Undetectable Macular Neovascularization on OCT Angiography in Age Related Macular Degeneration: Comparison between Different Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Image Acquisition and Analysis
OCTA Devices
2.3. Statistics
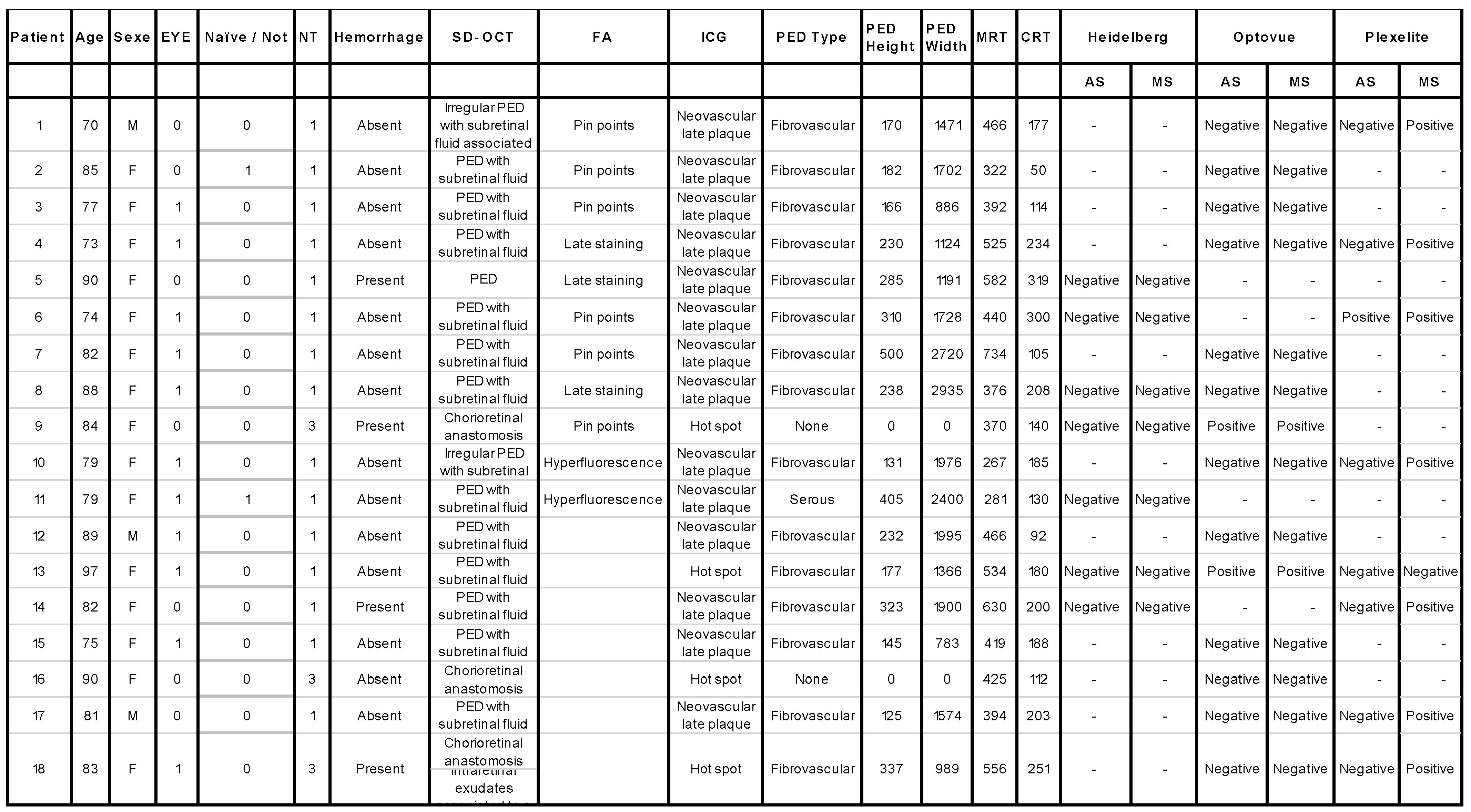
