Antibiotic Resistance of Helicobacter pylori in Patients with Peptic Ulcer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Esophagogastroduodenoscopy and Biopsies
2.3. H. pylori Culture and Antibiogram
2.4. Statistically Analysis
3. Results
3.1. Characteristics of Participants and Gastroduodenal Lesions
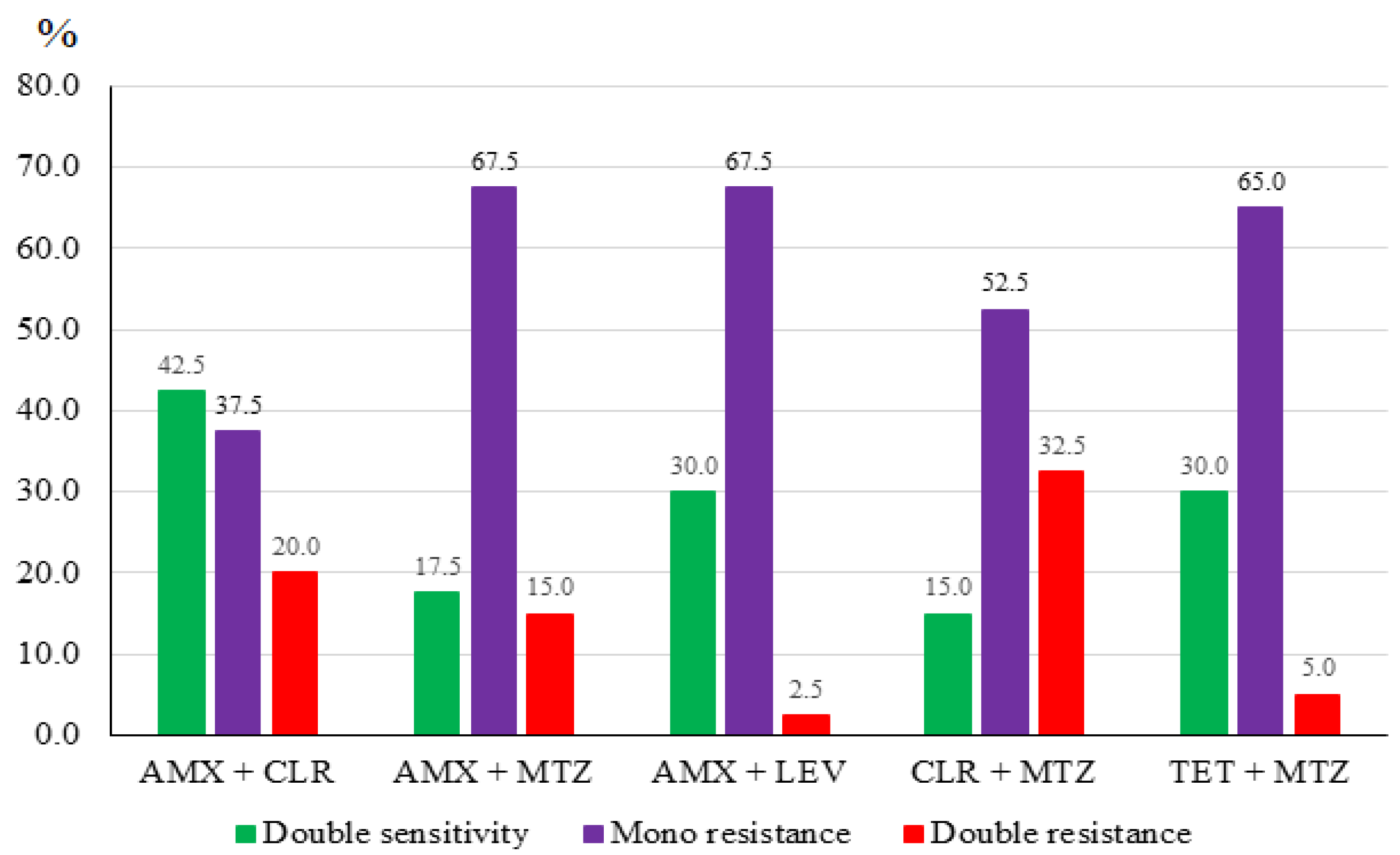
3.2. H. pylori Culture and Antibiotic Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter Pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eusebi, L.H.; Zagari, R.M.; Bazzoli, F. Epidemiology of Helicobacter Pylori Infection. Helicobacter 2014, 19 (Suppl. 1), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Li, M. Helicobacter Pylori Eradication for Preventing Gastric Cancer. World J. Gastroenterol. 2014, 20, 5660–5665. [Google Scholar] [CrossRef]
- Saleem, N.; Howden, C.W. Update on the Management of Helicobacter Pylori Infection. Curr. Treat. Options Gastroenterol. 2020, 18, 476–487. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Bazzoli, F.; El-Omar, E.; Graham, D.; Hunt, R.; Rokkas, T.; Vakil, N.; Kuipers, E.J. Current Concepts in the Management of Helicobacter Pylori Infection: The Maastricht III Consensus Report. Gut 2007, 56, 772–781. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.R.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T.; et al. Management of Helicobacter Pylori Infection—The Maastricht IV/Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Ho, T.T.M.; Nguyen-Hoang, T.-P.; Qumar, S.; Pham, T.T.D.; Bui, Q.N.; Bulach, D.; Nguyen, T.