Assessment of the In Vitro Cytotoxic Profile of Two Broad-Spectrum Antibiotics—Tetracycline and Ampicillin—On Pharyngeal Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cellular Viability Assessment
2.4. Cellular Morphology
2.5. Wound Healing Assay
2.6. Fluorescence Immunocytochemistry
2.7. Statistical Analysis
3. Results
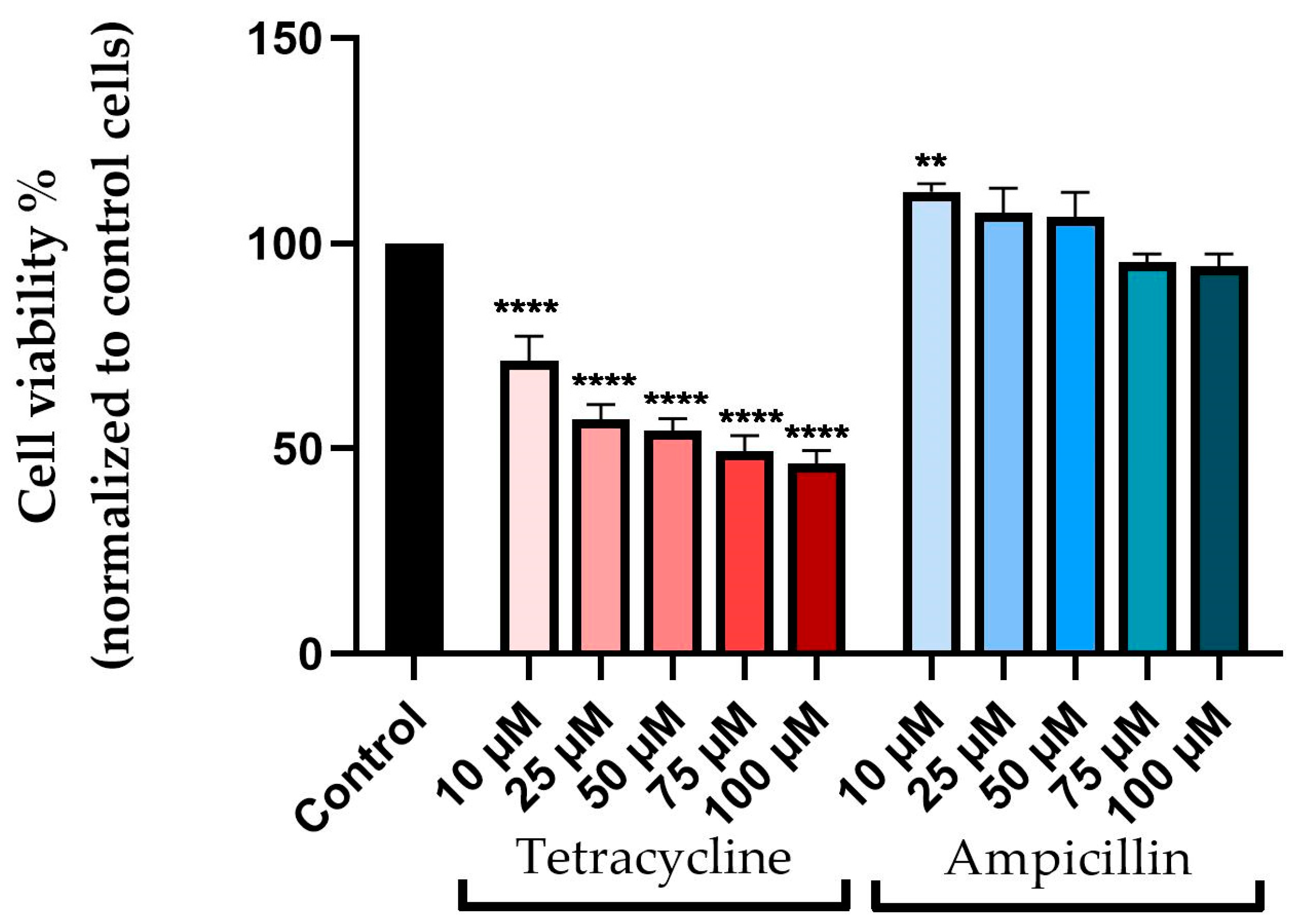
3.1. Cellular Viability Assessment
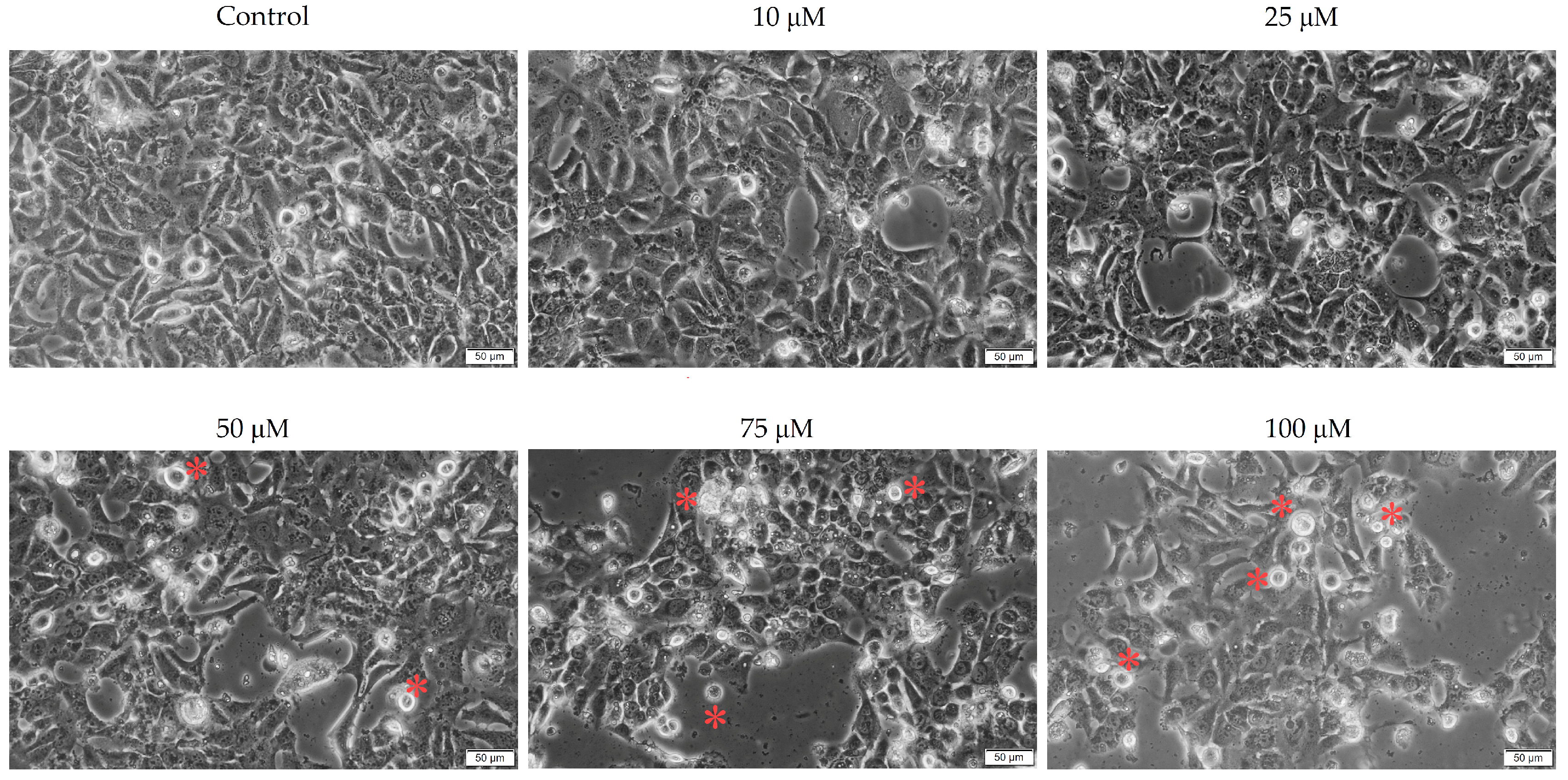
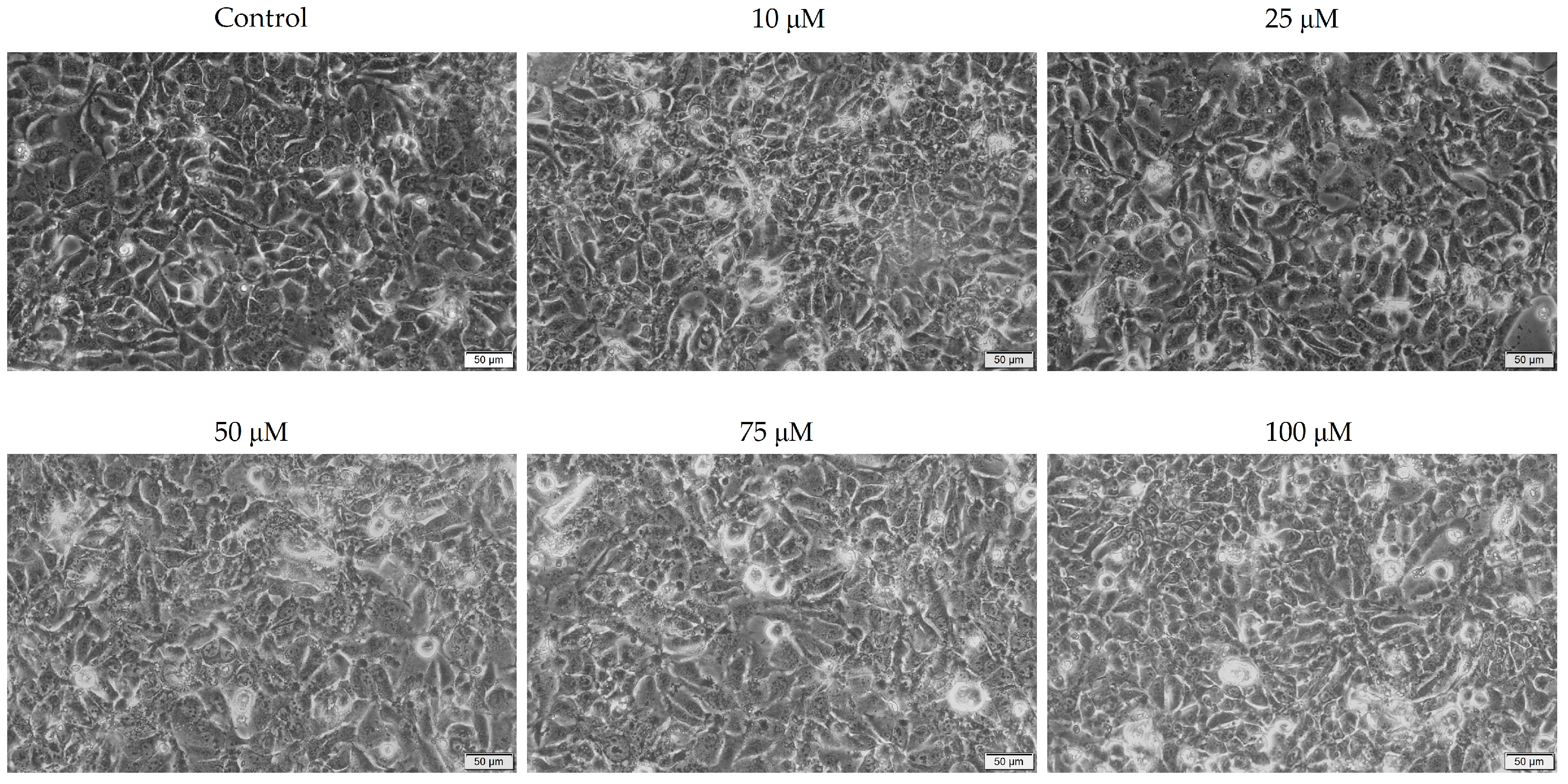
3.2. Cellular Morphology
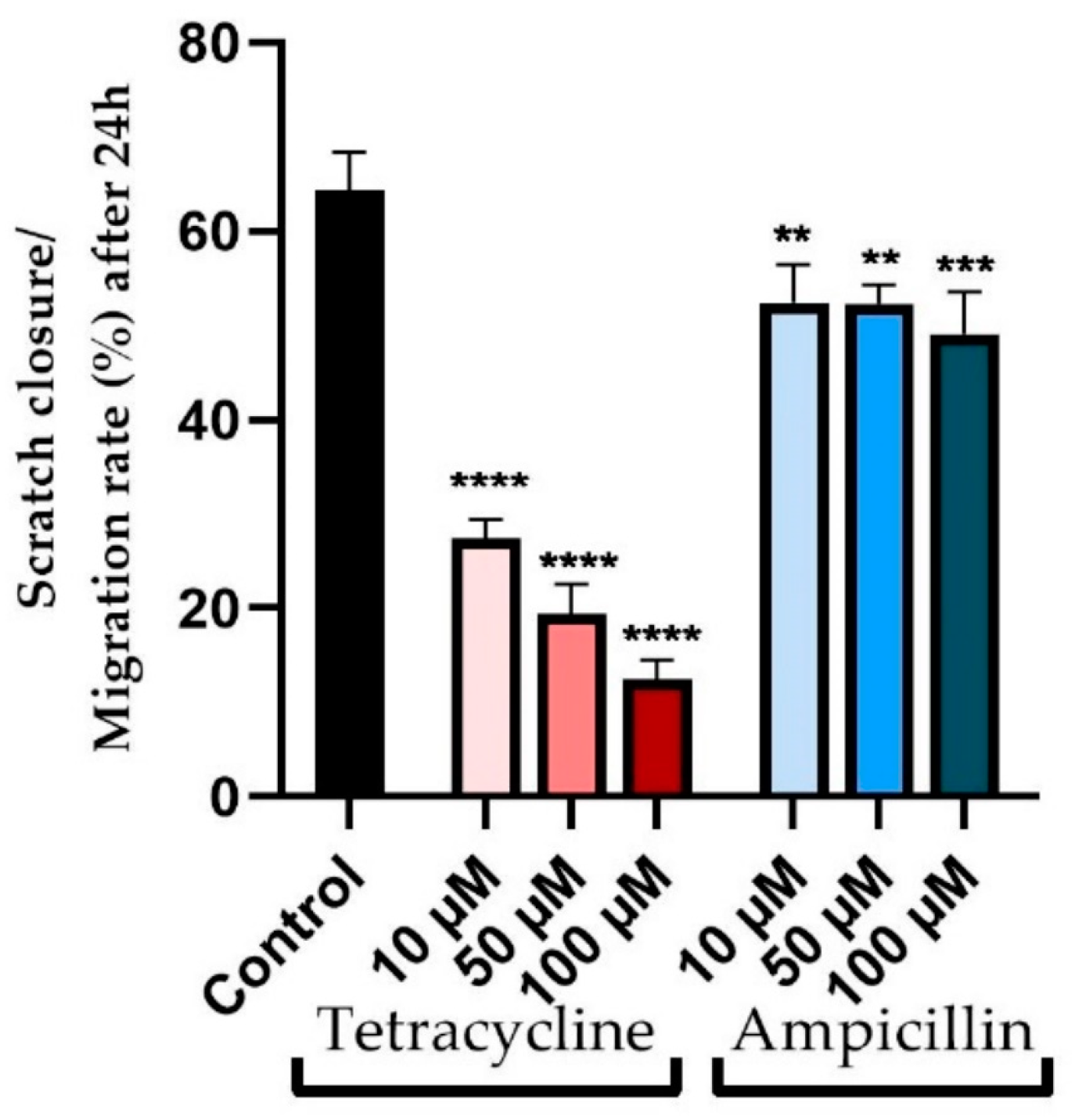
3.3. Wound Healing Assay
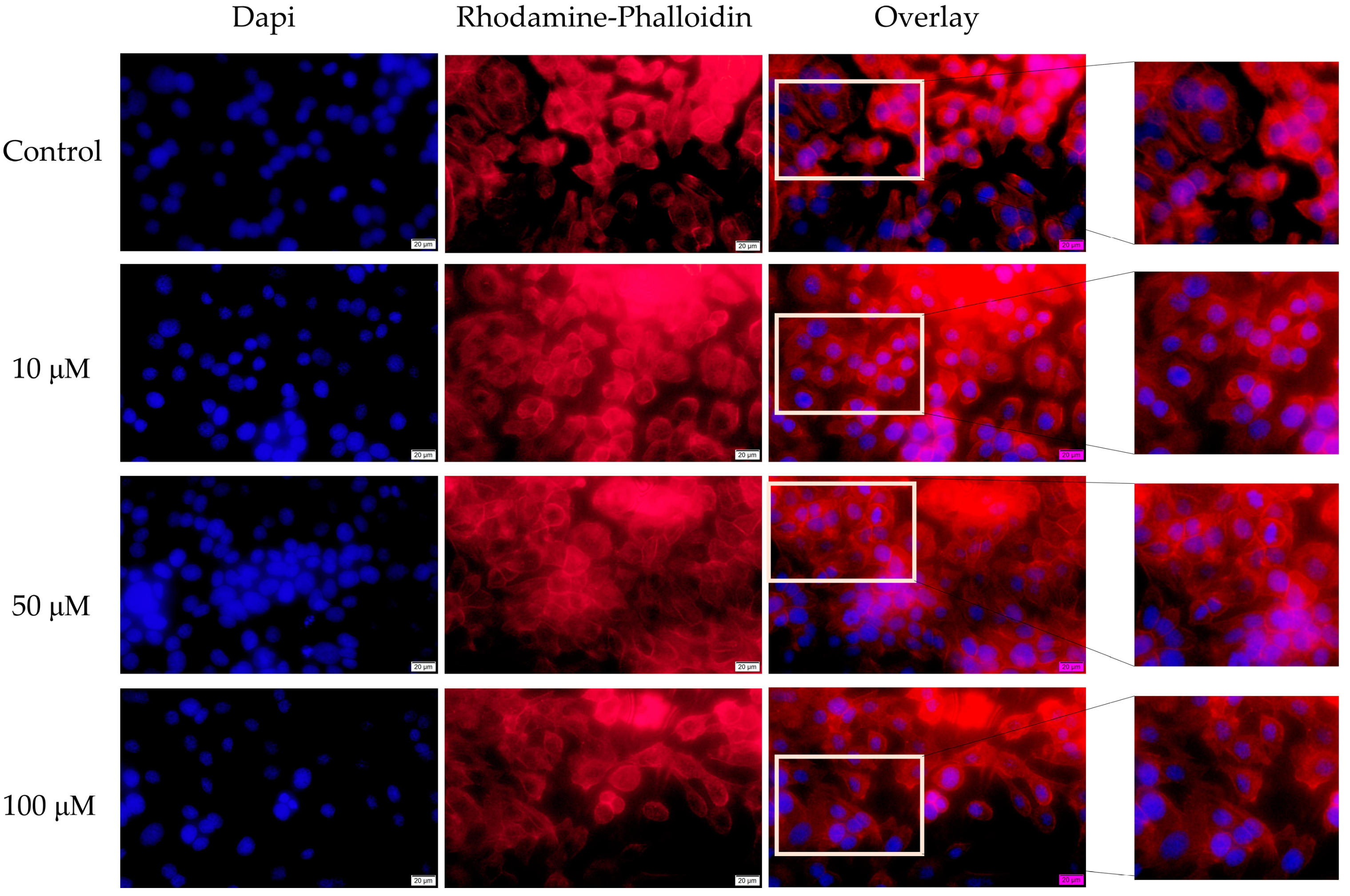
3.4. Fluorescence Immunocytochemistry
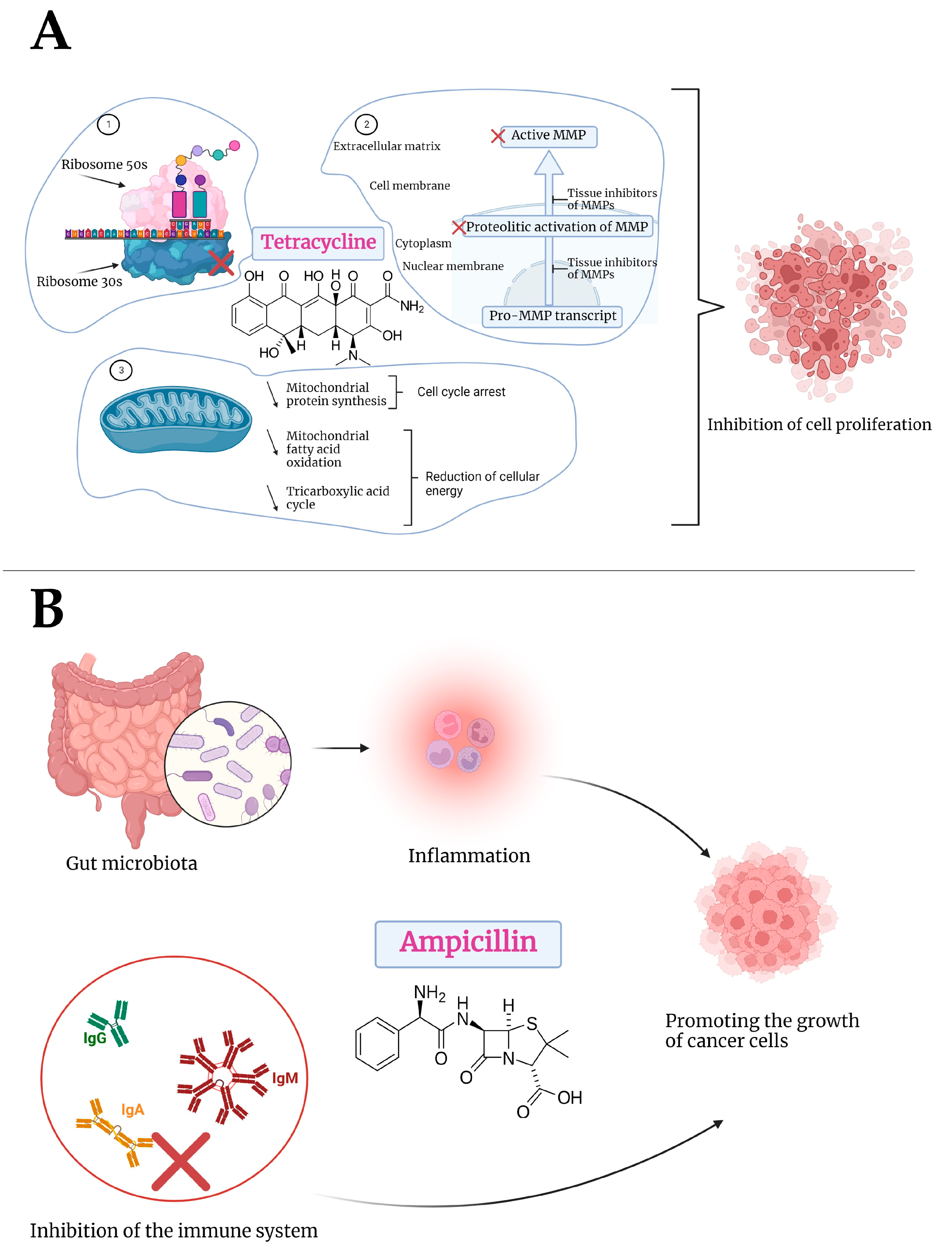
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Irani, S. New Insights into Oral Cancer-Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef]
- Ram, H.; Sarkar, J.; Kumar, H.; Konwar, R.; Bhatt, M.L.B.; Mohammad, S. Oral Cancer: Risk Factors and Molecular Pathogenesis. J. Maxillofac. Oral Surg. 2011, 10, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Nixon, I.J. Recent Advances in the Understanding and Management of Oropharyngeal Cancer. