Association of Blood MCP-1 Levels with Risk of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Plan
2.2. Recognizing of Articles
2.3. Suitability Criteria
2.4. Data Collection
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Characteristics
3.3. Pooled Analysis (Case vs. Control)
3.4. Pooled Analysis (Adults with Severe vs. Mild/Moderate OSA)
3.5. Subgroup Analysis
3.6. Meta-Regression
3.7. Sensitivity Analysis
3.8. Publication Bias
3.9. Trial Sequential Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet. Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Reitsma, M.B.; Murray, C.J.L. Health Effects of Overweight and Obesity in 195 Countries. N. Engl. J. Med. 2017, 377, 1496–1497. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Horvath, P.; Tarnoki, D.L.; Tarnoki, A.D.; Karlinger, K.; Lazar, Z.; Losonczy, G.; Kunos, L.; Bikov, A. Complement system activation in obstructive sleep apnea. J. Sleep Res. 2018, 27, e12674. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Mermigkis, C.; Tzanakis, N.; Kallergis, E.; Moniaki, V.; Mauroudi, E.; Schiza, S.E. Evaluation of inflammatory markers in a large sample of obstructive sleep apnea patients without comorbidities. Mediat. Inflamm. 2017, 2017, 4573756. [Google Scholar] [CrossRef]
- Chen, H.L.; Lin, H.C.; Lu, C.H.; Chen, P.C.; Huang, C.C.; Chou, K.H.; Su, M.C.; Friedman, M.; Chen, Y.W.; Lin, W.C. Systemic inflammation and alterations to cerebral blood flow in obstructive sleep apnea. J. Sleep Res. 2017, 26, 789–798. [Google Scholar] [CrossRef]
- DeMartino, T.; Ghoul, R.E.; Wang, L.; Bena, J.; Hazen, S.L.; Tracy, R.; Patel, S.R.; Auckley, D.; Mehra, R. Oxidative stress and inflammation differentially elevated in objective versus habitual subjective reduced sleep duration in obstructive sleep apnea. Sleep 2016, 39, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Chorostowska-Wynimko, J.; Kedzior, M.E. Role of immune system in the pathomechanism of obstructive sleep apnea. Pneumonol. I Alergol. Pol. 2008, 76, 101–110. [Google Scholar] [CrossRef]
