Vestibular Rehabilitation Therapy and Corticosteroids for Vestibular Neuritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
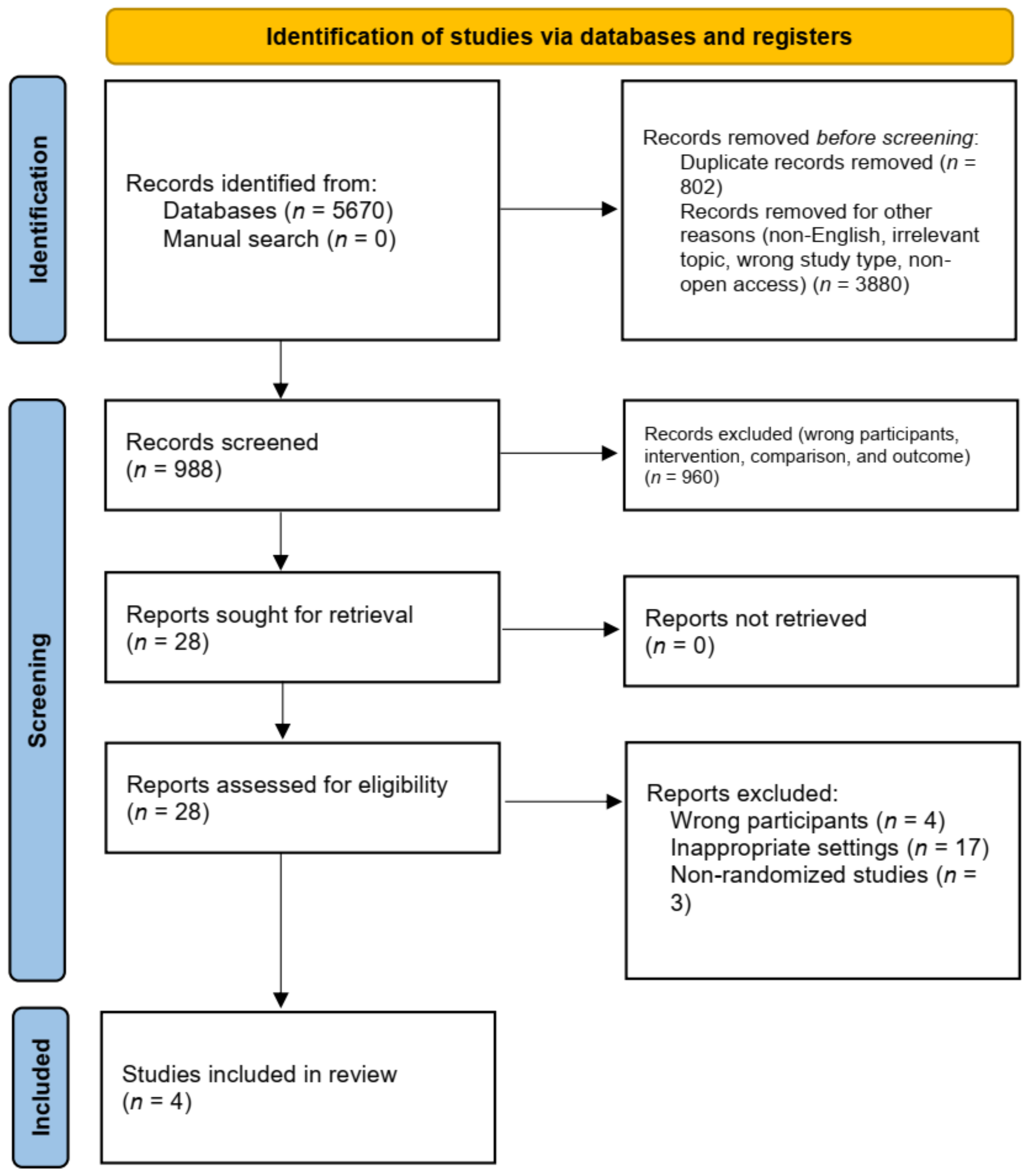
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Statistics
3. Results
3.1. General Characteristics and Narrative Synthesis of Included Studies
3.1.1. Participants
3.1.2. Intervention
3.1.3. Outcomes
3.2. Meta-Analysis of Vestibular Rehabilitation Therapy (VRT) vs. Corticosteroid Therapy (CT)
3.3. Meta-Analysis of Corticosteroid Therapy (CT) vs. Corticosteroid and Vestibular Rehabilitation Therapy Combination (CT + VRT)
3.4. Meta-Analysis of Vestibular Rehabilitation Therapy (VRT) vs. Corticosteroid and Vestibular Rehabilitation Therapy Combination (CT + VRT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamec, I.; Krbot Skorić, M.; Handžić, J.; Habek, M. Incidence, seasonality and comorbidity in vestibular neuritis. Neurol. Sci. 2015, 36, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Sekitani, T.; Imate, Y.; Inokuma, T.; Okuzono, Y.; Nishikawa, K. Vestibular neuronitis in aged patients: Results from an epidemiological survey by questionnaire in Japan. Acta Otolaryngol. Suppl. 1993, 503, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Goudakos, J.K.; Markou, K.D.; Franco-Vidal, V.; Vital, V.; Tsaligopoulos, M.; Darrouzet, V. Corticosteroids in the treatment of vestibular neuritis: A systematic review and meta-analysis. Otol. Neurotol. 2010, 31, 183–189. [Google Scholar] [CrossRef]
- Kitamura, K. Vestibular neuritis. Equilib. Res. 2018, 77, 3–10. [Google Scholar] [CrossRef]
- Rujescu, D.; Hartmann, A.M.; Giegling, I.; Konte, B.; Herrling, M.; Himmelein, S.; Strupp, M. Genome-Wide Association Study in Vestibular Neuritis: Involvement of the Host Factor for HSV-1 Replication. Front. Neurol. 2018, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Himmelein, S.; Lindemann, A.; Sinicina, I.; Horn, A.K.E.; Brandt, T.; Strupp, M.; Hüfner, K. Differential Involvement during Latent Herpes Simplex Virus 1 Infection of the Superior and Inferior Divisions of the Vestibular Ganglia: Implications for Vestibular Neuritis. J. Virol. 2017, 91, e00331-17. [Google Scholar] [CrossRef]
- Bronstein, A.M.; Lempert, T. Dizziness, a Practical Approach to Diagnosis and Management; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Uffer, D.S.; Hegemann, S.C. About the pathophysiology of acute unilateral vestibular deficit vestibular neuritis (VN) or peripheral vestibulopathy (PVP). J. Vestib. Res. 2016, 226, 311–317. [Google Scholar] [CrossRef]
- Goudakos, J.K. Corticosteroids vs. Corticosteroids Plus Antiviral Agents in the Treatment of Bell Palsy. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 558–564. [Google Scholar] [CrossRef]
- Brusaferri, F.C.L. Steroids for multiple sclerosis and optic neuritis: A meta-analysis of randomized controlled clinical trials. J. Neuro. 2000, 247, 435–442. [Google Scholar] [CrossRef]
