Atrial Pacing Affects Left Atrial Morphological and Functional Parameters Early after Pacemaker Implantation
Abstract
:1. Introduction
2. Materials and Methods
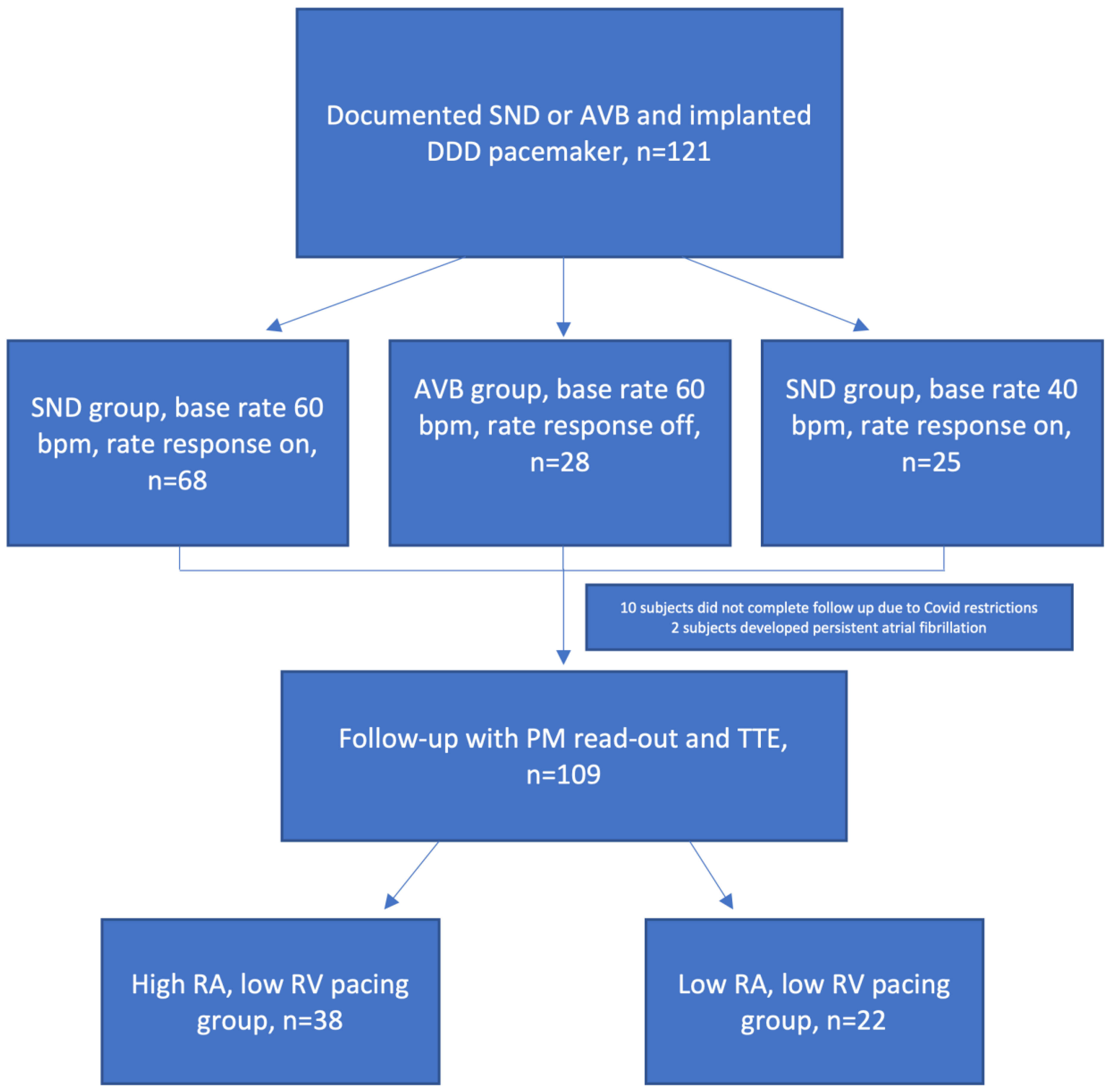
2.1. Study Population
2.2. Echocardiography
2.3. Left Atrium Evaluation
2.4. Follow-Up Visit Procedure
2.5. Statistical Analysis
3. Results
3.1. Baseline Parameters
3.2. Pacing Distribution
3.3. Comparison of High and Low Right Atrial Pacing with Low Right Ventricular Pacing Groups
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; A Barrabés, J.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidou, T.; Fox, D.J.; Brown, B.D. Bradycardia pacing. Medicine 2018, 46, 646–651. [Google Scholar] [CrossRef]
- Kerr, C.R.; Connolly, S.J.; Abdollah, H.; Roberts, R.S.; Gent, M.; Yusuf, S.; Gillis, A.M.; Tang, A.S.; Talajic, M.; Klein, G.J.; et al. Canadian Trial of Physiological Pacing: Effects of physiological pacing during long-term follow-up. Circulation 2004, 109, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Brenyo, A.; Goldenberg, I.; Barsheshet, A. The Downside of Right Ventricular Apical Pacing. Indian Pacing Electrophysiol. J. 2012, 12, 102–113. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Hellkamp, A.S.; Ellenbogen, K.A.; Greenspon, A.J.; Freedman, R.A.; Lee, K.L.; Lamas, G.A.; MOde Selection Trial Investigators. Adverse Effect of Ventricular Pacing on Heart Failure and Atrial Fibrillation Among Patients With Normal Baseline QRS Duration in a Clinical Trial of Pacemaker Therapy for Sinus Node Dysfunction. Circulation 2003, 107, 2932–2937. [Google Scholar] [CrossRef]
- Sharma, A.D.; Rizo-Patron, C.; Hallstrom, A.P.; O’Neill, G.P.; Rothbart, S.; Martins, J.B.; Roelke, M.; Steinberg, J.S.; Greene, H.L.; DAVID Investigators. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005, 2, 830–834. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Cook, J.R.; Epstein, A.E.; Greene, H.L.; Hallstrom, A.P.; Hsia, H.; Kutalek, S.P.; Sharma, A.; Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-Chamber Pacing or Ventricular Backup Pacing in Patients with an Implantable Defibrillator. JAMA 2002, 288, 3115–3123. [Google Scholar] [CrossRef]
- Xie, J.-M.; Fang, F.; Zhang, Q.; Chan, J.Y.-S.; Yip, G.W.-K.; Sanderson, J.E.; Lam, Y.-Y.; Yan, B.P.; Yu, C.-M. Left atrial remodeling and reduced atrial pump function after chronic right ventricular apical pacing in patients with preserved ejection fraction. Int. J. Cardiol. 2012, 157, 364–369. [Google Scholar] [CrossRef]