3. Results
3.1. Demographics
3.2. Global Comparison between Group 1 and Group 2
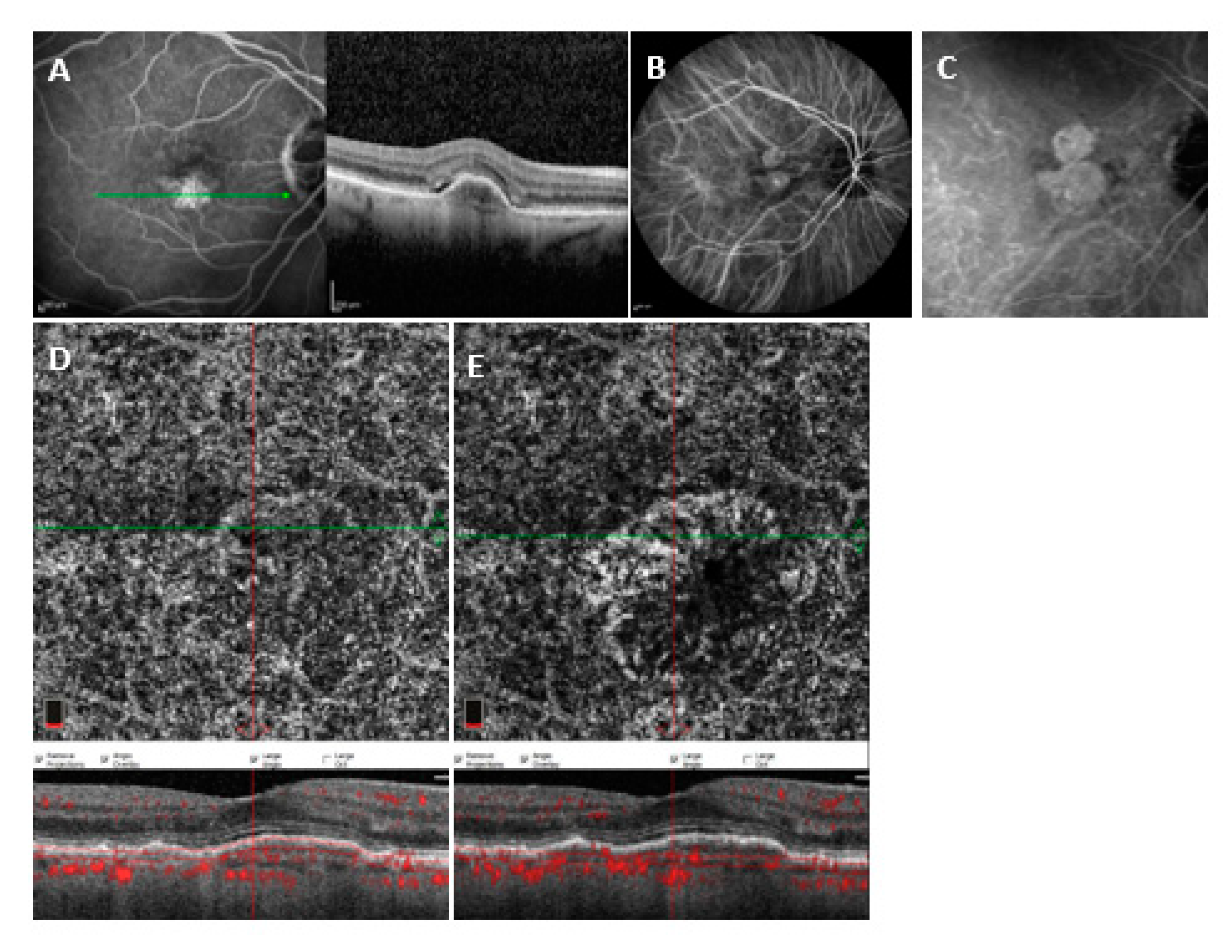
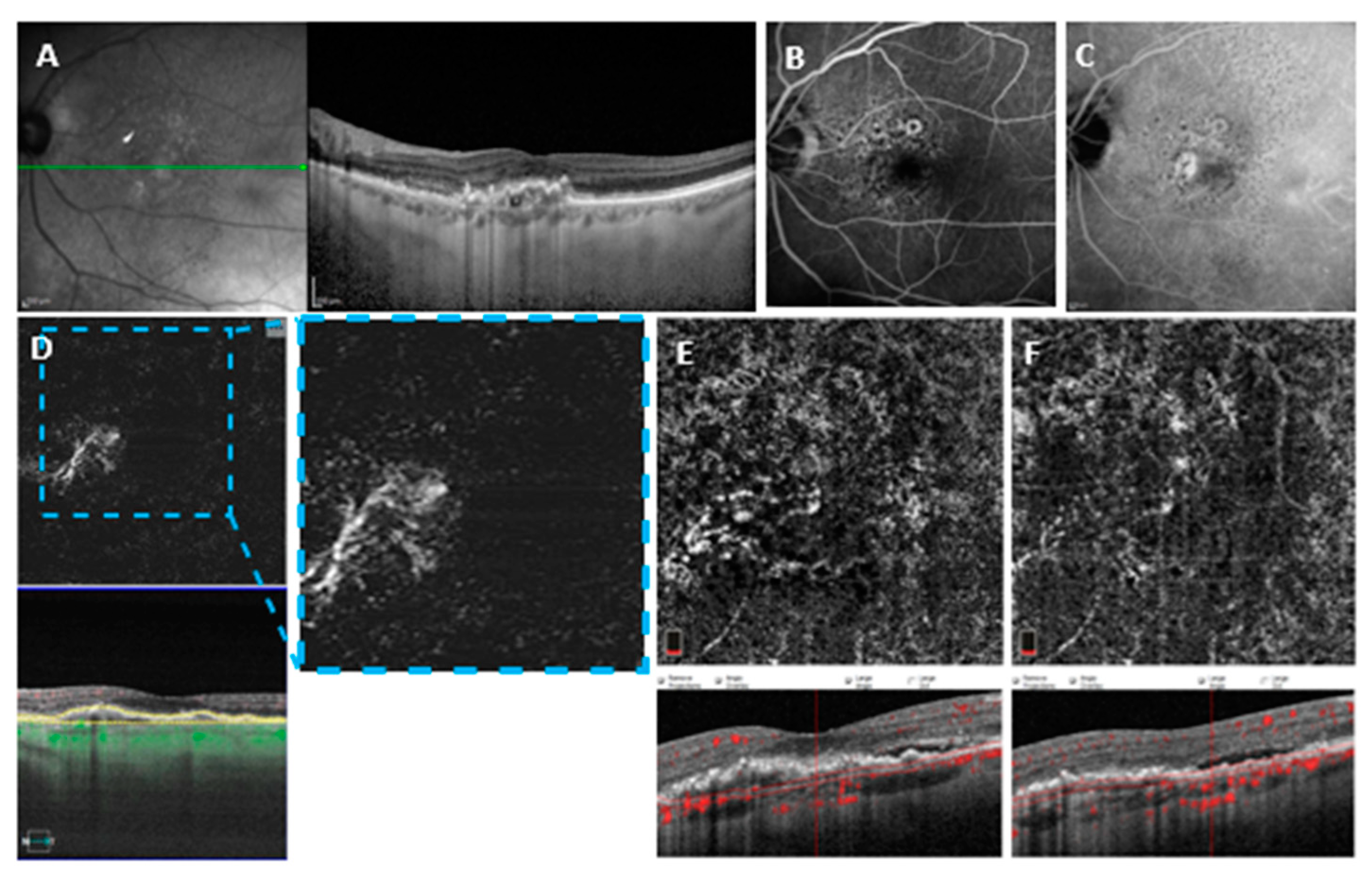
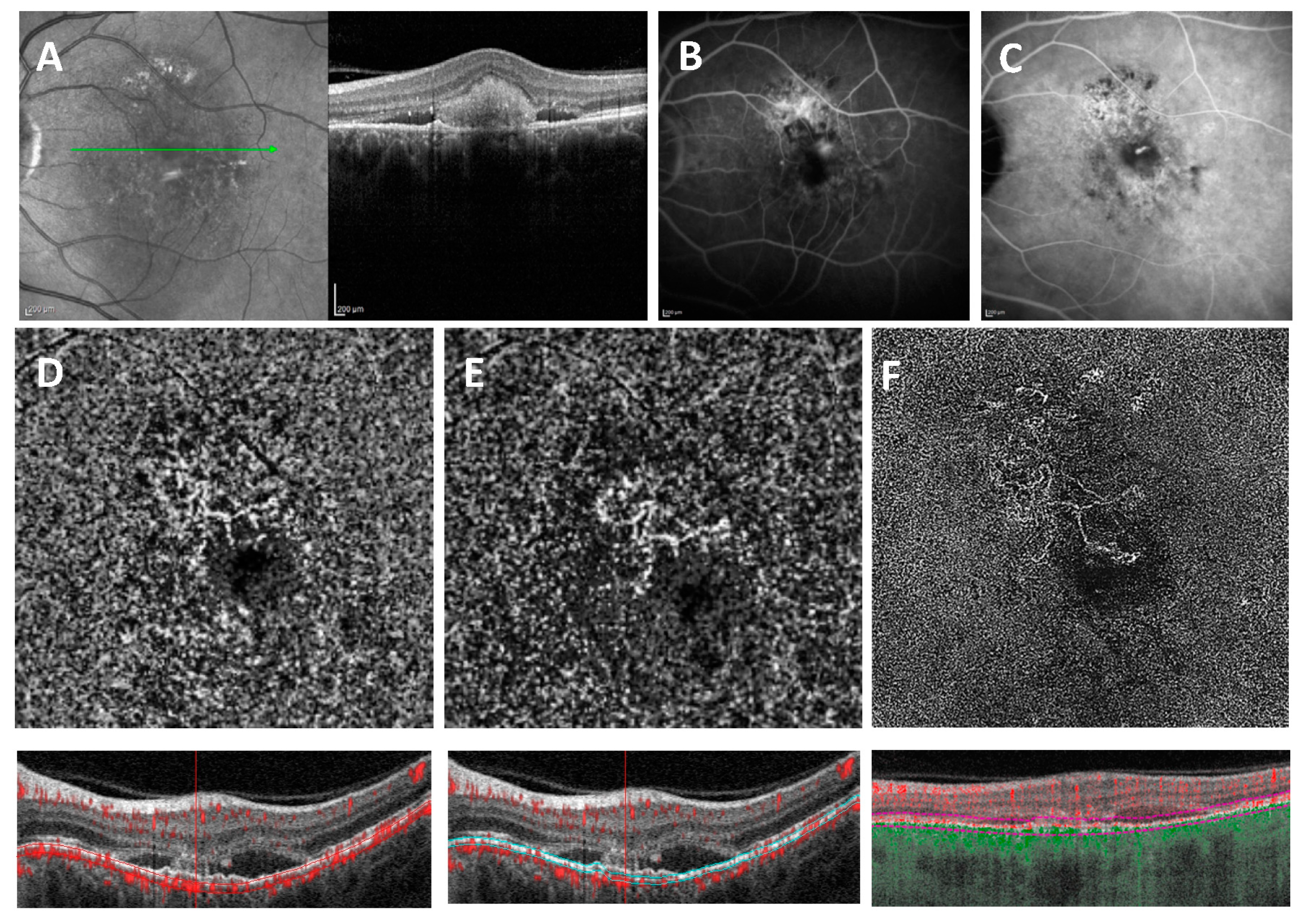
3.3. Variables Associated with MNV (un) Detectability on Different OCTA Devices
