (19.06%) | 17 (12.88%) | 0.240 | 1.000 | 0.293 | 0.128 |
| AGA–AGA, n (%) | 42 (63.64%) | 326 (66.80%) | 100 (75.56%) | 0.160 | 0.593 | 0.193 | 0.072 |
| AGA–SGA, n (%) | 22 (33.33%) | 138 (28.28%) | 30 (22.73%) | ||||
| SGA–SGA, n (%) | 2 (3.03%) | 24 (4.92%) | 2 (1.52%) | ||||
| Twin weight discordance, n (%) | 14 (21.21%) | 96 (19.67%) | 32 (24.24%) | 0.513 | 0.897 | 0.766 | 0.303 |
| Apgar score at 1 min (<7), n (%) | 23 (34.85%) | 116 (23.77%) | 34 (25.76%) | 0.149 | 0.072 | 0.244 | 0.720 |
| Apgar score at 5 min (<7), n (%) | 1 (1.52%) | 18 (3.69%) | 7 (5.30%) | 0.411 | 0.582 | 0.372 | 0.557 |
| NICU admission, (%) | 52 (78.79%) | 341 (70.16%) | 94 (71.21%) | 0.636 | 0.321 | 0.332 | 0.847 |
| Oxygen supply, n (%) | 33 (50.00%) | 222 (45.68%) | 67 (51.15%) | 0.478 | 0.597 | 0.999 | 0.311 |
| Intubation, n (%) | 17 (25.76%) | 75 (15.53%) | 17 (12.98%) | 0.060 | 0.056 | 0.041 * | 0.557 |
| RDS, n (%) | 15 (22.73%) | 64 (13.17%) | 12 (9.16%) | 0.030 * | 0.058 | 0.017 * | 0.276 |
| AGA–AGA (n = 234) | AGA–SGA or SGA–SGA (n = 109) | p Value | |
|---|---|---|---|
| Age, years | 31.67 ± 4.27 | 33.67 ± 3.97 | <0.001 * |
| Nulliparous, n (%) | 153 (65.38%) | 83 (76.15%) | 0.001 * |
| ART, n (%) | 114 (48.93%) | 71 (65.14%) | <0.001 * |
| Monochorionic, n (%) | 54 (23.08%) | 24 (22.02%) | 0.834 |
| Dichorionic, n (%) | 180 (76.92%) | 85 (77.98%) | |
| Preeclampsia, n (%) | 19 (8.12%) | 18 (16.51%) | 0.002 * |
| Gestational DM, n (%) | 32 (13.68%) | 14 (12.84%) | 0.859 |
| 50 g GTT | 131.83 ± 30.88 | 131.93 ± 29.28 | 0.976 |
| TSH | 2.04 ± 1.59 | 2.52 ± 2.28 | 0.007 * |
| Placenta previa, n (%) | 10 (2.14%) | 5 (4.59%) | 0.074 |
| Threatened preterm labor, n (%) | 162 (69.23%) | 45 (41.28%) | <0.001 * |
| PROM, n (%) | 68 (29.06%) | 18 (16.51%) | 0.001 * |
| Short cervix, n (%) | 126 (53.85%) | 28 (29.47%) | <0.001 * |
| Postpartum bleeding, n (%) | 15 (6.41%) | 6 (5.50%) | 0.772 |
| Cause of delivery, n (%) | <0.001 * | ||
| Elective | 76 (32.48%) | 46 (42.59%) | |
| Spontaneous | 129 (55.13%) | 33 (30.56%) | |
| Iatrogenic | 29 (12.39%) | 29 (26.85%) | |
| Gestational age at delivery, weeks | 34.37 ± 2.99 | 35.81 ± 2.24 | <0.001 * |
| Gender, male, n (%) | 244 (47.86%) | 107 (49.08%) | 0.507 |
| Birthweight, grams | 77.06 | 2117.34 ± 474.37 | 0.030 * |
| Birthweight percentile, % | 38.40 ± 19.41 | 15.47 ± 17.36 | <0.001 * |
| Placental weight, gram | 1057.80 ± 228.26 | 995.50 ± 200.74 | <0.001 * |
| Twin weight discordance, n (%) | 42 (8.97%) | 100 (45.87%) | <0.001 * |
| Apgar score at 1 min (<7), n (%) | 126 (26.92%) | 47 (21.56%) | 0.158 |
| Apgar score at 5 min (<7), n (%) | 17 (3.63%) | 9 (4.13%) | 0.919 |
| NICU admission, n (%) | 319 (68.45%) | 168 (77.06%) | 0.025 * |
| Maternal height, kg | 161.40 ± 5.10 | 159.85 ± 4.72 | <0.001 * |
| Pre-pregnancy weight, kg | 58.52 ± 11.07 | 55.83 ± 9.41 | 0.001 * |
| Weight at 15–18 weeks, kg | 61.25 ± 10.16 | 57.86 ± 8.61 | <0.001 * |
| Weight at 20–24 weeks, kg | 64.13 ± 9.75 | 61.87 ± 8.96 | 0.059 |
| Weight at 24–28 weeks, kg | 67.50 ± 10.21 | 64.11 ± 7.95 | 0.001 * |
| Weight at delivery, kg | 72.26 ± 12.30 | 69.65 ± 9.15 | 0.002 * |
| AGA–AGA (n = 162) | AGA–SGA or SGA–SGA (n = 79) | p Value | |
|---|---|---|---|
| Gestational age at delivery (weeks) | 34.40 ± 3.02 | 35.99 ± 2.28 | <0.001 * |
| Birthweight (grams) | 2214.83 ± 555.64 | 2151.27 ± 443.72 | 0.176 |
| Birthweight percentile (%) | 37.88 ± 19.40 | 14.72 ± 16.57 | <0.001 * |
| Maternal height (cm) | 161.32 ± 5.22 | 160.14 ± 4.25 | 0.008 * |
| Pre-pregnancy weight (kg) | 55.10 ± 5.54 | 54.51 ± 5.21 | 0.263 |