-V.; Rahman, M. The Endemic Helicobacter Pylori Population in Southern Vietnam Has Both South East Asian and European Origins. Gut Pathog. 2021, 13, 57. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Bengtsson, C.; Phung, D.C.; Sörberg, M.; Granström, M. Seroprevalence of Helicobacter Pylori Infection in Urban and Rural Vietnam. Clin. Vaccine Immunol. 2005, 12, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Binh, T.T.; Tuan, V.P.; Dung, H.D.Q.; Tung, P.H.; Tri, T.D.; Thuan, N.P.M.; Tam, L.Q.; Nam, B.C.; Giang, D.A.; Hoan, P.Q.; et al. Molecular Epidemiology of Helicobacter Pylori Infection in a Minor Ethnic Group of Vietnam: A Multiethnic, Population-Based Study. Int. J. Mol. Sci. 2018, 19, 708. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, A.T.; Quach, D.T.; Pham, D.T.-H.; Cao, N.M.; Nguyen, U.T.-H.; Dang, A.N.-T.; Tran, M.A.; Quach, L.H.; Tran, K.T.; et al. Emergence of Amoxicillin Resistance and Identification of Novel Mutations of the Pbp1A Gene in Helicobacter Pylori in Vietnam. BMC Microbiol. 2022, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M.; Howe, R.A. BSAC Working Party on Susceptibility Testing BSAC Standardized Disc Susceptibility Testing Method (Version 10). J. Antimicrob. Chemother. 2011, 66, 2726–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bộ Y Tế. Cục Quản Lý Khám Chữa Bệnh. Quyết định số 6769/QĐ-BYT Ngày 08 Tháng 11 Năm 2018 Của Bộ Trưởng Bộ Y Tế Về Việc Ban Hành Tài…. Available online: https://kcb.vn/quy-trinh/quyet-dinh-so-6769-qd-byt-ngay-08-thang-11-nam-2018-cua-bo-t.html (accessed on 12 November 2022).