- Kim, J.S. When the Room Is Spinning: Experience of Vestibular Neuritis by a Neurotologist. Front. Neurol. 2020, 11, 157. [Google Scholar] [CrossRef] [Green Version]
- Goudakos, J.K.; Markou, K.D.; Psillas, G.; Vital, V.; Tsaligopoulos, M. Corticosteroids and vestibular exercises in vestibular neuritis. Single-blind randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 434–440. [Google Scholar] [CrossRef]
- Yoo, M.H.; Yang, C.J.; Kim, S.A.; Park, M.J.; Ahn, J.H.; Chung, J.W.; Park, H.J. Efficacy of steroid therapy based on symptomatic and functional improvement in patients with vestibular neuritis: A prospective randomized controlled trial. Eur. Arch. Otorhinolaryngol. 2017, 274, 2443–2451. [Google Scholar] [CrossRef]
- Ismail, E.I.; Morgan, A.E.; Abdel Rahman, A.M. Corticosteroids versus vestibular rehabilitation in long-term outcomes in vestibular neuritis. J. Vestib. Res. 2018, 28, 417–424. [Google Scholar] [CrossRef]
- Tokle, G.; Mørkved, S.; Bråthen, G.; Goplen, F.K.; Salvesen, Ø.; Arnesen, H.; Holmeslet, B.; Nordahl, S.H.G.; Wilhelmsen, K.T. Efficacy of Vestibular Rehabilitation Following Acute Vestibular Neuritis: A Randomized Controlled Trial. Otol. Neurotol. 2020, 41, 78–85. [Google Scholar] [CrossRef]
- Han, B.I.; Song, H.S.; Kim, J.S. Vestibular rehabilitation therapy: Review of indications, mechanisms, and key exercises. J. Clin. Neurol. 2011, 7, 184–196. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Hillier, S.L. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst. Rev. 2015, CD005397. [Google Scholar] [CrossRef]
- Ciriaco, M.; Ventrice, P.; Russo, G.; Scicchitano, M.; Mazzitello, G.; Scicchitano, F.; Russo, E. Corticosteroid-related central nervous system side effects. J. Pharmacol. Pharmacother. 2013, 4, S94–S98. [Google Scholar] [CrossRef]
- Shupak, A.; Issa, A.; Golz, A.; Kaminer, M.; Braverman, I. Prednisone treatment for vestibular neuritis. Otol. Neurotol. 2008, 29, 368–374. [Google Scholar] [CrossRef]
- Kim, J.C.; Cha, W.W.; Chang, D.S.; Lee, H.Y. The Effect of Intravenous Dexamethasone on the Nausea Accompanying Vestibular Neuritis: A Preliminary Study. Clin. Ther. 2015, 37, 2536–2542. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Review Manager (RevMan) [Computer Program], Version 5.4.1; The Cochrane Collaboration: London, UK, 2020.
- Furman, J.M.; Jacob, R.G. Jongkees’ formula re-evaluated: Order effects in the response to alternate binaural bithermal caloric stimulation using closed-loop irrigation. Acta Otolaryngol. 1993, 113, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Cass, S.P.; Clendaniel, R.A.; Fife, T.D.; Furman, J.M.; Getchius, T.S.D.; Goebel, J.A.; Shepard, N.T.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline: From the American Physical Therapy Association Neurology Section. J. Neurol Phys. Ther. 2016, 40, 124–155. [Google Scholar] [CrossRef] [PubMed]
- Herdman, S.; Whitney, S. Physical therapy treatment of vestibular hypofunction. In Vestibular Rehabilitation; Herdman, S., Clendaniel, R.A., Eds.; F.A. Davies: Philadelphia, PA, USA, 2014; pp. 394–431. [Google Scholar]