- Stefanadis, C.; Dernellis, J.; Toutouzas, P. A clinical appraisal of left atrial function. Eur. Heart J. 2001, 22, 22–36. [Google Scholar] [CrossRef]
- Karayannis, G.; Kitsios, G.; Kotidis, H.; Triposkiadis, F. Left atrial remodelling contributes to the progression of asymptomatic left ventricular systolic dysfunction to chronic symptomatic heart failure. Heart Fail. Rev. 2008, 13, 91–98. [Google Scholar] [CrossRef]
- Rossi, A.; Gheorghiade, M.; Triposkiadis, F.; Solomon, S.D.; Pieske, B.; Butler, J. Left Atrium in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2014, 7, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Ramaekers, J.; Verhaert, D.; Dupont, M.; Vandervoort, P.M.; Mullens, W. The Detrimental Effect of RA Pacing on LA Function and Clinical Outcome in Cardiac Resynchronization Therapy. JACC Cardiovasc. Imaging 2020, 13, 895–906. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left Atrial Size and Function. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Kurt, M.; Wang, J.; Torre-Amione, G.; Nagueh, S.F. Left Atrial Function in Diastolic Heart Failure. Circ. Cardiovasc. Imaging 2009, 2, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Rossi, A.; Cicoira, M.; Florea, V.G.; Golia, G.; Florea, N.D.; Khan, A.A.; Murray, S.T.; Nguyen, J.T.; O’Callaghan, P.; Anand, I.S.; et al. Chronic heart failure with preserved left ventricular ejection fraction: Diagnostic and prognostic value of left atrial size. Int. J. Cardiol. 2006, 110, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-Y.; Cheng, A.; Chang, K.-C.; Berger, R.D.; Agarwal, K.; Eulitt, P.; Corretti, M.; Tomaselli, G.; Calkins, H.; Kass, D.A.; et al. Influence of Atrial Function and Mechanical Synchrony on LV Hemodynamic Status in Heart Failure Patients on Resynchronization Therapy. JACC Cardiovasc. Imaging 2011, 4, 691–698. [Google Scholar] [CrossRef]
- Spodick, D.H. Effect of interatrial block on left atrial function. J. Cardiol. 2001, 38, 169–171. [Google Scholar]
- Pastore, G.; Aggio, S.; Baracca, E.; Fraccaro, C.; Picariello, C.; Roncon, L.; Corbucci, G.; Noventa, F.; Zanon, F. Hisian area and right ventricular apical pacing differently affect left atrial function: An intra-patients evaluation. Europace 2014, 16, 1033–1039. [Google Scholar] [CrossRef]