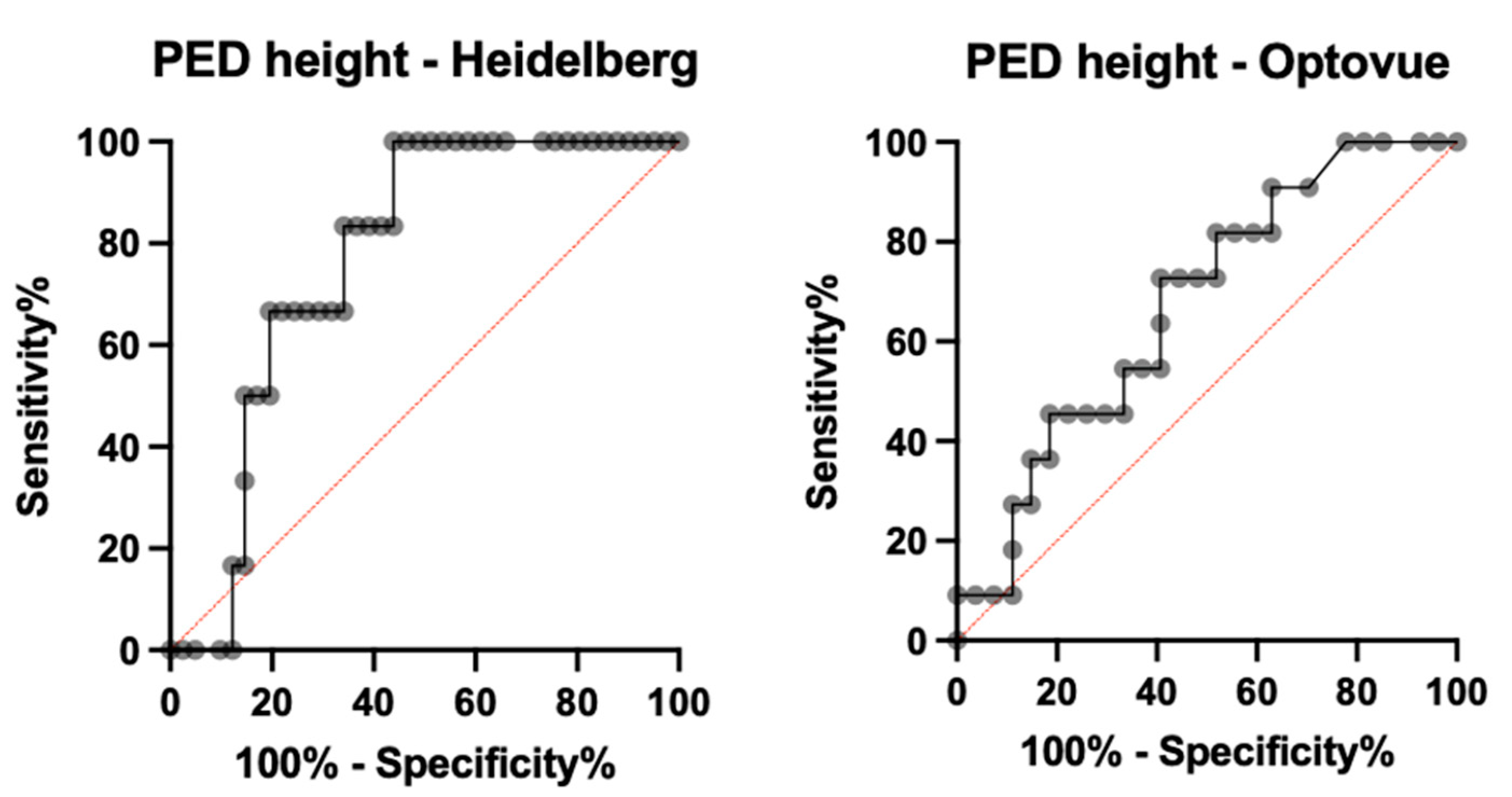
3.3.1. Heidelberg OCT2
3.3.2. Optovue OCTA
3.3.3. PlexElite OCTA
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Technical Characteristics of the Three Different OCTA Systems
| System | AngioVue™ | Spectralis® OCTA | SS OCT Angio™ |
| Manufactory | OptoVue, Fremont, CA, USA | Heidelberg Engineering, Heidelberg, Germany | 9000; Carl Zeiss Meditec, Inc., Dublin, CA, USA |
| Algorithm | SSADA | Full-spectrum probabilistic approach | OCTARA |
| OCT device | RTVue XR AVANTI Widefield; SD-OCT | Spectralis OCT2; SD-OCT | PlexElite |
| Optical source (nm) | Centered on 840 with a bandwidth of 50 | Centered on 870 with a bandwidth of 50 | Tunable laser centered on 1050 (invisible) |
| Scan speed (A-scan/s) | 70,000 | 85,000 | 100,000 |
Appendix B.