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Nenclares, P.; Bhide, S.A.; Sandoval-Insausti, H.; Pialat, P.; Gunn, L.; Melcher, A.; Newbold, K.; Nutting, C.M.; Harrington, K.J. Impact of Antibiotic Use during Curative Treatment of Locally Advanced Head and Neck Cancers with Chemotherapy and Radiotherapy. Eur. J. Cancer 2020, 131, 9–15. [Google Scholar] [CrossRef]
- Hu, B.; Elinav, E.; Huber, S.; Strowig, T.; Hao, L.; Hafemann, A.; Jin, C.; Wunderlich, C.; Wunderlich, T.; Eisenbarth, S.C. Microbiota-Induced Activation of Epithelial IL-6 Signaling Links Inflammasome-Driven Inflammation with Transmissible Cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 9862–9867. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut Microbiome Interactions with Drug Metabolism, Efficacy, and Toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Khan, A.A.; Sirsat, A.T.; Singh, H.; Cash, P. Microbiota and Cancer: Current Understanding and Mechanistic Implications. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2022, 24, 193–202. [Google Scholar] [CrossRef]
- Vogelmann, R.; Amieva, M.R. The Role of Bacterial Pathogens in Cancer. Curr. Opin. Microbiol. 2007, 10, 76–81. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef]