- Zou, C.; Song, J.; Li, H.; Huang, X.; Liu, Y.; Zhao, C.; Shi, X.; Yang, X. Right Ventricular Outflow Tract Septal Pacing Is Superior to Right Ventricular Apical Pacing. J. Am. Heart Assoc. 2015, 4, e001777. [Google Scholar] [CrossRef] [Green Version]
- Sommer, A.; Kronborg, M.B.; Nørgaard, B.; Gerdes, C.; Mortensen, P.T.; Nielsen, J.C. Left and right ventricular lead positions are imprecisely determined by fluoroscopy in cardiac resynchronization therapy: A comparison with cardiac computed tomography. Europace 2014, 16, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Sade, L.E.; Atar, I.; Özin, B.; Yüce, D.; Müderrisoğlu, H. Determinants of New-Onset Atrial Fibrillation in Patients Receiving CRT: Mechanistic Insights From Speckle Tracking Imaging. JACC Cardiovasc. Imaging 2016, 9, 99–111. [Google Scholar] [CrossRef]
- Adelstein, E.; Saba, S. Right atrial pacing and the risk of postimplant atrial fibrillation in cardiac resynchronization therapy recipients. Am. Heart J. 2008, 155, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 932–987. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.-M.; Nishimura, R.A.; Hayes, D.L. Difference in mechanical atrioventricular delay between atrial sensing and atrial pacing modes in patients with hypertrophic and dilated cardiomyopathy: An electrical hemodynamic catheterization study. J. Interv. Card. Electrophysiol. 2002, 6, 133–140. [Google Scholar] [CrossRef]

| SND Group a (n = 68) | AVB60 Group b (n = 28) | AVB40 Group c (n = 25) | p-Value | |
|---|---|---|---|---|
| Age, years | 75.1 ± 9.5 | 75.9 ± 11.3 | 69.3 ± 103 | 0.104 |
| Male, n (%) | 23 (33.8) | 14 (41.2) | 10 (52.6) | 0.332 |
| Body surface area | 1.91 ± 0.21 | 1.90 ± 0.25 | 1.98 ± 0.25 | 0.472 |
| Body mass index, kg/m2 | 28.9 ± 4.6 | 28.7 ± 6.6 | 30.5 ± 5.0 | 0.494 |
| Medical history | ||||
| Hypertension, n (%) | 63 (92.6) | 26 (92.3) | 25 (100) | 0.380 |
| Diabetes mellitus, n (%) | 5 (7.4) | 8 (28.6) | 6 (24.0) | 0.015 |
| Paroxysmal atrial fibrillation, n (%) | 27 (39.7) | 6 (21.4) | 11 (44.0) | 0.161 |
| Coronary artery disease, n (%) | 27 (39.7) | 9 (32.1) | 10 (40.0) | 0.766 |
| Chronic renal failure, n (%) | 3 (4.4) | 3 (10.7) | 3 (12.0) | 0.350 |
| Medications | ||||
| ACE inhibitors/ARB | 64 (94.1) | 27 (96.4) | 22 (88.0) | 0.438 |
| BAB | 51 (75.0) | 21 (75.0) | 22 (88.0) | 0.380 |
| MRA | 18 (26.5) | 7 (25.0) | 5 (20.0) | 0.814 |
| Other diuretic | 33 (48.5) | 14 (50.0) | 13 (52.0) | 0.956 |
