Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax
Abstract
1. Introduction
2. Materials and Methods
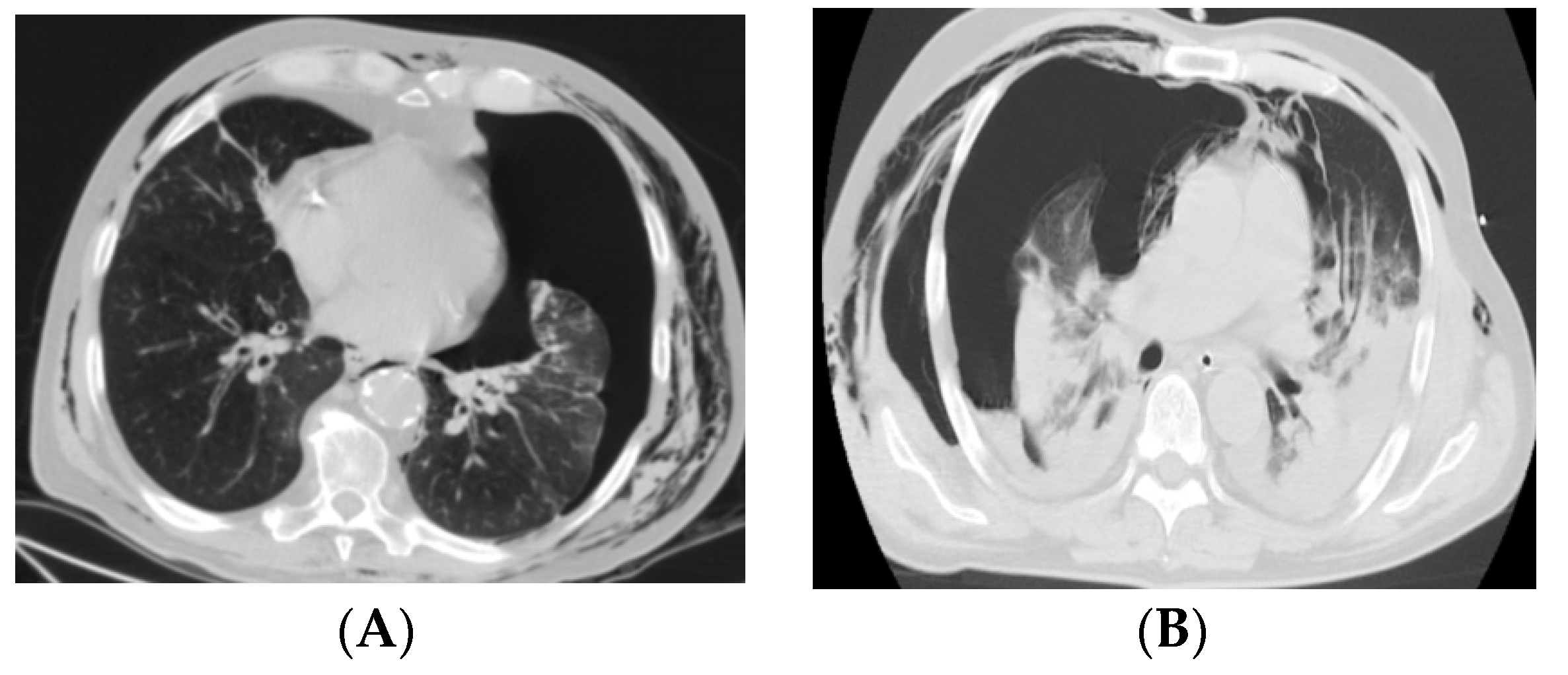
3. Results
4. Discussion
4.1. Treatment Methods for the Pleural Space Management
4.2. Interventional Management (Medical–Surgical Therapeutic Attitude)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsayed, H.H. Chapter Indications of Surgery in Pneumothorax. IntechOpen 2019, 2019, 1–9. [Google Scholar] [CrossRef][Green Version]
- Lu, H.; Stratton, C.W.; Tang, Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: The mystery and the miracle. J. Med. Virol. 2020, 20, 401–402. [Google Scholar] [CrossRef]
- Martinelli, A.W.; Ingle, T.; Newman, J.; Nadeem, I.; Jackson, K.; Lane, N.D.; Marciniak, S.J. COVID-19 and pneumothorax: A multicentre retrospective case series. Eur. Respir. J. 2020, 56, 2002697. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Shan, S.; Guangming, L.; Wei, L.; Xuedong, Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19: Case report and literature review. Rev. Inst. Med. Trop São Paulo 2020, 62, e76. [Google Scholar] [CrossRef]
- Chopra, A.; Al-Tarbsheh, A.H.; Shah, N.J.; Yaqoob, H.; Hu, K.; Feustel, P.; Ortiz-Pacheco, R.; Patel, K.M.; Oweis, J.; Kozlova, N.; et al. Pneumothorax in critically ill patients with COVID-19 infection: Incidence, clinical characteristics and outcomes in a case control multicenter study. Respir. Med. 2021, 184, 106464. [Google Scholar] [CrossRef]
- Yarmus, L.; Feller-Kopman, D. Pneumothorax in the critically ill patient. Chest 2012, 141, 1098–1105. [Google Scholar] [CrossRef]
- Ulutasa, H.; Celik, M.R.; Gulcek, I.; Kalkan, M.; Agara, M.; Kilicb, T.; Gulcek, E. Management of spontaneous pneumothorax in patients with COVID-19. Interact. Cardio Vascular Thorac. Surg. 2022, 34, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Jamous, F.; Meyer, N.; Buus, D.; Ateeli, H.; Taggart, K.; Devasahayam, J.; Hanson, T.; Alzoubaidi, M.; Nazir, J. Critical illness due to COVID-19: A description of the surge in a single center in Sioux Falls. S. D. Med. 2020, 73, 312–317. [Google Scholar]
