Evaluation of Macular Ganglion Cell-Inner Plexiform Layer in Children with Deprivational Amblyopia Who Underwent Unilateral Cataract Surgery
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Intraoperative Procedures
2.5. Examination
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simons, K. Amblyopia characterization, treatment, and prophylaxis. Surv. Ophthalmol. 2005, 50, 123–166. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; O’Keefe, M.; Lanigan, B. A follow-on study on vision-related quality of life assessment using the NEI-VFQ-25 in those with a history of unilateral and bilateral congenital cataracts. Acta Ophthalmol. 2018, 96, 596–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.E.; Hennig, A.; Trivedi, R.H.; Thomas, B.J.; Singh, S.K. Clinical characteristics and early postoperative outcomes of pediatric cataract surgery with IOL implantation from Lahan, Nepal. J. Pediatr. Ophthalmol. Strabismus 2011, 48, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yu, Y.S.; Kim, J.H.; Kim, S.J.; Choung, H.K. Visual function after primary posterior chamber intraocular lens implantation in pediatric unilateral cataract: Stereopsis and visual acuity. Korean J. Ophthalmol. 2007, 21, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xie, L.; Wu, X.; Tian, J. Long-term results of pediatric cataract surgery after delayed diagnosis. J. AAPOS 2012, 16, 65–69. [Google Scholar] [CrossRef]
- Hess, R.F.; Thompson, B.; Gole, G.; Mullen, K.T. Deficient responses from the lateral geniculate nucleus in humans with amblyopia. Eur. J. Neurosci. 2009, 29, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Hubel, D.H.; Wiesel, T.N. Binocular interaction in striate cortex of kittens reared with artificial squint. J. Neurophysiol. 1965, 28, 1041–1059. [Google Scholar] [CrossRef] [Green Version]
- von Noorden, G.K.; Crawford, M.L. The lateral geniculate nucleus in human strabismic amblyopia. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2729–2732. [Google Scholar]
- von Noorden, G.K.; Crawford, M.L.; Middleditch, P.R. Effect of lid suture on retinal ganglion cells in Macaca mulatta. Brain Res. 1977, 122, 437–444. [Google Scholar] [CrossRef]
- Chow, K.L.; Riesen, A.H.; Newell, F.W. Degeneration of retinal ganglion cells in infant chimpanzees reared in darkness. J. Comp. Neurol. 1957, 107, 27–42. [Google Scholar] [CrossRef]
- Rasch, E.; Swift, H.; Riesen, A.H.; Chow, K.L. Altered structure and composition of retinal cells in darkreared mammals. Exp. Cell Res. 1961, 25, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Fifková, E. Effect of visual deprivation and light on synapses of the inner plexiform layer. Exp. Neurol. 1972, 35, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sosula, L.; Glow, P.H. Increase in number of synapses in the inner plexiform layer of light deprived rat retinae: Quantitative electron microscopy. J. Comp. Neurol. 1971, 141, 427–451. [Google Scholar] [CrossRef]