| Non dihydropyridine CCB | 1 (1.5) | 0 (0) | 0 (0) | 0.675 |
| Statin | 28 (35.3) | 10 (35.6) | 10 (40.0) | 0.883 |
| LA volumetric parameters | ||||
| LAVmax, mL | 74.6 ± 20.5 | 71.6 ± 22.8 | 83.0 ± 28.4 | 0.359 |
| LAVmax index, mL/m2 | 38.9 ± 9.3 | 37.4 ± 9.5 | 40.6 ± 12.8 | 0.712 |
| LAVpre, mL | 54.2 ± 17.0 | 49.7 ± 17.7 | 57.4 ± 17.7 | 0.382 |
| LAVpre index, mL/m2 | 28.3 ± 8.0 | 25.9 ± 7.5 | 28.1 ± 7.9 | 0.544 |
| LAVmin, mL | 39.2 ± 14.0 | 37.4 ± 14.9 | 43.2 ± 15.6 | 0.379 |
| LAVmin index, mL/m2 | 20.5 ± 6.8 | 19.3 ± 6.3 | 21.1 ± 7.2 | 0.682 |
| LV parameters | ||||
| LVEDD, mm | 49.6 ± 4.7 | 49.7 ± 5.5 | 50.9 ± 4.9 | 0.655 |
| LVEDD index, mL/m2 | 26.1 ± 2.4 | 26.3 ± 2.6 | 25.2 ± 2.3 | 0.302 |
| LVEF, % | 58.4 ± 4.8 | 57.8 ± 4.7 | 59.2 ± 5.0 | 0.808 |
| E/A | 0.88 ± 0.40 | 0.80 ± 0.41 | 0.87 ± 0.30 | 0.983 |
| E/e′ | 8.7 ± 3.0 | 9.5 ± 4.6 | 11.1 ± 3.9 | 0.062 |
| LA functional parameters | ||||
| Total emptying fraction, % | 48.1 ± 8.4 | 48.9 ± 8.7 | 47.8 ± 7.6 | 0.894 |
| Passive emptying fraction, % | 27.6 ± 8.4 | 31.0 ± 9.6 | 29.5 ± 7.8 | 0.200 |
| Active emptying fraction, % | 28.2 ± 9.4 | 25.7 ± 8.6 | 25.9 ± 8.1 | 0.409 |
| LA strain parameters | ||||
| Reservoir strain, % | 23.5 ± 10.1 | 21.1 ± 7.3 | 22.1 ± 12.7 | 0.521 |
| Conduit strain, % | −10.7 ± 4.9 | −9.2 ± 6.5 | −11.0 ± 6.1 | 0.144 |
| Contractile strain, % | −12.8 ± 8.5 | −11.9 ± 6.9 | −11.1 ± 10.4 | 0.513 |
| Stiffness index | 0.50 ± 0.51 | 0.51 ± 0.32 | 0.78 ± 0.69 | 0.184 |
| High RA Low RV Group (n = 38) | Low RA Low RV Group (n = 22) | p-Value | |
|---|---|---|---|
| Age, years | 73.6 ± 10.2 | 69.0 ± 13.3 | 0.349 |
| Male, n (%) | 12 (31.7) | 8 (36.4) | 0.705 |
| Body surface area | 1.89 ± 0.2 | 2.0 ± 0.29 | 0.404 |
| Body mass index, kg/m2 | 28.4 ± 4.2 | 30.4 ± 6.4 | 0.312 |
| Medical history | |||
| Hypertension, n (%) | 35 (92.1) | 19 (86.4) | 0.475 |
| Diabetes mellitus, n (%) | 3 (7.9) | 6 (27.2) | 0.043 |
| Paroxysmal atrial fibrillation, n (%) | 15 (39.4) | 7 (31.8) | 0.553 |
| Coronary artery disease, n (%) | 14 (36.8) | 6 (27.3) | 0.449 |
| Chronic renal failure, n (%) | 2 (5.2) | 3 (13.6) | 0.258 |
| Medications | |||
| ACE inhibitors/ARB | 36 (94.7) | 2 (90.1) | 0.567 |
| BAB | 29 (76.3) | 16 (72.7) | 0.757 |
| MRA | 10 (26.3) | 5 (22.7) | 0.757 |
| Other diuretic | 17 (44.7) | 11 (50.0) | 0.694 |
| Non dihydropyridine CCB | 1 (2.6) | 0 (0) | 0.443 |
| Statin | 12 (31.6) | 8 (36.4) | 0.705 |