References
- Coscas, F.M.; Coscas, G.J.; Souied, E.H.; Soubrane, G. Confocal (en face) optical coherence tomography in vascularized retinal pigment epithelium detachment. Retin Cases Brief Rep. 2008, 2, 94–98. [Google Scholar] [CrossRef]
- Colantuono, D.; Souied, E.H.; Borrelli, E.; Capuano, V.; Amoroso, F.; Sacconi, R.; Jung, C.; Querques, G.; Miere, A. Quantitative deep vascular complex analysis of different AMD stages on optical coherence tomography angiography. Eur. J. Ophthalmol. 2020, 31, 2474–2480. [Google Scholar] [CrossRef]
- Lira, R.P.C.; Oliveira, C.L.D.A.; Marques, M.V.R.B.; Silva, A.R.; Pessoa, C.D.C. Adverse reactions of fluorescein angiography: A prospective study. Arq. Bras. Oftalmol. 2007, 70, 615–618. [Google Scholar] [CrossRef]
- Ellis, P.P.; Schoenberger, M.; Rendi, M.A. Antihistamines as prophylaxis against side reactions to intravenous fluorescein. Trans. Am. Ophthalmol. Soc. 1980, 78, 190. [Google Scholar]
- Souedan, V.; Souied, E.H.; Caillaux, V.; Miere, A.; El Ameen, A.; Blanco-Garavito, R. Sensitivity and specificity of optical coherence tomography angiography (OCT-A) for detection of choroidal neovascularization in real-life practice and varying retinal expertise level. Int. Ophthalmol. 2017, 38, 1051–1060. [Google Scholar] [CrossRef]
- Nikolopoulou, E.; Lorusso, M.; Ferrari, L.M.; Cicinelli, M.V.; Bandello, F.; Querques, G.; Ferrari, T.M. Optical Coherence Tomography Angiography versus Dye Angiography in Age-Related Macular Degeneration: Sensitivity and Specificity Analysis. BioMed Res. Int. 2018, 2018, 6724818. [Google Scholar] [CrossRef]
- Told, R.; Sacu, S.; Hecht, A.; Baratsits, M.; Eibenberger, K.; Kroh, M.E.; Rezar-Dreindl, S.; Schlanitz, F.G.; Weigert, G.; Pollreisz, A.; et al. Comparison of SD-Optical Coherence Tomography Angiography and Indocyanine Green Angiography in Type 1 and 2 Neovascular Age-related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Bonini Filho, M.A.; de Carlo, T.E.; Ferrara, D.; Adhi, M.; Baumal, C.R.; Witkin, A.J.; Reichel, E.; Duker, J.S.; Waheed, N.K. Association of Choroidal Neovascularization and Central Serous Chorioretinopathy with Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015, 133, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, A.; Sacconi, R.; Querques, L.; Marchese, A.; Capuano, V.; Rabiolo, A.; Corbelli, E.; Panozzo, G.; Miere, A.; Souied, E.; et al. Natural History of Treatment-Naïve Quiescent Choroidal Neovascularization in Age-Related Macular Degeneration Using OCT Angiography. Ophthalmol. Retin. 2018, 2, 922–930. [Google Scholar] [CrossRef]
- Cohen, S.Y.; Mrejen, S. Imaging of Exudative Age-Related Macular Degeneration: Toward a Shift in the Diagnostic Paradigm? Retina 2017, 37, 1625–1629. [Google Scholar] [CrossRef]
- Sacconi, R.; Battista, M.; Borrelli, E.; Miere, A.; Corbelli, E.; Capuano, V.; Querques, L.; Souied, E.H.; Bandello, F.; Querques, G. OCT-A characterisation of recurrent type 3 macular neovascularisation. Br. J. Ophthalmol. 2020, 105, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Miere, A.; Butori, P.; Cohen, S.Y.; Semoun, O.; Capuano, V.; Jung, C.; Souied, E.H. Vascular remodeling of choroidal neovascularization after anti-vascular endothelial growth factor therapy visualized on optical coherence tomography angiography. Retina 2019, 39, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Q.; Deegan, A.J.; Chang, J.; Wang, R.K. Comparing imaging capabilities of spectral domain and swept source optical coherence tomography angiography in healthy subjects and central serous retinopathy. Eye Vis. 2018, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Coscas, G.; Lupidi, M.; Coscas, F. Heidelberg Spectralis Optical Coherence Tomography Angiography: Technical Aspects. OCT Angiogr. Retin. Macular Dis. 2016, 56, 1–5. [Google Scholar]