- Weiskrantz, L. Sensory deprivation and the cat’s optic nervous system. Nature 1958, 181, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Arden, G.B.; Wooding, S.L. Pattern ERG in amblyopia. Investig. Ophthalmol. Vis. Sci. 1985, 26, 88–96. [Google Scholar]
- Arden, G.B.; Vaegan; Hogg, C.R.; Powell, D.J.; Carter, R.M. Pattern ERGs are abnormal in many amblyopes. Trans. Ophthalmol. Soc. UK 1980, 100, 453–460. [Google Scholar]
- Kim, Y.W.; Kim, S.J.; Yu, Y.S. Spectral-domain optical coherence tomography analysis in deprivational amblyopia: A pilot study with unilateral pediatric cataract patients. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 2811–2819. [Google Scholar] [CrossRef]
- Yilmaz Cinar, F.G.; Ozkan, G. Macular capillary system and ganglion cell-layer complex of the amblyopic eye with optical cohorence tomography angiography and optical cohorence tomography. Int. Ophthalmol. 2021, 41, 675–686. [Google Scholar] [CrossRef]
- Dereli Can, G. Quantitative analysis of macular and peripapillary microvasculature in adults with anisometropic amblyopia. Int. Ophthalmol. 2020, 40, 1765–1772. [Google Scholar] [CrossRef]
- Nishikawa, N.; Chua, J.; Kawaguchi, Y.; Ro-Mase, T.; Schmetterer, L.; Yanagi, Y.; Yoshida, A. Macular Microvasculature and Associated Retinal Layer Thickness in Pediatric Amblyopia: Magnification-Corrected Analyses. Investig. Ophthalmol. Vis. Sci. 2021, 62, 39. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, H.; Cheng, H.; Liu, Q.; Yuan, D. Evaluation of the Changes in Vessel Density and Retinal Thickness in Patients Who Underwent Unilateral Congenital Cataract Extraction by OCTA. Clin. Ophthalmol. 2020, 14, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Miki, A.; Goto, K.; Yamashita, T.; Takizawa, G.; Haruishi, K.; Ieki, Y.; Kiryu, J.; Yaoeda, K. Macular retinal and choroidal thickness in unilateral amblyopia using swept-source optical coherence tomography. BMC Ophthalmol. 2017, 17, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, P.; Ram, J.; Sukhija, J.; Singh, R.; Gupta, A. Retinal Nerve Fiber Layer and Macular Thickness Measurements in Children After Cataract Surgery Compared with Age-Matched Controls. Am. J. Ophthalmol. 2016, 166, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Chen, J.; Liu, Z.; Lin, Z.; Cao, Q.; Zhang, X.; Li, X.; Luo, L.; Lin, H.; Chen, W.; et al. Interocular anatomical and visual functional differences in pediatric patients with unilateral cataracts. BMC Ophthalmol. 2016, 16, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Smith, H.A.; Donaldson, D.L.; Haider, K.M.; Roberts, G.J.; Sprunger, D.T.; Neely, D.E.; Plager, D.A. Macular structural characteristics in children with congenital and developmental cataracts. J. AAPOS 2014, 18, 417–422. [Google Scholar] [CrossRef]