- Yao, W.; Wang, T.; Jiang, B.; Gao, F.; Wang, L.; Zheng, H.; Xiao, W.; Yao, S.; Mei, W.; Nishikawa, K. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: Lessons learnt and international expert recommendations. Br. J. Anaesth. 2020, 125, e28–e37. [Google Scholar] [CrossRef]
- Yang, F.; Shi, S.; Zhu, J.; Shi, J.; Dai, K.; Chen, X. Analysis of 92 deceased patients with COVID-19. J. Med. Virol. 2020, 92, 2511–2515. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.M.; Bintcliffe, O. ERS task force statement: Diagnosis and treatment of primary spontaneous pneumothorax. Eur. Respir. J. 2015, 46, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Aguinagalde, B.; Aranda, J.L.; Busca, P.; Martinez, I.; Royo, I.; Zabaleta, J. Working Group of the CPG for the Management of Patients with Spontaneous; SECT Clinical Practice Guideline on the Management of Patients with Spontaneous Pneumothorax. Cirugía Española 2018, 96, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.; Ciuche, A.; Horvat, T. Chapter Spontaneous Pneumothorax. Thorac. Surgery. Rom. Acad. 2008, 4, 261–269. [Google Scholar]
- Nistor, C.E.; Ciuche, A.; Stanciu-Găvan, C. Spontaneous Pneumothorax—Therapeutic Attitude—Brief Review. Int. J. Cardiovasc. Thorac. Surg. 2020, 6, 49–53. Available online: http://www.sciencepublishinggroup.com/j/ijcts (accessed on 13 May 2022). [CrossRef]
- Zantah, M.; Castillo, E.D.; Townsend, R.; Dikengil, F.; Criner, G.J. Pneumothorax in COVID-19 disease incidence and clinical characteristics. Respir. Res. 2020, 21, 236. [Google Scholar] [CrossRef]
- Soldati, G.; Testa, A.; Sher, S.; Pignataro, G.; La Sala, M.; Silveri, N.G. Occult traumatic pneumothorax: Diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008, 133, 204–211. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Sah, R. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease 2019 (COVID-19) Situation Report. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 1 June 2022).