- Luster, A.D. Chemokines—Chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998, 338, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Willimann, K. Chemokines: Role in inflammation and immune surveillance. Ann. Rheum. Dis. 2004, 63, ii84–ii89. [Google Scholar] [CrossRef]
- Lira, S.A.; Furtado, G.C. The biology of chemokines and their receptors. Immunol. Res. 2012, 54, 111–120. [Google Scholar] [CrossRef]
- Imani, M.M.; Sadeghi, M.; Tadakamadla, S.K.; Brühl, A.; Sadeghi Bahmani, D.; Taheri, M.; Brand, S. Polymorphisms of ATP-Binding Cassette, Sub-Family A, Member 4 (rs560426 and rs481931) and Non-Syndromic Cleft Lip/Palate: A Meta-Analysis. Life 2021, 11, 58. [Google Scholar] [CrossRef]
- Imani, M.M.; Lopez-Jornet, P.; López, E.P.; Ghanbari, F.; Sadeghi, M. Association of Betaine-Homocysteine S-Methyl Transferase (rs3797546 and rs3733890) polymorphisms with non-syndromic cleft lip/palate: A meta-analysis. Int. Orthod. 2019, 17, 643–651. [Google Scholar] [CrossRef]
- Imani, M.M.; Safaei, M.; Lopez-Jornet, P.; Sadeghi, M. A systematic review and meta-analysis on protective role of forkhead box E1 (FOXE1) polymorphisms in susceptibility to non-syndromic cleft lip/palate. Int. Orthod. 2019, 17, 437–445. [Google Scholar] [CrossRef]
- Imani, M.M.; Mozaffari, H.R.; Sharifi, R.; Sadeghi, M. Polymorphism of reduced folate carrier 1 (A80G) and non-syndromic cleft lip/palate: A systematic review and meta-analysis. Arch. Oral Biol. 2019, 98, 273–279. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Ohga, E.; Tomita, T.; Wada, H.; Yamamoto, H.; Nagase, T.; Ouchi, Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J. Appl. Physiol. 2003, 94, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Qin, Z.; Wang, W.; Kang, J. Effect of obstructive sleep apnea on carotid artery intima media thickness related to inflammation. Clin. Investig. Med. 2017, 40, E25–E33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.H.; Park, C.S.; Kim, B.G.; Kim, S.W.; Cho, J.H. Plasma levels of MCP-1 and adiponectin in obstructive sleep apnea syndrome. Arch. Otolaryngol.–Head Neck Surg. 2010, 136, 896–899. [Google Scholar] [CrossRef]