- Hansen, M.M.; Bach Holm, D.; Kessel, L. Associations between visual function and ultrastructure of the macula and optic disc after childhood cataract surgery. Acta Ophthalmol. 2022, 100, 640–647. [Google Scholar] [CrossRef]
- Park, K.A.; Park, D.Y.; Oh, S.Y. Analysis of spectral-domain optical coherence tomography measurements in amblyopia: A pilot study. Br. J. Ophthalmol. 2011, 95, 1700–1706. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, H.; Zheng, S. Thicknesses of Macular Inner Retinal Layers in Children with Anisometropic Amblyopia. Biomed. Res. Int. 2020, 19, 6853258. [Google Scholar] [CrossRef]
- Tugcu, B.; Araz-Ersan, B.; Kilic, M.; Erdogan, E.T.; Yigit, U.; Karamursel, S. The morpho-functional evaluation of retina in amblyopia. Curr. Eye Res. 2013, 38, 802–809. [Google Scholar] [CrossRef]
- Guagliano, R.; Barillà, D.; Bertone, C.; Montescani, S.; Verticchio Vercellin, A.C.; Arpa, C.; Tinelli, C.; De Angelis, G.; Quaranta, L. Evaluation of macular and optic nerve head parameters in amblyopic eyes: An optical coherence tomography study. Eur. J. Ophthalmol. 2022, 32, 1991–1996. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Hubel, D.H. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J. Neurophysiol. 1963, 26, 978–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Noorden, G.K. Histological studies of the visual system in monkeys with experimental amblyopia. Investig. Ophthalmol. 1973, 12, 727–738. [Google Scholar]
- Littmann, H. Determination of the real size of an object on the fundus of the living eye. Klin. Monbl. Augenheilkd. 1982, 180, 286–289. [Google Scholar] [CrossRef]
- Bennett, A.G.; Rudnicka, A.R.; Edgar, D.F. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes. Arch. Clin. Exp. Ophthalmol. 1994, 232, 361–367. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.W.; Im, S.K.; Lee, S.H.; Ahn, M.D. Effect of myopia on the thickness of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4075–4083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineles, S.L.; Demer, J.L. Bilateral abnormalities of optic nerve size and eye shape in unilateral amblyopia. Am. J. Ophthalmol. 2009, 148, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leguire, L.E.; Rogers, G.L.; Bremer, D.L. Amblyopia: The normal eye is not normal. J. Pediatr. Ophthalmol. Strabismus 1990, 27, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A. The use of the scanning laser ophthalmoscope in the evaluation of amblyopia (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 414–436. [Google Scholar]
- Hooks, B.M.; Chen, C. Critical periods in the visual system: Changing views for a model of experience-dependent plasticity. Neuron 2007, 56, 312–326. [Google Scholar] [CrossRef] [Green Version]
- Tian, N. Synaptic activity, visual experience and the maturation of retinal synaptic circuitry. J. Physiol. 2008, 586, 4347–4355. [Google Scholar] [CrossRef]