- Cali, F.; Cantone, M.; Cosentino, F.I.I.; Lanza, G.; Ruggeri, G.; Chiavetta, V.; Salluzzo, R.; Ragalmuto, A.; Vinci, M.; Ferri, R. Interpreting Genetic Variants: Hints from a Family Cluster of Parkinson’s Disease. J. Parkinson’s Dis. 2019, 9, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human Oral Microbiota and Its Modulation for Oral Health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef]
- Guarneri, C.; Bevelacqua, V.; Polesel, J.; Falzone, L.; Cannavò, P.S.; Spandidos, D.A.; Malaponte, G.; Libra, M. NF-κB Inhibition Is Associated with OPN/MMP-9 Downregulation in Cutaneous Melanoma. Oncol. Rep. 2017, 37, 737–746. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-KB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, G.; Guo, Y.; Zhao, Y.; Zhao, M.; Lai, Y.; Sui, P.; Shi, T.; Guo, W.; Huang, Z. Variations in Oral Microbiota Composition Are Associated with a Risk of Throat Cancer. Front. Cell. Infect. Microbiol. 2019, 9, 205. [Google Scholar] [CrossRef]
- Gong, H.; Shi, Y.; Zhou, X.; Wu, C.; Cao, P.; Xu, C.; Hou, D.; Wang, Y.; Zhou, L. Microbiota in the Throat and Risk Factors for Laryngeal Carcinoma. Appl. Environ. Microbiol. 2014, 80, 7356–7363. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells Are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Brink, A. Antibiotic Resistance and Virulence. Int. J. Infect. Dis. 2014, 21, 64. [Google Scholar] [CrossRef]
- Torralba, M.G.; Aleti, G.; Li, W.; Moncera, K.J.; Lin, Y.-H.; Yu, Y.; Masternak, M.M.; Golusinski, W.; Golusinski, P.; Lamperska, K.; et al. Oral Microbial Species and Virulence Factors Associated with Oral Squamous Cell Carcinoma. Microb. Ecol. 2021, 82, 1030–1046. [Google Scholar] [CrossRef]
- Xia, D.; Yang, X.; Liu, W.; Shen, F.; Pan, J.; Lin, Y.; Du, N.; Sun, Y.; Xi, X. Over-Expression of CHAF1A in Epithelial Ovarian Cancer can Promote Cell Proliferation and Inhibit Cell Apoptosis. Biochem. Biophys. Res. Commun. 2017, 486, 191–197. [Google Scholar] [CrossRef]
- Reuter, G. The Lactobacillus and Bifidobacterium Microflora of the Human Intestine: Composition and Succession. Curr. Issues Intest. Microbiol. 2001, 2, 43–53. [Google Scholar] [PubMed]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for Cancer Treatment: A Double-Edged Sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Nelson, M.L.; Levy, S.B. The