- Perrini, S.; Cignarelli, A.; Quaranta, V.N.; Falcone, V.A.; Kounaki, S.; Porro, S.; Ciavarella, A.; Ficarella, R.; Barbaro, M.; Genchi, V.A. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight 2017, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Hai-rong, B.; Qin, Y.; Xiao-ju, L.; Xiao-ya, W. Changes of serum interleukin-8 and monocyte chemoattractant protein-1 levels in patients with obstructive sleep apnea hypopnea syndrome. Chin. J. Clin. Rehabil. 2005, 9, 71–73. [Google Scholar]
- Silva, W.A.; Almeida-Pititto, B.; Santos, R.B.; Aielo, A.N.; Giatti, S.; Parise, B.K.; Souza, S.P.; Vivolo, S.F.; Lotufo, P.A.; Bensenor, I.M. Obstructive sleep apnea is associated with lower adiponectin and higher cholesterol levels independently of traditional factors and other sleep disorders in middle-aged adults: The ELSA-Brasil cohort. Sleep Breath. 2021, 25, 1935–1944. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Morgan, R.L.; Thayer, K.A.; Bero, L.; Bruce, N.; Falck-Ytter, Y.; Ghersi, D.; Guyatt, G.; Hooijmans, C.; Langendam, M.; Mandrioli, D. GRADE: Assessing the quality of evidence in environmental and occupational health. Environ. Int. 2016, 92, 611–616. [Google Scholar] [CrossRef]
- Morgan, R.L.; Thayer, K.A.; Santesso, N.; Holloway, A.C.; Blain, R.; Eftim, S.E.; Goldstone, A.E.; Ross, P.; Guyatt, G.; Schünemann, H.J. Evaluation of the risk of bias in non-randomized studies of interventions (ROBINS-I) and the ‘target experiment’concept in studies of exposures: Rationale and preliminary instrument development. Environ. Int. 2018, 120, 382–387. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Imberger, G.; Thorlund, K.; Gluud, C.; Wetterslev, J. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: An empirical review. BMJ Open 2016, 6, e011890. [Google Scholar] [CrossRef] [PubMed]
- Wetterslev, J.; Jakobsen, J.C.; Gluud, C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 2017, 17, 1–18. [Google Scholar] [CrossRef]