| Weight at 15–18 weeks | 58.42 ± 6.37 | 56.19 ± 5.64 | 0.004 * |
| Weight at 20–24 weeks | 62.75 ± 6.91 | 61.23 ± 5.51 | 0.091 |
| Weight at 24–28 weeks | 65.54 ± 6.93 | 63.27 ± 5.23 | 0.003 * |
| Weight at delivery (kg) | 69.49 ± 8.38 | 69.12 ± 5.95 | 0.578 |
| Weight gain during the pregnancy (kg) | 14.39 ± 5.88 | 14.61 ± 4.76 | 0.658 |
| Weight gain during the 1st trimester (kg) | 2.89 ± 3.49 | 2.47 ± 2.90 | 0.289 |
| Weight gain during the 2nd trimester (kg) | 7.21 ± 2.87 | 6.50 ± 2.04 | 0.043 * |
| Weight gain during the 3rd trimester (kg) | 5.00 ± 3.67 | 6.27 ± 2.96 | 0.003 * |
| AGA–AGA (n = 49) | AGA–SGA or SGA–SGA (n = 16) | p Value | |
|---|---|---|---|
| Gestational age at delivery (weeks) | 34.54 ± 2.82 | 35.52 ± 1.97 | 0.032 * |
| Birthweight (grams) | 2269.13 ± 565.92 | 2140.94 ± 498.56 | 0.255 |
| Birthweight percentile (%) | 41.64 ± 20.16 | 21.81 ± 23.28 | <0.001 * |
| Maternal height (cm) | 160.97 ± 5.09 | 158.38 ± 6.25 | 0.020 * |
| Pre-pregnancy weight (kg) | 74.42 ± 8.95 | 70.62 ± 10.90 | 0.051 |
| Weight at 15–18 weeks | 75.84 ± 8.49 | 70.43 ± 5.79 | 0.001 * |
| Weight at 20–24 weeks | 77.43 ± 9.26 | 74.83 ± 6.19 | 0.315 |
| Weight at 24–28 weeks | 82.85 ± 9.85 | 75.61 ± 6.07 | 0.001 * |
| Weight at delivery (kg) | 86.42 ± 11.06 | 80.29 ± 11.77 | 0.008 * |
| Weight gain during the pregnancy (kg) | 12.01 ± 5.77 | 9.67 ± 4.02 | 0.013 * |
| Weight gain during the 1st trimester (kg) | 2.92 ± 2.99 | 2.27 ± 2.69 | 0.354 |
| Weight gain during the 2nd trimester (kg) | 4.40 ± 2.02 | 4.57 ± 1.55 | 0.751 |
| Weight gain during the 3rd trimester (kg) | 3.73 ± 3.04 | 3.96 ± 2.09 | 0.765 |
| AGA–AGA (n = 42) | AGA–SGA or SGA–SGA (n = 24) | p Value | |
|---|---|---|---|
| Gestational age at delivery (weeks) | 33.49 ± 3.09 | 35.11 ± 2.24 | 0.028 * |
| Birthweight (grams) | 1963.21 ± 519.92 | 1896.67 ± 580.37 | 0.633 |
| Birthweight percentile (%) | 35.83 ± 20.34 | 12.54 ± 11.94 | <0.001 * |
| Maternal height (cm) | 162.48 ± 3.82 | 159.67 ± 5.30 | 0.015 * |
| Pre-pregnancy weight (kg) | 45.81 ± 3.54 | 44.25 ± 4.72 | 0.133 |
| Weight at 15–18 weeks | 50.17 ± 4.03 | 46.78 ± 4.35 | 0.022 * |
| Weight at 20–24 weeks | 51.06 ± 3.11 | 50.00 ± 4.76 | 0.470 |
| Weight at 24–28 weeks | 56.33 ± 4.61 | 54.30 ± 5.01 | 0.215 |
| Weight at delivery (kg) | 58.59 ± 6.88 | 57.74 ± 5.88 | 0.616 |
| Weight gain during the pregnancy (kg) | 12.78 ± 5.02 | 13.49 ± 5.97 | 0.605 |
| Weight gain during the 1st trimester (kg) | 2.99 ± 2.38 | 3.21 ± 2.76 | 0.804 |
| Weight gain during the 2nd trimester (kg) | 6.57 ± 1.65 | 6.33 ± 3.45 | 0.830 |
| Weight gain during the 3rd trimester (kg) | 4.49 ± 4.10 | 3.69 ± 2.89 | 0.512 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Kim, H.M.; Cha, H.-H.; Seong, W.J. Correlation between Maternal Weight Gain in Each Trimester and Fetal Growth According to Pre-Pregnancy Maternal Body Mass Index in Twin Pregnancies. Medicina 2022, 58, 1209. https://doi.org/10.3390/medicina58091209
Kim MJ, Kim HM, Cha H-H, Seong WJ. Correlation between Maternal Weight Gain in Each Trimester and Fetal Growth According to Pre-Pregnancy Maternal Body Mass Index in Twin Pregnancies. Medicina. 2022; 58(9):1209. https://doi.org/10.3390/medicina58091209
Chicago/Turabian StyleKim, Mi Ju, Hyun Mi Kim, Hyun-Hwa Cha, and Won Joon Seong. 2022. "Correlation between Maternal Weight Gain in Each Trimester and Fetal Growth According to Pre-Pregnancy Maternal Body Mass Index in Twin Pregnancies" Medicina 58, no. 9: 1209. https://doi.org/10.3390/medicina58091209





