Metastatic Renal Cell Carcinoma to the Soft Tissue 27 Years after Radical Nephrectomy: A Case Report
Abstract
:1. Introduction
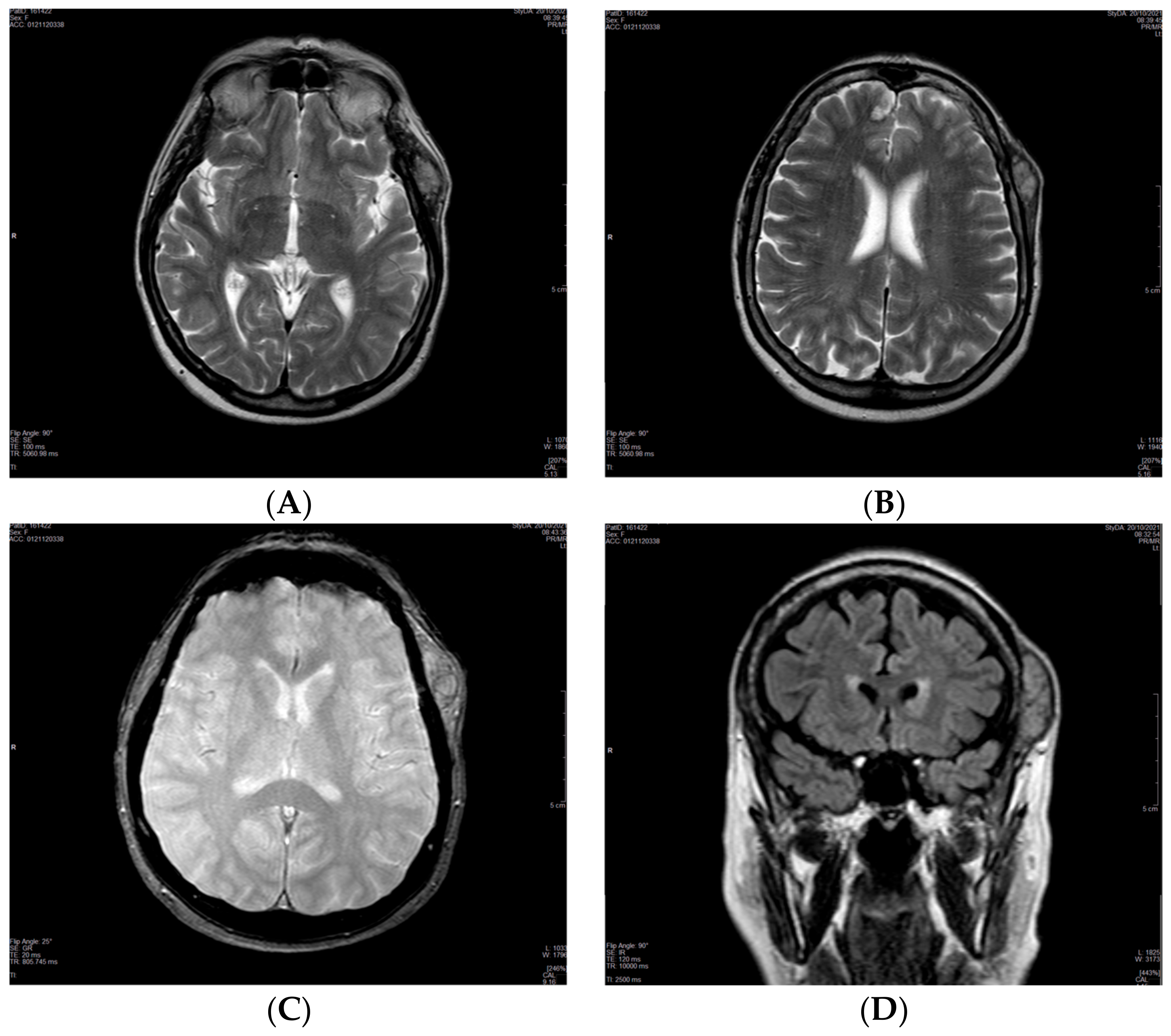
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, E.C.; Evans, C.P.; Lara, P.N. Renal cell carcinoma: Current status and emerging therapies. Cancer Treat. Rev. 2007, 33, 299–313. [Google Scholar] [CrossRef]
- Staderini, F.; Cianchi, F.; Badii, B.; Skalamera, I.; Fiorenza, G.; Foppa, C.; Qirici, E.; Perigli, G. A unique presentation of a renal clear cell carcinoma with atypical metastases. Int. J. Surg. Case Rep. 2015, 11, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Tosco, L.; Van Poppel, H.; Frea, B.; Gregoraci, G.; Joniau, S. Survival and Impact of Clinical Prognostic Factors in Surgically Treated Metastatic Renal Cell Carcinoma. Eur. Urol. 2013, 63, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Gay, H.A.; Cavalieri, R.; Allison, R.R.; Finley, J.; Quan, W.D., Jr. Complete response in a cutaneous facial metastatic nodule from renal cell carcinoma after hypofractionated radiotherapy. Dermatol. Online J. 2007, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Gutiérrez, G.; Fuentes-Valencia, A.; Salaverría-Cáceres, J.; Vela-Jiménez, G. Metástasis al cuero cabelludo de carcinoma de células claras [Metastasis to scalp of clear cell carcinoma]. Actas Urol. Esp. 2010, 34, 923–924. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Matias, M.; Casa-Nova, M.; Borges-Costa, J.; Ribeiro, L. Unusual head metastasis of kidney cancer. BMJ Case Rep. 2013, 2013, bcr2013200004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feola, A.; De Simone, M.; Ciamarra, P.; Sica, S.; Buonomo, C.; Carfora, A.; Campobasso, C.P. Chronic Encapsulated Intracerebral Hematoma as an Occasional Finding in Sudden Cardiac Death. Healthcare 2022, 10, 2053. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P. Head and Neck Surgery Residency during COVID-19 Pandemic. Lessons from Southern Italy. Transl. Med. UniSa 2020, 23, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, G. The Dormant Cancer Cell. BMJ 1954, 2, 607–610. [Google Scholar] [CrossRef]
- Udagawa, T. Tumor dormancy of primary and secondary cancers. Apmis 2008, 116, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Citro, M.; Caputo, M.; Pisanti, S.; Martinelli, R. Psychological Stress and Cancer: New Evidence of An Increasingly Strong Link. Transl. Med. UniSa 2020, 23, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F. Influence of diet on metastasis and tumor dormancy. Clin. Exp. Metastasis 2008, 26, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Eisen, T.; Porta, C.; Patard, J.J.; Khoo, V.; Algaba, F.; Mulders, P.; Kataja, V.; ESMO Guidelines Working Group. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii65–vii71. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Yang, S.-F.; Ke, H.-L.; Lee, K.-S.; Huang, C.-H.; Wu, W.-J. Renal Cell Carcinoma Presenting with Skull Metastasis: A Case Report and Literature Review. Kaohsiung J. Med. Sci. 2007, 23, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Kandemir, N.O.; Barut, F.; Yılmaz, K.; Tokgöz, H.; Hosnuter, M.; Ozdamar, S.O. Renal Cell Carcinoma Presenting with Cutaneous Metastasis: A Case Report. Case Rep. Med. 2010, 2010, 913734. [Google Scholar] [CrossRef]