History of the Tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Nowrouzian, F.; Adlerberth, I.; Wold, A.E. Tetracycline Resistance in Escherichia coli and Persistence in the Infantile Colonic Microbiota. Antimicrob. Agents Chemother. 2006, 50, 156–161. [Google Scholar] [CrossRef]
- Beutlich, J.; Jahn, S.; Malorny, B.; Hauser, E.; Hühn, S.; Schroeter, A.; Rodicio, M.R.; Appel, B.; Threlfall, J.; Mevius, D.; et al. Antimicrobial Resistance and Virulence Determinants in European Salmonella Genomic Island 1-Positive Salmonella Enterica Isolates from Different Origins. Appl. Environ. Microbiol. 2011, 77, 5655–5664. [Google Scholar] [CrossRef]
- Kroon, A.M.; Dontje, B.H.; Holtrop, M.; Van den Bogert, C. The Mitochondrial Genetic System as a Target for Chemotherapy: Tetracyclines as Cytostatics. Cancer Lett. 1984, 25, 33–40. [Google Scholar] [CrossRef]
- Koltai, T.; Researcher, I. Tetracyclines against Cancer. A Review. 2015. Available online: https://www.researchgate.net/publication/285051696_TETRACYCLINES_AGAINST_CANCER_A_REVIEW?channel=doi&linkId=565b076508aefe619b24250e&showFulltext=true (accessed on 6 September 2022).