- Told, R.; Ginner, L.; Hecht, A.; Sacu, S.; Leitgeb, R.; Pollreisz, A.; Schmidt-Erfurth, U. Comparative study between a spectral domain and a high-speed single-beam swept source OCTA system for identifying choroidal neovascularization in AMD. Sci. Rep. 2016, 6, 38132. [Google Scholar] [CrossRef]
- Soomro, T.; Talks, J. The use of optical coherence tomography angiography for detecting choroidal neovascularization, compared to standard multimodal imaging. Eye 2018, 32, 661–672. [Google Scholar] [CrossRef]
- Unterhuber, A.; Povazay, B.; Hermann, B.; Sattmann, H.; Chavez-Pirson, A.; Drexler, W. In vivo retinal optical coherence tomography at 1040 nm-enhanced penetration into the choroid. Opt. Express 2005, 13, 3252–3258. [Google Scholar] [CrossRef]
- Yeo, J.H.; Chung, H.; Kim, J.T. Swept-Source Optical Coherence Tomography Angiography According to the Type of Choroidal Neovascularization. J. Clin. Med. 2019, 8, 1272. [Google Scholar] [CrossRef]
- Corvi, F.; Cozzi, M.; Barbolini, E.; Nizza, D.; Belotti, M.; Staurenghi, G.; Giani, A. Comparison Between Several Optical Coherence Tomography Angiography Devices and Indocyanine Green Angiography of Choroidal Neovascularization. Retina 2020, 40, 873–880. [Google Scholar] [CrossRef]
- Inoue, M.; Jung, J.J.; Balaratnasingam, C.; Dansingani, K.K.; Dhrami-Gavazi, E.; Suzuki, M.; De Carlo, T.E.; Shahlaee, A.; Klufas, M.A.; El Maftouhi, A.; et al. A Comparison Between Optical Coherence Tomography Angiography and Fluorescein Angiography for the Imaging of Type 1 Neovascularization. Investig. Opthalmol. Vis. Sci. 2016, 57, OCT314–OCT323. [Google Scholar] [CrossRef]
- Gong, J.; Yu, S.; Gong, Y.; Wang, F.; Sun, X. The Diagnostic Accuracy of Optical Coherence Tomography Angiography for Neovascular Age-Related Macular Degeneration: A Comparison with Fundus Fluorescein Angiography. J. Ophthalmol. 2016, 2016, 7521478. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.S.; Freund, K.B.; Balaratnasingam, C.; Simhaee, D.; Yannuzzi, L.A. Imaging of Pigment Epithelial Detachments with Optical Coherence Tomography Angiography. Retina 2018, 38, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Filho, M.A.B.; Chin, A.T.; Adhi, M.; Ferrara, D.; Baumal, C.R.; Witkin, A.J.; Reichel, E.; Duker, J.S.; Waheed, N.K. Spectral-Domain Optical Coherence Tomography Angiography of Choroidal Neovascularization. Ophthalmology 2015, 122, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Aragona, E.; Bordato, A.; Amato, A.; Saladino, A.; Bandello, F.; Parodi, M.B. High Reflectivity and Low Reflectivity Properties on OCTA Influence the Detection of Macular Neovascularization in AMD. Front. Phys. 2021, 9, 694035. [Google Scholar] [CrossRef]
- Kuehlewein, L.; Bansal, M.; Lenis, T.L.; Iafe, N.A.; Sadda, S.R.; Bonini Filho, M.A.; De Carlo, T.E.; Waheed, N.K.; Duker, J.S.; Sarraf, D. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2015, 160, 739–748.e2. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, A.; Semoun, O.; Caillaux, V.; Jung, C.; Lay, B.; Miere, A.; Souied, E.H.; Blanco-Garavito, R. Reliability and Reproducibility of Pigment Epithelial Detachment Volume Measurements in AMD Using a New Tool: ReVAnalyzer. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, e242–e249. [Google Scholar] [CrossRef] [PubMed]