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Takamizawa, A.; Kinoshita, O.; Kubo, K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology 2006, 11, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.-W.; Sun, H.-L.; Yang, Y.-X.; Jia, Y.-F.; Huang, M.-L.; Du, Y.-H.; Qin, Y.-W.; Fang, F.; Zhang, M.; Wei, Y.-X. The association between circulating APRIL levels and severity of obstructive sleep apnea in Chinese adults. Clin. Chim. Acta 2020, 508, 161–169. [Google Scholar] [CrossRef]
- Niu, J.; Kolattukudy, P.E. Role of MCP-1 in cardiovascular disease: Molecular mechanisms and clinical implications. Clin. Sci. 2009, 117, 95–109. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Niu, J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ. Res. 2012, 110, 174–189. [Google Scholar] [CrossRef]
- Uchiyama, T.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Shobatake, R.; Ota, H.; Takeda, M.; Ohbayashi, C.; Takasawa, S. Intermittent hypoxia up-regulates CCL2, RETN, and TNFα mRNAs in adipocytes via down-regulation of miR-452. Int. J. Mol. Sci. 2019, 20, 1960. [Google Scholar] [CrossRef]
- Lee, M.Y.; Wang, Y.; Mak, J.C.; Ip, M.S. Intermittent hypoxia induces NF-κB-dependent endothelial activation via adipocyte-derived mediators. Am. J. Physiol.-Cell Physiol. 2016, 310, C446–C455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gileles-Hillel, A.; Alonso-Álvarez, M.L.; Kheirandish-Gozal, L.; Peris, E.; Cordero-Guevara, J.A.; Terán-Santos, J.; Martinez, M.G.; Jurado-Luque, M.J.; Corral-Peñafiel, J.; Duran-Cantolla, J. Inflammatory markers and obstructive sleep apnea in obese children: The NANOS study. Mediat. Inflamm. 2014, 2014, 605280. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.E.; Knight, B.; Pastel, E.; McCulloch, L.; Patel, B.; Shore, A.; Kos, K. Adipose tissue is influenced by hypoxia of obstructive sleep apnea syndrome independent of obesity. Diabetes Metab. 2017, 43, 240–247. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.-i.