- Kaushik, D.; Mohan, M.; Borade, D.M.; Swami, O.C. Ampicillin: Rise Fall and Resurgence. J. Clin. Diagn. Res. 2014, 8, ME01–ME03. [Google Scholar] [CrossRef]
- Katzung, B.G.; Trevor, A.J. Basic & Clinical Pharmacology; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Greer, L.G.; Roberts, S.W.; Sheffield, J.S.; Rogers, V.L.; Hill, J.B.; Mcintire, D.D.; Wendel, G.D. Ampicillin Resistance and Outcome Differences in Acute Antepartum Pyelonephritis. Infect. Dis. Obstet. Gynecol. 2008, 2008, 891426. [Google Scholar] [CrossRef]
- Billström, H.; Lund, B.; Sullivan, A.; Nord, C.E. Virulence and Antimicrobial Resistance in Clinical Enterococcus Faecium. Int. J. Antimicrob. Agents 2008, 32, 374–377. [Google Scholar] [CrossRef] [Green Version]
- Agents, A.; Andry, G. Antimicrobial Prophylaxis for Major Head and Neck Surgery in Cancer Patients: Sulbactam-Ampicillin versus Clindamycin-Amikacin. Antimicrob. Agents Chemother. 1992, 36, 2014–2019. [Google Scholar] [CrossRef]
- Mohammed, T.J.; Al-Ibadi, I.N.A. Ampicillin Inhibition Effect on HCT116 Cell Line. Al-Qadisiyah J. Pure Sci. 2017, 22, 185–190. [Google Scholar]
- Bartlett, J.G. Antibiotic Use in Relation to the Risk of Breast Cancer. Infect. Dis. Clin. Pract. 2004, 12, 263. [Google Scholar] [CrossRef]
- Detroit 562-CCL-138|ATCC. Available online: https://www.atcc.org/products/ccl-138 (accessed on 6 September 2022).
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab—Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef] [PubMed]
- Saman, D.M. A Review of the Epidemiology of Oral and Pharyngeal Carcinoma: Update. Head Neck Oncol. 2012, 4, 1. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The Salivary Microbiota as a Diagnostic Indicator of Oral Cancer: A Descriptive, Non-Randomized Study of Cancer-Free and Oral Squamous Cell Carcinoma Subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Aghazadeh, M.; Kafil, H.S. Role of Oral Microbiome on Oral Cancers, a Review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The Microbiome and Cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016, 70, 395–411. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The Inflammatory Microenvironment and Microbiome in Prostate Cancer Development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef]
- Frank, D.N.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.M.; Robertson, C.E.; Liu, Y.; Wang, H.; Levens, C.L.; et al. A Dysbiotic Microbiome Promotes Head and Neck Squamous Cell Carcinoma. Oncogene 2022, 41, 1269–1280. [Google Scholar] [CrossRef]
- Quintana, F.J. The Aryl Hydrocarbon Receptor: A Molecular Pathway for the Environmental Control of the Immune Response. Immunology 2013, 138, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Sherr, D.H. Aryl Hydrocarbon Receptor Control of Adaptive Immunity. Pharmacol. Rev. 2013, 65, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Stanford, E.A.; Ramirez-Cardenas, A.; Wang, Z.; Novikov, O.; Alamoud, K.; Koutrakis, P.; Mizgerd, J.P.; Genco, C.A.; Kukuruzinska, M.; Monti, S.; et al. Role for the Aryl Hydrocarbon Receptor and Diverse Ligands in Oral Squamous Cell Carcinoma Migration and Tumorigenesis. Mol. Cancer Res. 2016, 14, 696–706. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Hernández, D.L. Letter to the Editor: Use of Antibiotics, Gut Microbiota, and Risk of Type 2 Diabetes: Epigenetics Regulation. J. Clin. Endocrinol. Metab. 2016, 101, L62–L63. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S. Anticancer Antibiotics BT. In New Approaches to Natural Anticancer Drugs; Saeidnia, S., Ed.; Springer: Cham, Switzerland, 2015; pp. 51–66. ISBN 978-3-319-14027-8. [Google Scholar]
- Levy, A. Correlation between In-Vitro and In-Vivo Studies Based on Pharmacokinetic Considerations. Am. J. Biomed. Sci. Res. 2020, 8, 48–50. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and Pharmacodynamics of the Tetracyclines Including Glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef]