- Damron, T.A.; Heiner, J. Distant Soft Tissue Metastases: A Series of 30 New Patients and 91 Cases from the Literature. Ann. Surg. Oncol. 2000, 7, 526–534. [Google Scholar] [CrossRef]
- Chin, W.; Cao, L.; Liu, X.; Ye, Y.; Liu, Y.; Yu, J.; Zheng, S. Metastatic renal cell carcinoma to the pancreas and subcutaneous tissue 10 years after radical nephrectomy: A case report. J. Med. Case Rep. 2020, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Bacik, J.; Schwartz, L.H.; Reuter, V.; Russo, P.; Marion, S.; Mazumdar, M. Prognostic Factors for Survival in Previously Treated Patients with Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2004, 22, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, H.R.; Kamyab, K.; Daneshpazhooh, M. Cutaneous metastasis of renal cell carcinoma: A case report. Dermatol. Online J. 2012, 18, 12. [Google Scholar] [CrossRef]
- Klatte, T.; Seligson, D.B.; LaRochelle, J.; Shuch, B.; Said, J.W.; Riggs, S.B.; Zomorodian, N.; Kabbinavar, F.F.; Pantuck, A.J.; Belldegrun, A.S. Molecular Signatures of Localized Clear Cell Renal Cell Carcinoma to Predict Disease-Free Survival after Nephrectomy. Cancer Epidemiol. Biomark. Prev. 2009, 18, 894–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.P.; Weight, C.J.; Leibovich, B.C.; Thompson, R.H.; Costello, B.A.; Cheville, J.C.; Lohse, C.M.; Boorjian, S.A. Outcomes and Clinicopathologic Variables Associated with Late Recurrence After Nephrectomy for Localized Renal Cell Carcinoma. Urology 2011, 78, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Hongo, F.; Saitoh, M.; Tsuruyama, K.; Shimoo, K.; Yamamura, Y. Renal cell carcinoma with late skin metastasis: A case report. Hinyokika kiyo. Acta Urol. Jpn. 1999, 45, 415–417. (In Japanese) [Google Scholar]
- Antonelli, A.; Arrighi, N.; Corti, S.; Legramanti, S.; Zanotelli, T.; Cozzoli, A.; Cunico, S.C.; Simeone, C. Surgical treatment of atypical metastasis from renal cell carcinoma (RCC). BJU Int. 2012, 110, E559–E563. [Google Scholar] [CrossRef]
- Kerst, J.M.; Bex, A.; Mallo, H.; Dewit, L.; Haanen, J.B.A.G.; Boogerd, W.; Teertstra, H.J.; De Gast, G.C. Prolonged low dose IL-2 and thalidomide in progressive metastatic renal cell carcinoma with concurrent radiotherapy to bone and/or soft tissue metastasis: A phase II study. Cancer Immunol. Immunother. 2005, 54, 926–931. [Google Scholar] [CrossRef]
- Paolino, G.; Cardone, M.; Didona, D.; Moliterni, E.; Losco, L.; Corsetti, P.; Schipani, G.; Lopez, T.; Calvieri, S.; Bottoni, U. Prognostic factors in head and neck melanoma according to facial aesthetic units. G Ital. Dermatol. Venereol. 2020, 155, 41–45. [Google Scholar] [CrossRef]
- Kawakita, D.; Matsuo, K. Alcohol and head and neck cancer. Cancer Metastasis Rev. 2017, 36, 425–434. [Google Scholar] [CrossRef]
- Lo Torto, F.; Redi, U.; Cigna, E.; Losco, L.; Marcasciano, M.; Casella, D.; Ciudad, P.; Ribuffo, D. Nasal Reconstruction with Two Stages Versus Three Stages Forehead Fap: What is Better for Patients with High Vascular Risk? J. Craniofacial Surg. 2020, 31, e57–e60. [Google Scholar] [CrossRef]