- Teggi, R.; Caldirola, D.; Fabiano, B.; Recanati, P.; Bussi, M. Rehabilitation after acute vestibular disorders. J. Laryngol. Otol. 2009, 123, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Krebs, D.E.; Gill-Body, K.M.; Riley, P.O.; Parker, S.W. Double-blind, placebo-controlled trial of rehabilitation for bilateral vestibular hypofunction: Preliminary report. Otolaryngol. Head Neck Surg. 1993, 109, 735–741. [Google Scholar] [CrossRef]
- Cowland, J.L.; Wrisley, D.M.; Walker, M.; Strasnick, B.; Jacobson, J.T. Efficacy of vestibular rehabilitation. Otolaryngol. Head Neck Surg. 1998, 118, 49–54. [Google Scholar] [CrossRef]
- Fishman, J.M.; Burgess, C.; Waddell, A. Corticosteroids for the treatment of idiopathic acute vestibular dysfunction (vestibular neuritis). Cochrane Database Syst. Rev. 2011, 5, 1465–1858. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.Y.; Hwang, J.H.; Kim, K.S. Vestibular Neuritis with Minimal Canal Paresis: Characteristics and Clinical Implication. Clin. Exp. Otorhinolaryngol. 2017, 10, 148–152. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Curthoys, I.S. A clinical sign of canal paresis. Arch. Neurol. 1988, 45, 737–739. [Google Scholar] [CrossRef]
- Fetter, M.; Dichgans, J. Vestibular neuritis spares the inferior division of the vestibular nerve. Brain 1996, 119, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Aw, S.T.; Fetter, M.; Cremer, P.D.; Karlberg, M.; Halmagyi, G.M. Individual semicircular canal function in superior and inferior vestibular neuritis. Neurology 2001, 57, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Zamyslowska-Szmytke, E.; Politanski, P.; Jozefowicz-Korczynska, M. Dizziness Handicap Inventory in Clinical Evaluation of Dizzy Patients. Int. J. Environ. Res. Public Health 2021, 18, 2210. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Pourbakht, A.; Bahrami, E.; Jalaie, S.; Bayat, A. Effect of Early Vestibular Rehabilitation on Vertigo and Unsteadiness in Patients with Acute and Sub-Acute Head Trauma. Iran. J. Otorhinolaryngol. 2018, 30, 85–90. [Google Scholar]
- Zhu, C.; Li, Y.; Ju, Y.; Zhao, X. Dizziness handicap and anxiety depression among patients with benign paroxysmal positional vertigo and vestibular migraine. Medicine 2020, 99, e23752. [Google Scholar] [CrossRef]
- Wang, J.; Gong, J.; Sui, X.; Wang, L.; Zhu, L.; Sun, D. An effect analysis of vestibular rehabilitation training in vertigo treatment. Am. J. Transl. Res. 2021, 13, 3494–3500. [Google Scholar]
- Yardley, L.; Donovan-Hall, M.; Smith, H.E.; Walsh, B.M.; Mullee, M.; Bronstein, A.M. Effectiveness of primary care-based vestibular rehabilitation for chronic dizziness. Ann. Intern. Med. 2004, 141, 598–605. [Google Scholar] [CrossRef]
- Eagger, S.; Luxon, L.M.; Davies, R.A.; Coelho, A.; Ron, M.A. Psychiatric morbidity in patients with peripheral vestibular disorder: A clinical and neuro-otological study. J. Neurol. Neurosurg. Psych. 1992, 55, 383–387. [Google Scholar] [CrossRef]