- Emmerson, A.M.; Cox, D.A.; Lees, L.J. Pharmacokinetics of Sulbactam and Ampicillin Following Oral Administration of Sultamicillin with Probenecid. Eur. J. Clin. Microbiol. 1983, 2, 340–344. [Google Scholar] [CrossRef]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-Inactivating Enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Bogert, C.; van Kernebeek, G.; de Leij, L.; Kroon, A.M. Inhibition of Mitochondrial Protein Synthesis Leads to Proliferation Arrest in the G1-Phase of the Cell Cycle. Cancer Lett. 1986, 32, 41–51. [Google Scholar] [CrossRef]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Nagasue, N. Tetracycline Analogues (Doxycycline and COL-3) Induce Caspase-Dependent and -Independent Apoptosis in Human Colon Cancer Cells. Int. J. Cancer 2006, 118, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Fares, M.; Maguire, K.R.; Sidén, A.; Potácová, Z. Cytotoxic Effects of Tetracycline Analogues (Doxycycline, Minocycline and COL-3) in Acute Myeloid Leukemia HL-60 Cells. PLoS ONE 2014, 9, e114457. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Bonuccelli, G.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P. Vitamin C and Doxycycline: A Synthetic Lethal Combination Therapy Targeting Metabolic Flexibility in Cancer Stem Cells (CSCs). Oncotarget 2017, 8, 67269–67286. [Google Scholar] [CrossRef]
- Funahara, M.; Yanamoto, S.; Ueda, M.; Suzuki, T.; Ota, Y.; Nishimaki, F.; Kurita, H.; Yamakawa, N.; Kirita, T.; Okura, M.; et al. Prevention of Surgical Site Infection after Oral Cancer Surgery by Topical Tetracycline: Results of a Multicenter Randomized Control Trial. J. Clin. Oncol. 2018, 36, 6080. [Google Scholar] [CrossRef]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Fujii, T.; Nagasue, N. Doxycycline Inhibits Cell Proliferation and Invasive Potential: Combination Therapy with Cyclooxygenase-2 Inhibitor in Human Colorectal Cancer Cells. J. Lab. Clin. Med. 2004, 143, 207–216. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Li, L.; Li, C. Doxycycline Inhibits Proliferation and Induces Apoptosis of Both Human Papillomavirus Positive and Negative Cervical Cancer Cell Lines. Can. J. Physiol. Pharmacol. 2016, 94, 526–533. [Google Scholar] [CrossRef]
- Lamb, R.; Ozsvari, B.; Lisanti, C.L.; Tanowitz, H.B.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Antibiotics That Target Mitochondria Effectively Eradicate Cancer Stem Cells, across Multiple Tumor Types: Treating Cancer like an Infectious Disease. Oncotarget 2015, 6, 4569–4584. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Mekkawy, A.H.; Badar, S.; Morris, D.L. Minocycline Inhibits Growth of Epithelial Ovarian Cancer. Gynecol. Oncol. 2012, 125, 433–440. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Badar, S.; Morris, D.L.; Pourgholami, M.H. Minocycline Targets the NF-ΚB Nexus through Suppression of TGF-Β1-TAK1-IκB Signaling in Ovarian CancerRegulation of NF-ΚB Pathway By Minocycline. Mol. Cancer Res. 2013, 11, 1279–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, G.; Liao, Z.; Cai, L.; Wei, R.; Sun, L. The Combined Effects of Celecoxib and Minocycline Hydrochloride on Inhibiting the Osseous Metastasis of Breast Cancer in Nude Mice. Cancer Biother. Radiopharm. 2008, 23, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yan, Y.; Li, Z.; Qian, L.; Gong, Z. The Antibiotic Drug Tigecycline: A Focus on Its Promising Anticancer Properties. Front. Pharmacol. 2016, 7, 473. [Google Scholar] [CrossRef]
- Hirasawa, K.; Moriya, S.; Miyahara, K.; Kazama, H.; Hirota, A.; Takemura, J.; Abe, A.; Inazu, M.; Hiramoto, M.; Tsukahara, K.; et al. Macrolide Antibiotics Exhibit Cytotoxic Effect under Amino Acid-Depleted Culture Condition by Blocking Autophagy Flux in Head and Neck Squamous Cell Carcinoma Cell Lines. PLoS ONE 2016, 11, e0164529. [Google Scholar] [CrossRef] [PubMed]
- Gouzos, M.; Ramezanpour, M.; Bassiouni, A.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Antibiotics Affect ROS Production and Fibroblast Migration in an In-Vitro Model of Sinonasal Wound Healing. Front. Cell. Infect. Microbiol. 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Salimiaghdam, N.; Singh, L.; Schneider, K.; Nalbandian, A.; Chwa, M.; Atilano, S.R.; Bao, A.; Kenney, M.C. Potential Adverse Effects of Ciprofloxacin and Tetracycline on ARPE-19 Cell Lines. BMJ Open Ophthalmol. 2020, 5, e000458. [Google Scholar] [CrossRef]
- Ishikawa, C.; Tsuda, T.; Konishi, H.; Nakagawa, N.; Yamanishi, K. Tetracyclines Modulate Protease-Activated Receptor 2-Mediated Proinflammatory Reactions in Epidermal Keratinocytes. Antimicrob. Agents Chemother. 2009, 53, 1760–1765. [Google Scholar] [CrossRef]
- McKee, A.M.; Kirkup, B.M.; Madgwick, M.; Fowler, W.J.; Price, C.A.; Dreger, S.A.; Ansorge, R.; Makin, K.A.; Caim, S.; Le Gall, G.; et al. Antibiotic-Induced Disturbances of the Gut Microbiota Result in Accelerated Breast Tumor Growth. iScience 2021, 24, 103012. [Google Scholar] [CrossRef]
- Zhang, J.; Haines, C.; Watson, A.J.M.; Hart, A.R.; Platt, M.J.; Pardoll, D.M.; Cosgrove, S.E.; Gebo, K.A.; Sears, C.L. Oral Antibiotic Use and Risk of Colorectal Cancer in the United Kingdom, 1989–2012: A Matched Case-Control Study. Gut 2019, 68, 1971–1978. [Google Scholar] [CrossRef]