- Chiummariello, S.; Dessy, L.; Buccheri, E.; Gagliardi, D.; Menichini, G.; Alfano, C.; Scuderi, N. An approach to managing non-melanoma skin cancer of the nose with mucosal invasion: Our experience. Acta Oto-Laryngologica 2008, 128, 915–919. [Google Scholar] [CrossRef]
- Younes, E.; Haas, G.P.; Dezso, B.; Ali, E.; Maughan, R.L.; Kukuruga, M.A.; Montecillo, E.; Pontes, J.; Hillman, G.G. Local Tumor Irradiation Augments the Response to IL-2 Therapy in a Murine Renal Adenocarcinoma. Cell. Immunol. 1995, 165, 243–251. [Google Scholar] [CrossRef]
- Dezso, B.; Haas, G.P.; Hamzavi, F.; Kim, S.; Montecillo, E.J.; Benson, P.D.; Pontes, J.E.; Maughan, R.L.; Hillman, G.G. The mechanism of local tumor irradiation combined with interleukin 2 therapy in murine renal carcinoma: Histological evaluation of pulmonary me-tastases. Clin. Cancer Res. 1996, 2, 1543–1552. [Google Scholar] [PubMed]
- Terada, T. Cutaneous metastasis of renal cell carcinoma: A report of two cases. Int. J. Clin. Exp. Pathol. 2012, 5, 175–178. [Google Scholar] [PubMed]


| Time | Event |
|---|---|
| 1993 | Right radical nephrectomy caused by renal cell carcinoma. |
| November 2020 | Swelling in the left temporal region for 2 months. |
| 24 November 2020 | Ultrasonography: hypoechoic oval mass lesion measuring 30 × 10 mm. |
| 27 November 2020 | Otolaryngological examination: soft and movable mass lesion was reported. Surgical excision was refused by the patient. |
| October 2021 | Plastic surgery visit due to the growth of the mass: hard and non-movable mass. |
| 20 October 2021 | MRI of the brain: single 2.5 × 5 cm soft tissue temporal lesion. Suspected benign lesion. |
| 2 November 2021 | Surgical resection attempted and not accomplished due to massive bleeding. |
| 13 January 2022 | Cytological examination without diagnosis. |
| February 2022 | Biopsy of the lesion: metastasis of renal cell carcinoma. |
| 7 March 2022 | The patient was directed to the Cancer Board for evaluation. |
| 5 April 2022 | Total body CT scan: absence of involvement of other sites. |
| June 2022 | Drug treatment with Pazopanib 800 mg/daily started. Dose was reduced to 200 mg/daily and 400 mg/daily on alternate days due to blood pressure peaks. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marra, C.; Losco, L.; Ceccaroni, A.; Pentangelo, P.; Troisi, D.; Alfano, C. Metastatic Renal Cell Carcinoma to the Soft Tissue 27 Years after Radical Nephrectomy: A Case Report. Medicina 2023, 59, 150. https://doi.org/10.3390/medicina59010150
Marra C, Losco L, Ceccaroni A, Pentangelo P, Troisi D, Alfano C. Metastatic Renal Cell Carcinoma to the Soft Tissue 27 Years after Radical Nephrectomy: A Case Report. Medicina. 2023; 59(1):150. https://doi.org/10.3390/medicina59010150
Chicago/Turabian StyleMarra, Caterina, Luigi Losco, Alessandra Ceccaroni, Paola Pentangelo, Donato Troisi, and Carmine Alfano. 2023. "Metastatic Renal Cell Carcinoma to the Soft Tissue 27 Years after Radical Nephrectomy: A Case Report" Medicina 59, no. 1: 150. https://doi.org/10.3390/medicina59010150