- Schuerger, R.J.; Balaban, C.D. Immunohistochemical demonstration of regionally selective projections from locus coeruleus to the vestibular nuclei in rats. Exp. Brain Res. 1993, 92, 351–359. [Google Scholar] [CrossRef]
- Pollak, L.; Klein, C.; Stryjer, R.; Kossych, V.; Rabey, J.M. Anxiety in the first attack of vertigo. Otolaryngol. Head Neck Surg. 2003, 128, 829–834. [Google Scholar] [CrossRef]
- Yuan, Q.; Yu, L.; Shi, D.; Ke, X.; Zhang, H. Anxiety and depression among patients with different types of vestibular peripheral vertigo. Medicine 2015, 94, e453. [Google Scholar] [CrossRef] [PubMed]
- Kozak, H.H.; Dündar, M.A.; Uca, A.U.; Uguz, F.; Turgut, K.; Altas, M.; Tekin, G.; Aziz, S.K. Anxiety, Mood, and Personality Disorders in Patients with Benign Paroxysmal Positional Vertigo. Noro Psikiyatr Ars. 2018, 55, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.B.; Park, I.S.; Hong, S.J.; Kim, H.; Hong, S.M. Clinical Analysis of Dizzy Patients with High Levels of Depression and Anxiety. J. Audiol. Otol. 2016, 20, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Piker, E.G.; Kaylie, D.M.; Garrison, D.; Tucci, D.L. Hospital Anxiety and Depression Scale: Factor Structure, Internal Consistency and Convergent Validity in Patients with Dizziness. Audiol. Neurootol. 2015, 20, 394–399. [Google Scholar] [CrossRef]
- Piker, E.G.; Jacobson, G.P.; McCaslin, D.L.; Grantham, S.L. Psychological comorbidities and their relationship to self-reported handicap in samples of dizzy patients. J. Am. Acad. Audiol. 2008, 19, 337–347. [Google Scholar] [CrossRef]
- Morettin, M.; Mariotto, L.D.; Filho, O.A.C. Evaluation of the Effectiveness of Rehabilitaion Vestibular in Patients with Vestibular Dysfunction. Int. Arch. Otorhinolaryngol. 2007, 11, 284–292. [Google Scholar]
- Lacour, M.; Tighilet, B. Plastic events in the vestibular nuclei during vestibular compensation: The brain orchestration of a “deafferentation” code. Restor. Neurol. Neurosci. 2010, 28, 19–35. [Google Scholar] [CrossRef]
- Yan, T.; Zong, F.; Han, X.; Wang, X.; Li, Q.; Qiao, R.; Zhang, H. Vestibular Neuritis in Patients Among Different Age Groups: Clinical Features and Outcomes. J. Am. Acad. Audiol. 2020, 31, 629–635. [Google Scholar] [CrossRef]






| Author, Publication Year | Study Design | Population | Sample Size | Standard of Care (SoC) | Studied Arms | Primary Outcomes | Follow Up, Months | Findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | VRT | Combination | CT | VRT | Combination | |||||||
| Goudakos et al., 2014 [12] | RCT | Patients (18–80 years old) with VN | 20 | 20 | NR | Dimenhydrinate Proton pump inhibitor SoC for 3 days | 25 days dexamethasone | 3 weeks VRT | NR | Clinical (EEV, DHI), canal paresis, otolith (VEMP) recovery | 1, 6, 12 | Both arms exhibit equal improvement in clinical, canal, and otolith parameters, ns Better canal paresis recovery in the first month (CT vs. VR, 0/20 vs. 2/20) Significantly higher ratio of CT group with complete disease resolution at 6 month follow-up (p < 0.05) |