- Hameed, M.; Jamal, W.; Yousaf, M.; Thomas, M.; Haq, I.; Ahmed, S.; Ahmad, M.; Khatib, M. Case report Pneumothorax In COVID-19 Pneumonia: A case series. Respir. Med. Case Rep. 2020, 31, 101265. [Google Scholar] [CrossRef]
- MacDuff, A.; Arnold, A.; Harvey, J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010, BTS guidelines. Thorax 2010, 65 (Suppl. 2), ii4–ii17. [Google Scholar] [CrossRef]
- Luh, S.P. Review: Diagnosis and treatment of primary spontaneous pneumothorax. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2010, 10, 735–744. [Google Scholar] [CrossRef]
- Vallejo, F.A.G.; Romero, R.; Mejia, M.; Quijano, E. Chapter Primary Spontaneous Pneumothorax, a Clinical Challenge. IntechOpen 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Stodghill, J.D.; Collins, D.T.; Mahajan, A.K.; Khandhar, S.J. Review Article Primary spontaneous pneumothorax: A pathway to practice. AME Med. J. 2019, 4, 8. [Google Scholar] [CrossRef]
- Nistor, C.; Ciuche, A.; Davidescu, M.; Horvat, T. Pleurotomy—A surgical intervention at the hand of the general surgeon. Chirurgia 2009, 104, 323–328. [Google Scholar]
- Gogakos, A.; Barbetakis, N.; Lazaridis, G.; Papaiwannou, A.; Karavergou, A.; Lampaki, S.; Baka, S.; Mpoukovinas, I.; Karavasilis, V.; Zarogoulidis, P. Heimlich valve and pneumothorax; Annals of Translational Medicine. Ann. Transl. Med. 2015, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nadler, R.; Schwartz, D.; Tien, H.; Andrew, P.; Glassberg, E. Needle thoracostomy for tension pneumothorax: The Israeli Defense Forces experience. J. Can. Chir. 2015, 58, 118–124. [Google Scholar] [CrossRef]
- Nistor, C.; Ranetti, A.E.; Ciuche, A.; Pantile, D.; Constantin, L.M.; Brîncoveanu, R. Betadine in Chemichal Pleurodesis. Farmacia 2014, 62, 897–907. [Google Scholar]
- Chou, S.H.; Li, H.P.; Lee, Y.L.; Lee, J.Y.; Chiang, H.H.; Tsai, D.L.; Huang, M.F.; Lin, T.E. Video-assisted thoracoscopic surgery for postoperative recurrent primary spontaneous pneumothorax. J. Thorac. Dis. 2014, 6, 52–55. [Google Scholar] [CrossRef]
- Hallifax, R.J.; Yousuf, A.; Jones, H.E.; Corcoran, J.P.; Psallidas, I.; Rahman, N.M. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: A systematic review. Respir. Res. BMJ Thorax 2017, 72, 1121–1131. [Google Scholar] [CrossRef]
- Ciuche, A.; Nistor, C.; Pantile, D.; Horvat, T. Minimally Invasive Surgical Treatment of Malignant Pleural Effusions. Mædica—A J. Clin. Med. 2011, 6, 262–267. [Google Scholar]
- Porcel, J.M. Chest Tube Drainage of the Pleural Space: A Concise Review for Pulmonologists. Tuberc. Respir. Dis. 2018, 81, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y.; Song, J.; Mo, L.; Jiang, T. One-port video-assisted thoracic surgery versus three-port videoassisted thoracic surgery for primary spontaneous pneumothorax: A metaanalysis. Surg. Endosc. 2017, 31, 17–24. [Google Scholar] [CrossRef]
- Ng, C.; Maier, H.T.; Kocher, F.; Jud, S.; Lucciarini, P.; Öfner, D.; Schmid, T.; Augustin, F. VATS Partial Pleurectomy Versus VATS Pleural Abrasion: Significant Reduction in Pneumothorax Recurrence Rates after Pleurectomy. World J. Surg. 2018, 42, 3256–3262. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.R.; Ricketts, W.; Fotheringham, T. Use of an antiviral filter attached to a pleural drain bottle to prevent aerosol contamination with SARS-CoV-2. Clin. Med. 2020, 20, e60–e61. [Google Scholar] [CrossRef] [PubMed]
- British Thoracic Society. Pleural Services during the COVID-19 Pandemic—Revised. Available online: www.brit-thoracic.org.uk/about-us/COVID-19-information-for-the-respiratory-community/#guidance-on-pleural-services-during-COVID-19-pandemic (accessed on 21 May 2020).