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; García-Río, F. Sleep apnoea, insulin resistance and diabetes: The first step is in the fat. Eur. Respir. J. 2017, 49, 1700179. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, E.; Theorell-Haglöw, J.; Svensson, M.; Gislason, T.; Berne, C.; Janson, C. Sleep apnea and glucose metabolism: A long-term follow-up in a community-based sample. Chest 2012, 142, 935–942. [Google Scholar] [CrossRef]
- Boring, L.; Gosling, J.; Cleary, M.; Charo, I.F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998, 394, 894–897. [Google Scholar] [CrossRef]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.-C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A.; Luster, A.D.; Luscinskas, F.W.; Rosenzweig, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–723. [Google Scholar] [CrossRef]
- Lin, J.; Kakkar, V.; Lu, X. Impact of MCP-1 in atherosclerosis. Curr. Pharm. Des. 2014, 20, 4580–4588. [Google Scholar] [CrossRef]
- Harrington, J.R. The role of MCP-1 in atherosclerosis. Stem Cells 2000, 18, 65–66. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Almendros, I.; Farré, N. Obstructive sleep apnea and atherosclerosis: Both the gut microbiome and hypercapnia matter. Am. J. Respir. Cell Mol. Biol. 2017, 57, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Pépin, J.-L.; Arnaud, C.; Baguet, J.-P.; Dematteis, M.; Mach, F. Obstructive sleep apnea and atherosclerosis. Prog. Cardiovasc. Dis. 2009, 51, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, I.; Kaaman, M.; Olsson, T.; Tan, G.D.; Bickerton, A.S.; Wåhlén, K.; Andersson, J.; Nordström, E.A.; Blomqvist, L.; Sjögren, A. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 5834–5840. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Cereda, E.; Morricone, L.; Coman, C.; Corsi, M.M.; Ambrosi, B. Monocyte chemoattractant protein 1: A possible link between visceral adipose tissue-associated inflammation and subclinical echocardiographic abnormalities in uncomplicated obesity. Eur. J. Endocrinol. 2005, 153, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Inadera, H.; Egashira, K.; Takemoto, M.; Ouchi, Y.; Matsushima, K. Increase in circulating levels of monocyte chemoattractant protein-1 with aging. J. Interferon Cytokine Res. 1999, 19, 1179–1182. [Google Scholar] [CrossRef]