| Yoo et al., 2016 a [13] | RCT | Patients (19–80 years old) with VN | NR | 15 | 14 | Ginkgo biloba extract, intravenous or oral diazepam VRT for at least a month until no VRT-evoked dizziness is observed. | NR | As SoC | 14 days methylprednisolone 14 days ranitidine VRT as SoC | Improvement in objective (CT, SOT, vHIT) and subjective (DHI) parameters | 1, 6 | Equal improvement in objective parameters in both groups, ns Equal improvement in subjective parameters in both groups, ns |
| Ismail et al., 2018 [14] | RCT | Patients (20–50 years old) with VN | 20 | 20 | 20 | Dimenhydrinate for a maximum of 3 days | 2 weeks methylprednisolone, H2 blocker | 6 weeks VRT | CT + VRT protocol | Improvement in objective (CT, VEMP) and subjective (DHI) indicators | 1, 3, 6, 12 | Equal improvement in objective parameters in both groups, ns Equal improvement in subjective parameters in both groups, ns |
| Tokle et al., 2020 a [15] | RCT | Patients (18–70 years) with VN | 27 | NR | 3 months 27 12 months 26 | 10 days prednisolone | As SoC | NR | 12 months VRT + SoC | Perceived dizziness during head motion, HADS, DHI, UCLA-DQ, VAS, walking speed, and standing balance | 3, 12 | Significant improvement in overall perceived dizziness score, favoring toward combination group at 3 and 12 month follow-ups (p = 0.007, p = 0.001) Equal improvement in HADS, DHI, UCLA-DQ, VAS, walking speed, and standing balance, ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidayati, H.B.; Imania, H.A.N.; Octaviana, D.S.; Kurniawan, R.B.; Wungu, C.D.K.; Rida Ariarini, N.N.; Srisetyaningrum, C.T.; Oceandy, D. Vestibular Rehabilitation Therapy and Corticosteroids for Vestibular Neuritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2022, 58, 1221. https://doi.org/10.3390/medicina58091221
Hidayati HB, Imania HAN, Octaviana DS, Kurniawan RB, Wungu CDK, Rida Ariarini NN, Srisetyaningrum CT, Oceandy D. Vestibular Rehabilitation Therapy and Corticosteroids for Vestibular Neuritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina. 2022; 58(9):1221. https://doi.org/10.3390/medicina58091221
Chicago/Turabian StyleHidayati, Hanik Badriyah, Hana Aqilah Nur Imania, Dinda Sella Octaviana, Roy Bagus Kurniawan, Citrawati Dyah Kencono Wungu, Ni Nengah Rida Ariarini, Cempaka Thursina Srisetyaningrum, and Delvac Oceandy. 2022. "Vestibular Rehabilitation Therapy and Corticosteroids for Vestibular Neuritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Medicina 58, no. 9: 1221. https://doi.org/10.3390/medicina58091221
APA StyleHidayati, H. B., Imania, H. A. N., Octaviana, D. S., Kurniawan, R. B., Wungu, C. D. K., Rida Ariarini, N. N., Srisetyaningrum, C. T., & Oceandy, D. (2022). Vestibular Rehabilitation Therapy and Corticosteroids for Vestibular Neuritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina, 58(9), 1221. https://doi.org/10.3390/medicina58091221