| COVID-19 Associated Pneumothorax n = 34 | Pneumothorax without COVID-19 n = 42 | p-Value | |
|---|---|---|---|
| Age variation (y-o) | 30–84 | 15–68 | N/A |
| Mean age (y-o) | 58.29 | 41.13 | <0.0001 * |
| Sex ratio (M:F) | 26:8 | 33:9 | N/A |
| Comorbidities | COVID-19 Associated Pneumothorax n = 34 | Pneumothorax without COVID-19 n = 42 | Statistic Test | p-Value |
|---|---|---|---|---|
| No comorbidity | 0 (0%) | 16 (38.09%) | OR = 0.023 | 0.009 |
| Smoker | 16 (47.05%) | 34 (80.95%) | OR = 0.209 | 0.002 |
| COPD | 21 (61.76%) | 0 (0%) | OR = 135.37 | 0.0008 |
| Asthma | 6 (17.64%) | 0 (0%) | OR = 19.38 | 0.04 |
| Bronchiectasis | 26 (76.47%) | 0 (0%) | OR = 265 | 0.0002 |
| Systemic hypertension | 18 (52.94%) | 0 (0%) | OR = 95.3 | 0.001 |
| Pulmonary emphysema | 5 (14.7%) | 26 (61.9%) | OR = 0.106 | 0.0001 |
| Tachycardia | 14 (41.17%) | 0 (0%) | OR = 60.12 | 0.005 |
| Heart disease | 16 (47.05%) | 0 (0%) | OR = 75.81 | 0.003 |
| COVID-19 Associated Pneumothorax n = 34 | Pneumothorax without COVID-19 n = 42 | Statistic Test | p-Value | |||
|---|---|---|---|---|---|---|
| Haematology | Range | Average | Range | Average | ||
| WBC (k/microL) | 4.20–24.32 | 14.3 | 3.80–8.76 | 7.8 | t-test = 10.29 | <0.0001 |
| D_Dimer (ng/mL) | 305–720 | 505 | 0–250 | 125 | t-test = 16.4 | <0.0001 |
| Blood chemistry | Range | Average | Range | Average | ||
| Ferritin (mg/dL) | 356–833 | 606 | 20.00–250 | 140 | t-test = 20.11 | <0.0001 |
| CRP (mg/L) | 28.08–71.3 | 58.1 | 0.00–5.00 | 3 | t-test = 15.44 | <0.0001 |
| LD-P (U/L) | 283.69–387.07 | 303.8 | 125.00–220.00 | 176.2 | t-test = 4.55 | <0.0001 |
| γGT (U/L) | 63.02–98.75 | 74.6 | 11.00–59.00 | 23.9 | t-test = 4.94 | <0.0001 |
| Imaging Diagnosis | ||||||
| Right pneumothorax | 25 | 23 | OR = 2.29 | 0.09 | ||
| Left pneumothorax | 9 | 19 | OR = 0.43 | 0.09 | ||
| COVID-19 Associated Pneumothorax n = 34 | Pneumothorax without COVID-19 n = 42 | Statistic Test | p-Value | |
|---|---|---|---|---|
| Dyspnea | 34 (100%) | 42 (100%) | OR = 0.81 | 0.91 |
| Persistent cough | 34 (100%) | 1 (2.38%) | OR = 2.49 | 0.57 |
| Fever | 34 (100%) | 0 (0%) | OR = 5865 | <0.0001 |
| Chest pain | 23 (67.64%) | 42 (100%) | OR = 0.024 | 0.01 |
| Therapeutic Attitude | COVID-19 Associated Pneumothorax n = 34 | Pneumothorax without COVID-19 n = 42 | Statistic Test | p-Value |
|---|---|---|---|---|
| Chest tube drainage | 34 | 12 | OR = 168.36 | 0.0005 |
| Video assisted (VATS) procedures * | 0 | 30 | OR = 0.005 | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, C.-E.; Pantile, D.; Stanciu-Gavan, C.; Ciuche, A.; Moldovan, H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina 2022, 58, 1242. https://doi.org/10.3390/medicina58091242
Nistor C-E, Pantile D, Stanciu-Gavan C, Ciuche A, Moldovan H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina. 2022; 58(9):1242. https://doi.org/10.3390/medicina58091242
Chicago/Turabian StyleNistor, Claudiu-Eduard, Daniel Pantile, Camelia Stanciu-Gavan, Adrian Ciuche, and Horatiu Moldovan. 2022. "Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax" Medicina 58, no. 9: 1242. https://doi.org/10.3390/medicina58091242
APA StyleNistor, C.-E., Pantile, D., Stanciu-Gavan, C., Ciuche, A., & Moldovan, H. (2022). Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina, 58(9), 1242. https://doi.org/10.3390/medicina58091242