| LV parameters | |||
| LVEDD, mm | 49.2 ± 4.5 | 49.6 ± 5.3 | 0.600 |
| LVEDD index, mL/m2 | 26.2 ± 2.2 | 25.4 ± 2.2 | 0.358 |
| LV EF, % | 58.4 ± 4.9 | 58.2 ± 5.0 | 0.798 |
| E/A | 0.89 ± 0.37 | 0.84 ± 3.4 | 0.406 |
| E/e′ | 8.4 ± 2.6 | 8.7 ± 3.7 | 0.842 |
| High RA Low RV Group | Low RA Low RV Group | p-Value | |
|---|---|---|---|
| LA volumetric parameters | |||
| LAVmax, mL | 73.2 ± 17.3 | 72.5 ± 27.2 | 0.916 |
| LAVmax index, mL/m2 | 38.7 ± 7.9 | 36.5 ± 11.9 | 0.433 |
| LAVpre, mL | 53.7 ± 14.3 | 50.2 ± 20.2 | 0.479 |
| LAVpre index, mL/m2 | 28.3 ± 6.8 | 25.2 ± 8.8 | 0.164 |
| LAVmin, mL | 38.2 ± 11.8 | 35.6 ± 16.1 | 0.511 |
| LAVmin index, mL/m2 | 20.2 ± 5.9 | 17.9 ± 7.3 | 0.230 |
| LA functional parameters | |||
| Total emptying fraction, % | 48.1 ± 8.2 | 51.6 ± 7.2 | 0.979 |
| Passive emptying fraction, % | 26.7 ± 8.2 | 31.1 ± 7.6 | 0.586 |
| Active emptying fraction, % | 29.2 ± 8.7 | 29.7 ± 8.6 | 0.903 |
| LA strain parameters | |||
| Reservoir strain, % | 25.9 ± 10.3 | 23.7 ± 9.5 | 0.493 |
| Conduit strain, % | −11.9 ± 5.3 | −11.0 ± 3.7 | 0.565 |
| Contractile strain, % | −14.0 ± 9.0 | −12.7 ± 7.6 | 0.633 |
| Stiffness index | 0.41 ± 0.27 | 0.45 ± 0.32 | 0.639 |
| Baseline | 1 Month | 3 Months | p-Value Baseline vs. 1 Month | p-Value Baseline vs. 3 Months | |
|---|---|---|---|---|---|
| LA volumetric parameters | |||||
| LAVmax, mL | 73.2 ± 17.3 | 77.8 ± 21.1 | 75.8 ± 20.1 | 0.442 | 0.367 |
| LAVmax index, mL/m2 | 38.7 ± 7.9 | 41.0 ± 10.4 | 40.1 ± 10.0 | 0.424 | 0.376 |
| LAVpre, mL | 53.7 ± 14.3 | 55.5 ± 16.2 | 57.3 ± 17.5 | 0.294 | 0.161 |
| LAVpre index, mL/m2 | 28.3 ± 6.8 | 29.3 ± 8.2 | 30.2 ± 8.4 | 0.261 | 0.186 |
| LAVmin, ml | 38.2 ± 11.8 | 41.3 ± 14.6 | 42.7 ± 13.7 | 0.169 | 0.038 |
| LAVmin index, mL/m2 | 20.2 ± 5.9 | 21.7 ± 7.3 | 22.6 ± 7.5 | 0.190 | 0.039 |
| LA functional parameters | |||||
| Total emptying fraction, % | 48.1 ± 8.2 | 47.6 ± 8.6 | 44.9 ± 9.8 | 0.678 | 0.033 |
| Passive emptying fraction, % | 26.7 ± 8.2 | 28.6 ± 9.4 | 24.5 ± 9.7 | 0.398 | 0.401 |
| Active emptying fraction, % | 29.2 ± 8.7 | 26.5 ± 8.5 | 25.7 ± 8.9 | 0.076 | 0.043 |
| LA strain parameters | |||||
| Reservoir strain, % | 25.9 ± 10.3 | 24.4 ± 9.5 | 21.1 ± 9.9 | 0.315 | 0.003 |
| Conduit strain, % | −11.9 ± 5.3 | −11.8 ± 6.4 | −10.0 ± 5.3 | 0.798 | 0.086 |
| Contractile strain, % | −14.0 ± 9.0 | −12.7 ± 7.0 | −11.1 ± 7.8 | 0.342 | 0.018 |
| Stiffness index | 0.41 ± 0.27 | 0.46 ± 0.33 | 0.67 ± 0.65 | 0.478 | 0.001 |