- Iocca, O.; Copelli, C.; Ramieri, G.; Zocchi, J.; Savo, M.; Di Maio, P. Antibiotic Prophylaxis in Head and Neck Cancer Surgery: Systematic Review and Bayesian Network Meta-Analysis. Head Neck 2022, 44, 254–261. [Google Scholar] [CrossRef]
- Veve, M.P.; Greene, J.B.; Williams, A.M.; Davis, S.L.; Lu, N.; Shnayder, Y.; Li, D.X.; Noureldine, S.I.; Richmon, J.D.; Lin, L.O.; et al. Multicenter Assessment of Antibiotic Prophylaxis Spectrum on Surgical Infections in Head and Neck Cancer Microvascular Reconstruction. Otolaryngol. Neck Surg. Off. J. Am. Acad. Otolaryngol. Neck Surg. 2018, 159, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, N.; Alam, H.; Khan, A.; Raza, K.; Sardar, M. Ampicillin Silver Nanoformulations against Multidrug Resistant Bacteria. Sci. Rep. 2019, 9, 6848. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, D.-J.; Pinho, M.G. Bacterial Cell Wall Synthesis: New Insights from Localization Studies. Microbiol. Mol. Biol. Rev. 2005, 69, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Hut, E.-F.; Radulescu, M.; Pilut, N.; Macasoi, I.; Berceanu, D.; Coricovac, D.; Pinzaru, I.; Cretu, O.; Dehelean, C. Two Antibiotics, Ampicillin and Tetracycline, Exert Different Effects in HT-29 Colorectal Adenocarcinoma Cells in Terms of Cell Viability and Migration Capacity. Curr. Oncol. 2021, 28, 2466–2480. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Haynes, K.; Mamtani, R.; Yang, Y.-X. Impact of Antibiotic Exposure on the Risk of Colorectal Cancer. Pharmacoepidemiol. Drug Saf. 2015, 24, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Perego, G.; Cabiddu, M.; Khakoo, S.; Oggionni, E.; Abeni, C.; Hahne, J.C.; Tomasello, G.; et al. Use of Antibiotics and Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cancers 2019, 11, 1174. [Google Scholar] [CrossRef]
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline Antibiotics Impair Mitochondrial Function and Its Experimental Use Confounds Research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef]
- Hanemaaijer, R.; van Lent, N.; Sorsa, T.; Salo, T.; Yrj, Ő.; Konttinen, T.; Lindeman, J. Inhibition of matrix metalloproteinases (MMPs) by tetracyclines BT. In Tetracyclines in Biology, Chemistry and Medicine; Nelson, M., Hillen, W., Greenwald, R.A., Eds.; Birkhäuser: Basel, Switzerland, 2001; pp. 267–281. ISBN 978-3-0348-8306-1. [Google Scholar]
- Fife, R.S.; Sledge, G.W.J.; Sissons, S.; Zerler, B. Effects of Tetracyclines on Angiogenesis In Vitro. Cancer Lett. 2000, 153, 75–78. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; Zhang, F.; Vlashi, E. Doxycycline Inhibits the Cancer Stem Cell Phenotype and Epithelial-to-Mesenchymal Transition in Breast Cancer. Cell Cycle 2017, 16, 737–745. [Google Scholar] [CrossRef]
- Lamb, R.; Fiorillo, M.; Chadwick, A.; Ozsvari, B.; Reeves, K.J.; Smith, D.L.; Clarke, R.B.; Howell, S.J.; Cappello, A.R.; Martinez-Outschoorn, U.E.; et al. Doxycycline Down-Regulates DNA-PK and Radiosensitizes Tumor Initiating Cells: Implications for More Effective Radiation Therapy. Oncotarget 2015, 6, 14005–14025. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pancu, D.F.; Racea, R.C.; Macasoi, I.; Sarau, C.A.; Pinzaru, I.; Poenaru, M.; Rusu, L.-C.; Dehelean, C.A.; Dinu, S. Assessment of the In Vitro Cytotoxic Profile of Two Broad-Spectrum Antibiotics—Tetracycline and Ampicillin—On Pharyngeal Carcinoma Cells. Medicina 2022, 58, 1289. https://doi.org/10.3390/medicina58091289
Pancu DF, Racea RC, Macasoi I, Sarau CA, Pinzaru I, Poenaru M, Rusu L-C, Dehelean CA, Dinu S. Assessment of the In Vitro Cytotoxic Profile of Two Broad-Spectrum Antibiotics—Tetracycline and Ampicillin—On Pharyngeal Carcinoma Cells. Medicina. 2022; 58(9):1289. https://doi.org/10.3390/medicina58091289
Chicago/Turabian StylePancu, Daniel Florin, Robert Cosmin Racea, Ioana Macasoi, Cristian Andrei Sarau, Iulia Pinzaru, Marioara Poenaru, Laura-Cristina Rusu, Cristina Adriana Dehelean, and Stefania Dinu. 2022. "Assessment of the In Vitro Cytotoxic Profile of Two Broad-Spectrum Antibiotics—Tetracycline and Ampicillin—On Pharyngeal Carcinoma Cells" Medicina 58, no. 9: 1289. https://doi.org/10.3390/medicina58091289
APA StylePancu, D. F., Racea, R. C., Macasoi, I., Sarau, C. A., Pinzaru, I., Poenaru, M., Rusu, L.-C., Dehelean, C. A., & Dinu, S. (2022). Assessment of the In Vitro Cytotoxic Profile of Two Broad-Spectrum Antibiotics—Tetracycline and Ampicillin—On Pharyngeal Carcinoma Cells. Medicina, 58(9), 1289. https://doi.org/10.3390/medicina58091289