- Arakelyan, A.; Petrkova, J.; Hermanova, Z.; Boyajyan, A.; Lukl, J.; Petrek, M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediat. Inflamm. 2005, 2005, 175–179. [Google Scholar] [CrossRef]
- Chen, J.; Lin, S.; Zeng, Y. An update on obstructive sleep apnea for atherosclerosis: Mechanism, diagnosis, and treatment. Front. Cardiovasc. Med. 2021, 8, 647071. [Google Scholar] [CrossRef]
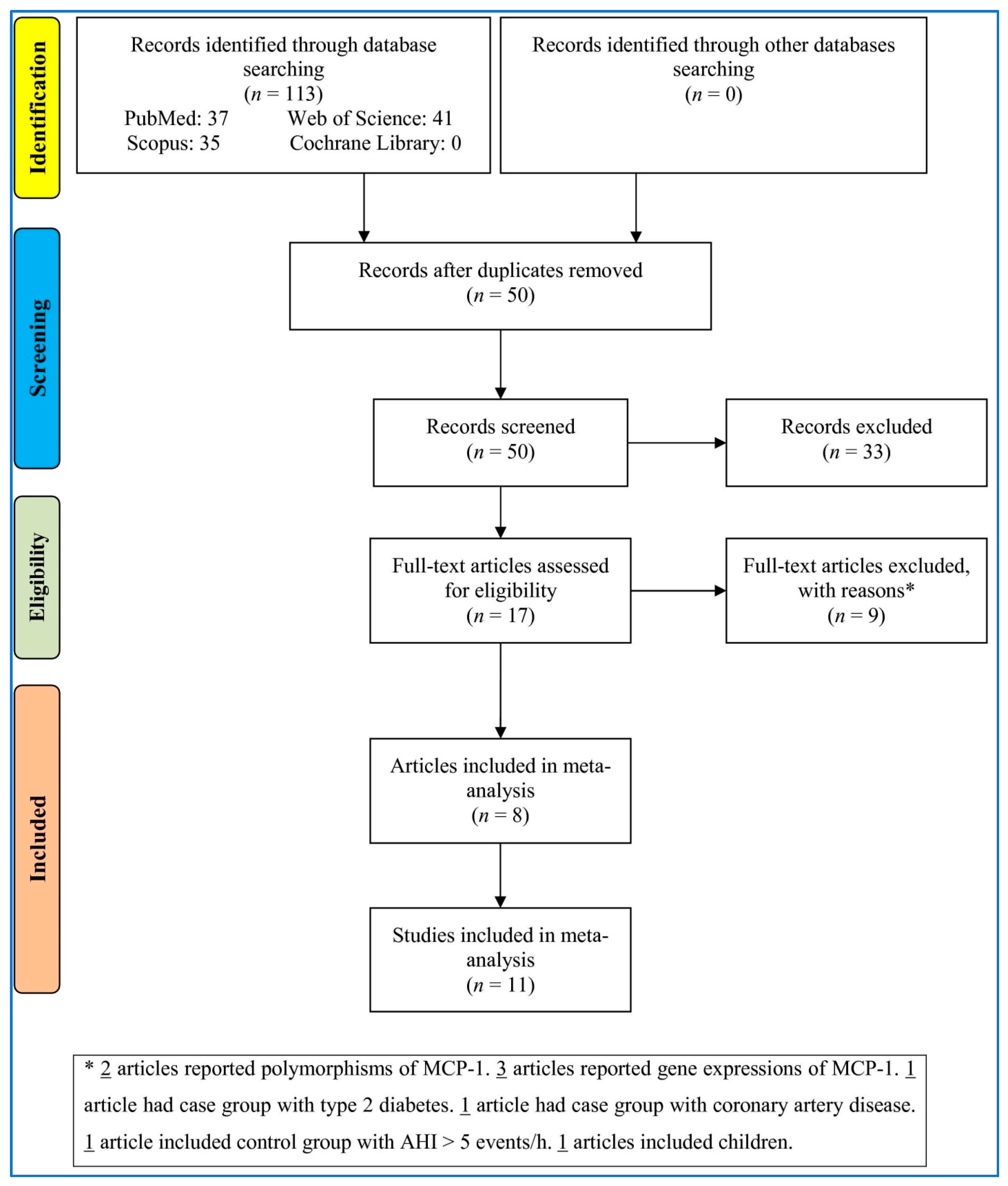
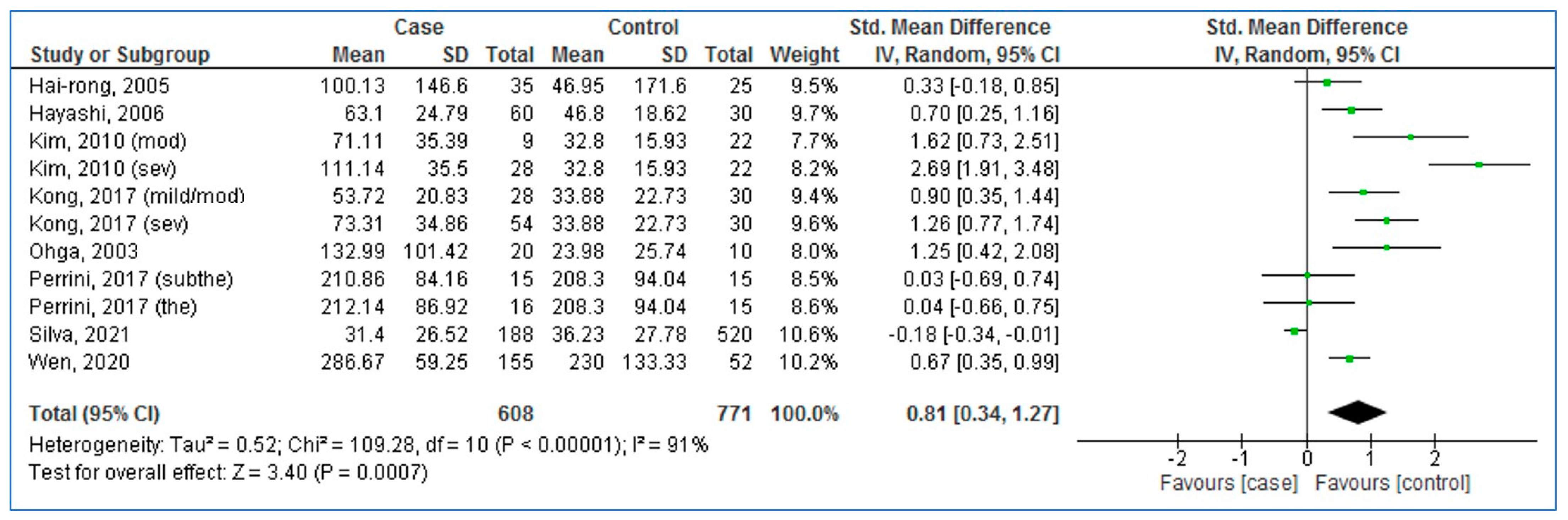
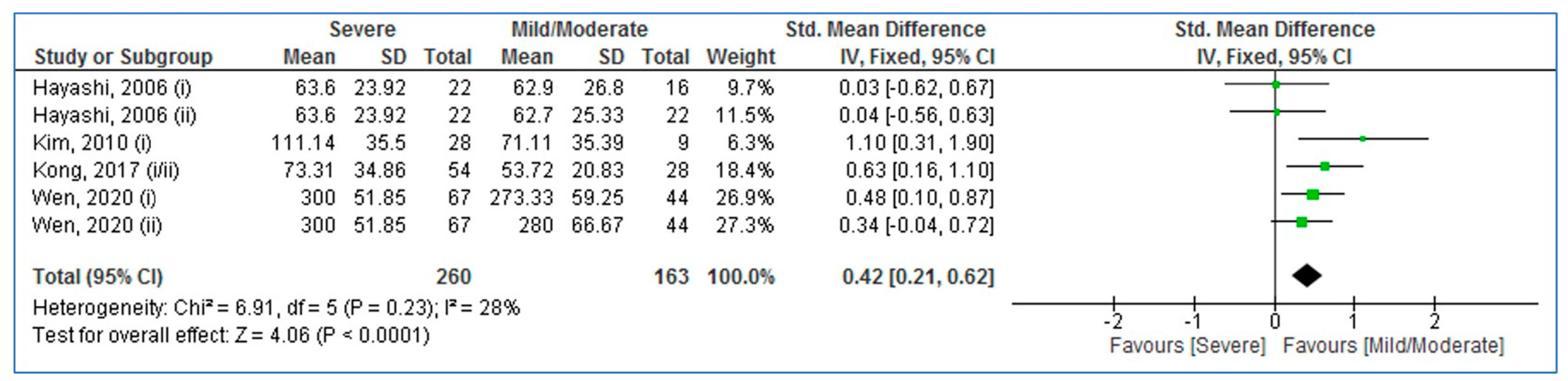

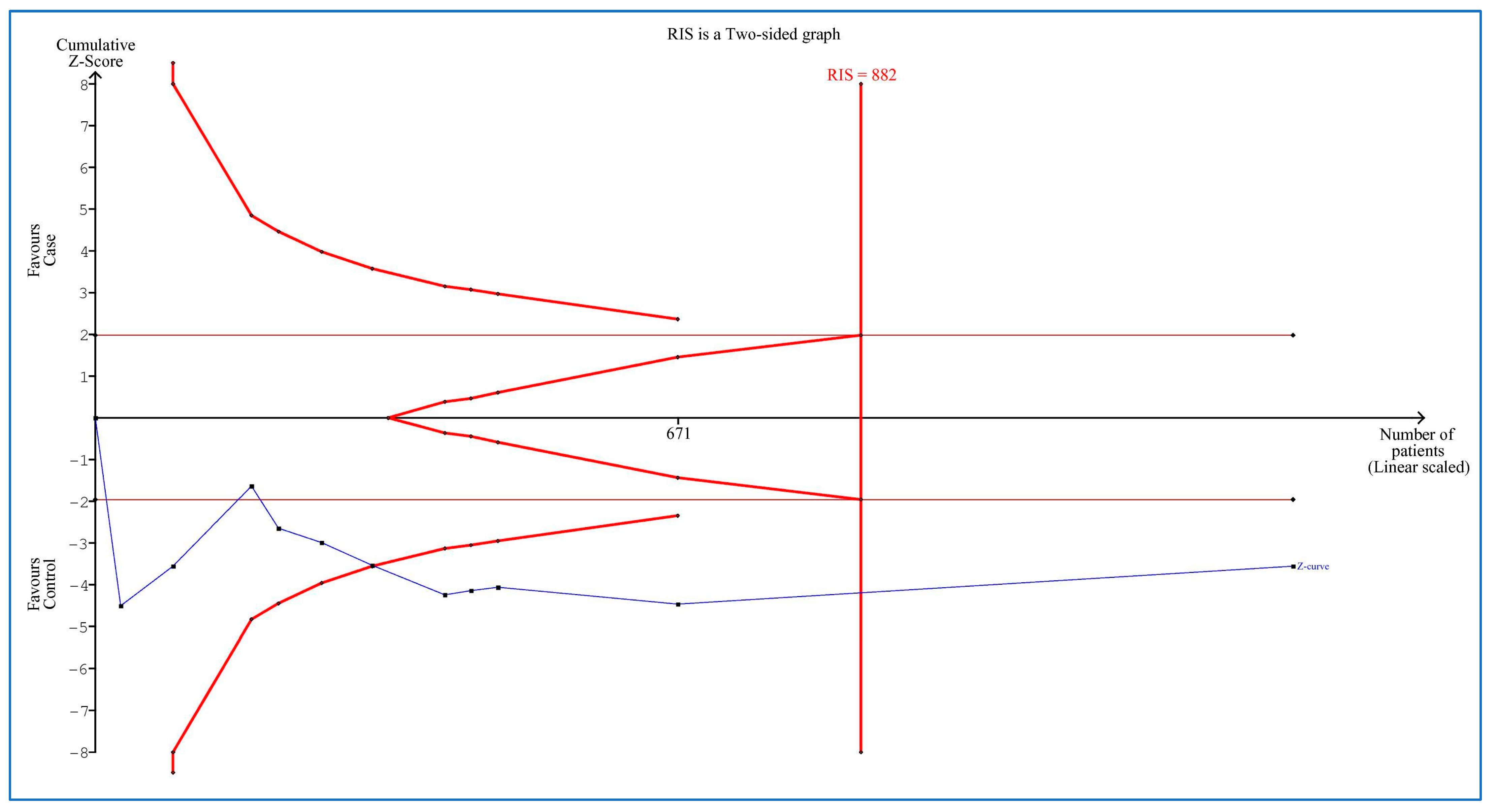
| First Author, Publication Year | Country | Ethnicity | Cases | Controls | Sampling | Quality Score | ||||||
| No. | Mean | No. | Mean | |||||||||
| AHI, events/h | BMI, kg/m2 | Age, Years | AHI, events/h | BMI, kg/m2 | Age, Years | |||||||
| Ohga, 2003 [21] | Japan | Asian | 20 | 38.9 | 29.4 | 47.8 | 10 | 3.1 | 28.4 | 48.9 | Serum | 9 |
| Hai-rong, 2005 [25] | China | Asian | 35 | ≥5 | 29.24 | 50 | 25 | <5 | 28.04 | 50 | Serum | 8 |
| Hayashi, 2006 [36] | Japan | Asian | 60 | 49.4 | 28.8 | 51.6 | 30 | 2.8 | 23.2 | 55 | Serum | 7 |
| Kim, 2010 * [23] | Korea | Asian | 9 | 14.40 | 24.43 | 38 | 22 | 1.25 | 23.88 | 26 | Serum | 6 |
| 28 | 52.71 | 28.69 | 42 | |||||||||
| Kong, 2017 ** [22] | China | Asian | 28 | ≥5 | 27.3 | 44.9 | 30 | <5 | 27.6 | 45.7 | Serum | 9 |
| 54 | ≥5 | 27.9 | 45.9 | |||||||||
| Perrini, 2017 *** [24] | Italy | Caucasian | 16 | 42.5 | 38 | 46.3 | 15 | 4.6 | 41.1 | 43.7 | Serum | 8 |
| 15 | 27.3 | 38.7 | 46.1 | |||||||||
| Wen, 2020 [37] | China | Asian | 155 | 28.53 | 23.59 | 52 | 52 | 3.00 | 27.21 | 49.67 | Plasma | 7 |
| Silva, 2021 [26] | Brazil | Mixed | 188 | 26.97 | 25.1 | 47 | 520 | 4.8 | 28.7 | 45 | Plasma | 5 |
| Variable, N | SMD | 95% CI | p-Value | I2, % | Pheterogeneity | |
| Min | Max | |||||