- Lee, H.; Purohit, R.; Patel, A.; Papageorgiou, E.; Sheth, V.; Maconachie, G.; Pilat, A.; McLean, R.J.; Proudlock, F.A.; Gottlob, I. In Vivo Foveal Development Using Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4537–4545. [Google Scholar] [CrossRef] [PubMed]
| Deprivational Amblyopic Eyes (22 Eyes) (Mean ± SD) | Fellow Non-Amblyopic Eyes (22 Eyes) (mean ± SD) | Normal Eyes (22 Eyes) (Mean ± SD) | p1-Value † | p2-Value ‡ | |
|---|---|---|---|---|---|
| Age (years) | 9.73 ± 2.85 | 9.36 ± 2.46 | - | 0.653 | |
| Male (n %) | 14 (63.6) | 12 (54.5) | - | 0.540 # | |
| Vision (LogMAR) | 0.75 ± 0.27 | 0.1 ± 0.13 | 0.04 ± 0.07 | 0.000 *§ | 0.000 *¦ |
| SE (D) | −1.51 ± 2.29 | −1.73 ± 2.22 | −1.86 ± 2.79 | 0.747 | 0.646 |
| Axial length (mm) | 23.89 ± 1.7 | 24.18 ± 1.77 | 24.54 ± 2.08 | 0.599 | 0.272 |
| OCT scan signal strength | 0.8 ± 0.1 | 0.81 ± 0.11 | 0.83 ± 0.13 | 0.916 | 0.496 |
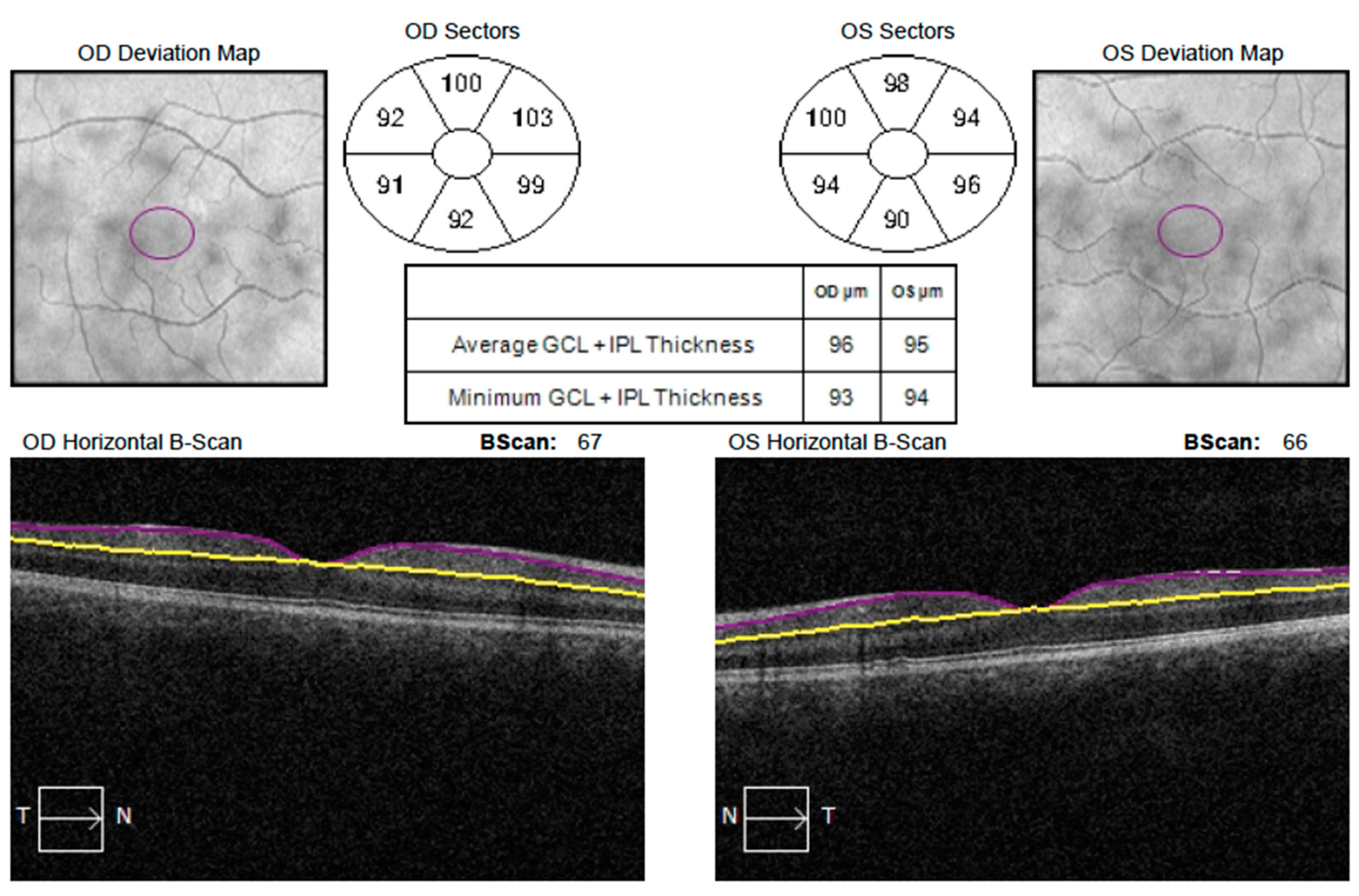
| GCIPL Thickness (μm) | Deprivational Amblyopic Eyes (22 Eyes) (Mean ± SD) | Fellow Non-Amblyopic Eyes (22 Eyes) (Mean ± SD) | Normal Eyes (22 Eyes) (Mean ± SD) | p1-Value † | p2-Value ‡ |
|---|---|---|---|---|---|
| Average | 70.68 ± 11.28 | 77.50 ± 6.72 | 81.73 ± 5.18 | 0.038 *§ | 0.0005 *¦ |
| Minimum | 62.68 ± 13.20 | 70.36 ± 7.61 | 74.50 ± 5.47 | 0.023 * | 0.0004 * |
| Superior | 78.68 ± 10.06 | 80.45 ± 7.76 | 84.18 ± 5.84 | 0.869 § | 0.103 ¦ |
| Superonasal | 76.86 ± 9.89 | 81.27 ± 7.47 | 83.09 ± 6.04 | 0.236 § | 0.056 ¦ |
| Inferonasal | 74.09 ± 13.38 | 79.32 ± 7.73 | 81.64 ± 4.45 | 0.265 § | 0.116 ¦ |
| Inferior | 68.23 ± 13.53 | 73.68 ± 8.87 | 78.36 ± 5.77 | 0.173 § | 0.008 *¦ |
| Inferotemporal | 70.54 ± 11.33 | 76.86 ± 7.45 | 79.82 ± 5.29 | 0.025 *§ | 0.003 *¦ |
| Superotemporal | 69.59 ± 11.56 | 77.36 ± 8.64 | 79.73 ± 6.07 | 0.007 *§ | 0.001 *¦ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świerczyńska, M.; Tronina, A.; Filipek-Janiszewski, B.; Filipek, E. Evaluation of Macular Ganglion Cell-Inner Plexiform Layer in Children with Deprivational Amblyopia Who Underwent Unilateral Cataract Surgery. Medicina 2023, 59, 13. https://doi.org/10.3390/medicina59010013
Świerczyńska M, Tronina A, Filipek-Janiszewski B, Filipek E. Evaluation of Macular Ganglion Cell-Inner Plexiform Layer in Children with Deprivational Amblyopia Who Underwent Unilateral Cataract Surgery. Medicina. 2023; 59(1):13. https://doi.org/10.3390/medicina59010013
Chicago/Turabian StyleŚwierczyńska, Marta, Agnieszka Tronina, Bartosz Filipek-Janiszewski, and Erita Filipek. 2023. "Evaluation of Macular Ganglion Cell-Inner Plexiform Layer in Children with Deprivational Amblyopia Who Underwent Unilateral Cataract Surgery" Medicina 59, no. 1: 13. https://doi.org/10.3390/medicina59010013
APA StyleŚwierczyńska, M., Tronina, A., Filipek-Janiszewski, B., & Filipek, E. (2023). Evaluation of Macular Ganglion Cell-Inner Plexiform Layer in Children with Deprivational Amblyopia Who Underwent Unilateral Cataract Surgery. Medicina, 59(1), 13. https://doi.org/10.3390/medicina59010013