| Baseline | 1 Month | 3 Months | p-Value Baseline vs. 1 Month | p-Value Baseline vs. 3 Months | |
|---|---|---|---|---|---|
| LA volumetric parameters | |||||
| LAVmax, mL | 72.5 ± 27.2 | 77.8 ± 23.9 | 81.5 ± 21.4 | 0.245 | 0.286 |
| LAVmax index, mL/m2 | 36.5 ± 11.9 | 39.5 ± 10.4 | 39.6 ± 9.1 | 0.187 | 0.213 |
| LAVpre, ml | 50.2 ± 20.2 | 56.2 ± 18.4 | 56.2 ± 18.4 | 0.191 | 0.505 |
| LAVpre index, mL/m2 | 25.2 ± 8.8 | 28.3 ± 8.7 | 27.2 ± 8.1 | 0.191 | 0.477 |
| LAVmin, mL | 35.6 ± 16.1 | 38.2 ± 13.5 | 39.2 ± 12.6 | 0.408 | 0.594 |
| LAVmin index, mL/m2 | 17.9 ± 7.3 | 19.4 ± 6.4 | 19.0 ± 5.5 | 0.301 | 0.625 |
| LA functional parameters | |||||
| Total emptying fraction, % | 51.6 ± 7.2 | 50.6 ± 7.6 | 52.2 ± 7.8 | 0.660 | 0.534 |
| Passive emptying fraction, % | 31.1 ± 7.6 | 30.1 ± 8.5 | 31.8 ± 9.5 | 0.460 | 0.824 |
| Active emptying fraction, % | 29.7 ± 8.6 | 31.7 ± 8.7 | 29.8 ± 8.0 | 0.334 | 0.790 |
| LA strain parameters | |||||
| Reservoir strain | 23.7 ± 9.5 | 23.7 ± 9.1 | 24.2 ± 10.2 | 0.925 | 0.575 |
| Conduit strain | −11.0 ± 3.7 | −12.4 ± 7.4 | −13.8 ± 8.0 | 0.778 | 0.161 |
| Contractile strain | −12.7 ± 7.6 | −11.2 ± 7.2 | −10.0 ± 4.8 | 0.683 | 0.093 |
| Stiffness | 0.45 ± 0.32 | 0.51 ± 0.39 | 0.39 ± 0.18 | 0.518 | 0.334 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viezelis, M.; Neverauskaite-Piliponiene, G.; Marcinkeviciene, A.; Teleisyte, E.; Kazakevicius, T.; Zabiela, V.; Kviesulaitis, V.; Jurkevicius, R.; Puodziukynas, A. Atrial Pacing Affects Left Atrial Morphological and Functional Parameters Early after Pacemaker Implantation. Medicina 2022, 58, 1283. https://doi.org/10.3390/medicina58091283
Viezelis M, Neverauskaite-Piliponiene G, Marcinkeviciene A, Teleisyte E, Kazakevicius T, Zabiela V, Kviesulaitis V, Jurkevicius R, Puodziukynas A. Atrial Pacing Affects Left Atrial Morphological and Functional Parameters Early after Pacemaker Implantation. Medicina. 2022; 58(9):1283. https://doi.org/10.3390/medicina58091283
Chicago/Turabian StyleViezelis, Mindaugas, Gintare Neverauskaite-Piliponiene, Agne Marcinkeviciene, Eligija Teleisyte, Tomas Kazakevicius, Vytautas Zabiela, Vilius Kviesulaitis, Renaldas Jurkevicius, and Aras Puodziukynas. 2022. "Atrial Pacing Affects Left Atrial Morphological and Functional Parameters Early after Pacemaker Implantation" Medicina 58, no. 9: 1283. https://doi.org/10.3390/medicina58091283