| Ethnicity | ||||||
| Asian (8) | 1.10 | 0.69 | 1.52 | <0.00001 | 79 | <0.0001 |
| Caucasian (2) | 0.03 | –0.47 | 0.54 | 0.89 | 0 | 0.98 |
| Mixed (1) | –0.18 | –0.34 | –0.01 | 0.04 | - | - |
| Sample size | ||||||
| <100 (9) | 0.95 | 0.47 | 1.43 | <0.0001 | 81 | <0.00001 |
| ≥100 (2) | 0.24 | –0.59 | 1.07 | 0.58 | 95 | <0.00001 |
| Sampling | ||||||
| Serum (9) | 0.95 | 0.47 | 1.43 | <0.0001 | 81 | <0.00001 |
| Plasma (4) | 0.24 | −0.59 | 1.07 | 0.58 | 95 | <0.00001 |
| Variable | Point Estimate | Standard Error | Lower Limit | Upper Limit | Z-Value | p-Value |
| Publication year | −0.06450 | 0.01084 | −0.08575 | −0.04325 | −5.94886 | <0.00001 |
| Mean age of adults with OSA | −0.03085 | 0.02243 | −0.07481 | 0.01311 | −1.37525 | 0.16905 |
| Mean age of controls | −0.03391 | 0.01188 | −0.05720 | −0.01063 | −2.85432 | 0.00431 |
| Mean BMI of adults with OSA | 0.02835 | 0.01804 | −0.00701 | 0.06370 | 1.57128 | 0.00612 |
| Mean BMI of controls | −0.07558 | 0.01744 | −0.10976 | −0.04139 | −4.33352 | 0.00001 |
| Mean AHI of adults with OSA | 0.04198 | 0.00826 | 0.02579 | 0.05816 | 5.08275 | <0.00001 |
| Sample size | −0.00165 | 0.00020 | −0.00204 | −0.00126 | −8.29125 | <0.00001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imani, M.M.; Sadeghi, M.; Mohammadi, M.; Brühl, A.B.; Sadeghi-Bahmani, D.; Brand, S. Association of Blood MCP-1 Levels with Risk of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1266. https://doi.org/10.3390/medicina58091266
Imani MM, Sadeghi M, Mohammadi M, Brühl AB, Sadeghi-Bahmani D, Brand S. Association of Blood MCP-1 Levels with Risk of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Medicina. 2022; 58(9):1266. https://doi.org/10.3390/medicina58091266
Chicago/Turabian StyleImani, Mohammad Moslem, Masoud Sadeghi, Mehdi Mohammadi, Annette Beatrix Brühl, Dena Sadeghi-Bahmani, and Serge Brand. 2022. "Association of Blood MCP-1 Levels with Risk of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis" Medicina 58, no. 9: 1266. https://doi.org/10.3390/medicina58091266