- Mrejen, S.; Giocanti-Auregan, A.; Tabary, S.; Cohen, S.Y. Sensitivity of 840-nm spectral domain optical coherence tomography angiography in detecting type 1 neovascularization according to the height of the associated pigment epithelial detachment. Retina 2019, 39, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Bailey, S.T.; Wilson, D.J.; Tan, O.; Klein, M.L.; Flaxel, C.J.; Potsaid, B.; Liu, J.J.; Lu, C.D.; Kraus, M.F.; et al. Quantitative Optical Coherence Tomography Angiography of Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmology 2014, 121, 1435–1444. [Google Scholar] [CrossRef] [Green Version]

| Group 1 | Group 2 | p | Total | |
|---|---|---|---|---|
| Total Eyes n, (%) | 90 (83.3%) | 18 (16.7%) | N/A | 108 |
| Age, years, mean ± SD | 80.7 ± 8.2 | 82.1 ± 7 | 0.450 | 80.9 ± 8 |
| Sex (Female/Male) n, (%) | 60/30 67% | 15/3 83% | N/A | 75/33 69.7% |
| Eye (Right/Left) n, (%) | 39/51 (43%/57%) | 7/11 (39%/61%) | 0.726 | 46/62 (42.6%/57.4%) |
| Naïve/Not n, (%) | 77/13 (85%/15%) | 16/2 (89%/11%) | 0.709 | 93/15 (86.1%/13.9%) |
| Hemorrhage n, (%) | 48 (53%) | 4 (22%) | 0.016 p Val < 0.05 | 52 (48.2%) |
| Neovessels n, (%) | ||||
| Type I | 37 (41.1%) | 15 (83.3%) | 0.02 | 52 (48.1%) |
| Type II | 27 (30%) | 0 | p Val < 0.05 | 27 (25%) |
| Type III | 26 (28.9%) | 3 (16.7%) | 29 (26.9%) | |
| PED Height, µm, mean ± SD 95% Conf. Interval, µm | 151.9 ± 135 (137–189) | 219.8 ± 128 (156–283) | 0.0163 p Val < 0.05 | 163.23 ± 135.52 |
| PED Width, µm, mean ± SD | 1469 ± 1164 | 1485 ± 804 | 0.791 | 1471 ± 1109 |
| MRT, µm, mean ± SD | 451 ± 129 | 474 ± 107 | 0.453 | 455 ± 125 |
| Choroidal Thickness | 187 ± 90 | 177 ± 71 | 0.904 | 185 ± 87 |
| OCTA Device n, (%) Heidelberg OCT2 AngioVue PlexElite | 51 (43.6%) 40 (34.2%) 26 (22.2%) | 7 (35%) 12 (60%) 1 (5%) | 58 (42.3%) 52 (37.9%) 27 (19.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filali Ansary, M.; Crincoli, E.; Semoun, O.; Uzzan, J.; Amoroso, F.; Jung, C.; Miere, A.; Souied, E. Undetectable Macular Neovascularization on OCT Angiography in Age Related Macular Degeneration: Comparison between Different Devices. Medicina 2022, 58, 1246. https://doi.org/10.3390/medicina58091246
Filali Ansary M, Crincoli E, Semoun O, Uzzan J, Amoroso F, Jung C, Miere A, Souied E. Undetectable Macular Neovascularization on OCT Angiography in Age Related Macular Degeneration: Comparison between Different Devices. Medicina. 2022; 58(9):1246. https://doi.org/10.3390/medicina58091246
Chicago/Turabian StyleFilali Ansary, Meryem, Emanuele Crincoli, Oudy Semoun, Joel Uzzan, Francesca Amoroso, Camille Jung, Alexandra Miere, and Eric Souied. 2022. "Undetectable Macular Neovascularization on OCT Angiography in Age Related Macular Degeneration: Comparison between Different Devices" Medicina 58, no. 9: 1246. https://doi.org/10.3390/medicina58091246
APA StyleFilali Ansary, M., Crincoli, E., Semoun, O., Uzzan, J., Amoroso, F., Jung, C., Miere, A., & Souied, E. (2022). Undetectable Macular Neovascularization on OCT Angiography in Age Related Macular Degeneration: Comparison between Different Devices. Medicina, 58(9), 1246. https://doi.org/10.3390/medicina58091246