- Le, L.T.T.; Nguyen, T.A.; Nguyen, N.A.; Nguyen, Y.T.H.; Nguyen, H.T.B.; Nguyen, L.T.; Vi, M.T.; Nguyen, T. Helicobacter Pylori Eradication Efficacy of Therapy Based on the Antimicrobial Susceptibility in Children with Gastritis and Peptic Ulcer in Mekong Delta, Vietnam. Children 2022, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Le, L.T.T.; Nguyen, T.A.; Nguyen, N.A.; Nguyen, Y.T.H.; Nguyen, H.T.B.; Nguyen, L.T.; Vi, M.T.; Nguyen, T. Antibiotic Resistance of Helicobacter Pylori in Children with Gastritis and Peptic Ulcers in Mekong Delta, Vietnam. Healthcare 2022, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 8.0. 2018. Available online: http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/ (accessed on 12 November 2022).
- Al Humayed, M.B.S.; SBIM, A. Low Recovery Rate of Helicobacter Pylori from Positive CLO Test Patients Suffering from Dyspepsia. Bahrain Med. Bull. 2015, 37, 117–120. [Google Scholar] [CrossRef]
- Galoș, F.; Năstase, G.; Boboc, C.; Coldea, C.; Anghel, M.; Orzan, A.; Bălgrădean, M. A Study of the Correlation between Bacterial Culture and Histological Examination in Children with Helicobacter Pylori Gastritis; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Campuzano-Maya, G. Hematologic Manifestations of Helicobacter Pylori Infection. World J. Gastroenterol. 2014, 20, 12818–12838. [Google Scholar] [CrossRef]
- Binh, T.T.; Shiota, S.; Nguyen, L.T.; Ho, D.D.Q.; Hoang, H.H.; Ta, L.; Trinh, D.T.; Fujioka, T.; Yamaoka, Y. The Incidence of Primary Antibiotic Resistance of Helicobacter Pylori in Vietnam. J. Clin. Gastroenterol. 2013, 47, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Park, J.M.; Cheung, D.Y.; Oh, J.H. Comparison of 7- and 14-Day Eradication Therapy for Helicobacter Pylori with First- and Second-Line Regimen: Randomized Clinical Trial. J. Korean Med. Sci. 2020, 35, e33. [Google Scholar] [CrossRef]
- Mao, H.V.; Lak, B.V.; Long, T.; Chung, N.Q.; Thang, D.M.; Hop, T.V.; Chien, N.N.; Hoan, P.Q.; Henley, K.S.; Perez-Perez, G.I.; et al. Omeprazole or Ranitidine Bismuth Citrate Triple Therapy to Treat Helicobacter Pylori Infection: A Randomized, Controlled Trial in Vietnamese Patients with Duodenal Ulcer. Aliment. Pharmacol. Ther. 2000, 14, 97–101. [Google Scholar] [CrossRef]
- Hoang, B. Efficacy of Sequential Regimens for the Eradication of Helicobacter Pylori in Patients with Peptic Ulcer Disease. J. Med. Hochiminh City 2011, 15, 303–307. [Google Scholar]
- Dao, L.V.; Dao, H.V.; Nguyen, H.T.; Vu, V.T.; Tran, A.T.N.; Dat, V.Q.; Hoang, L.B.; Nguyen, H.T.V.; Nguyen, T.D. Helicobacter Pylori Infection and Eradication Outcomes among Vietnamese Patients in the Same Households: Findings from a Non-Randomized Study. PLoS ONE 2021, 16, e0260454. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter Pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Katelaris, P.; Sugano, K.; Ang, T.L.; Hunt, R.; Talley, N.J.; Lam, S.K.; Xiao, S.-D.; Tan, H.J.; Wu, C.-Y.; et al. Second Asia-Pacific Consensus Guidelines for Helicobacter Pylori Infection. J. Gastroenterol. Hepatol. 2009, 24, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Kocsmár, É.; Buzás, G.M.; Szirtes, I.; Kocsmár, I.; Kramer, Z.; Szijártó, A.; Fadgyas-Freyler, P.; Szénás, K.; Rugge, M.; Fassan, M.; et al. Primary and Secondary Clarithromycin Resistance in Helicobacter Pylori and Mathematical Modeling of the Role of Macrolides. Nat. Commun. 2021, 12, 2255. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lu, H.; Yamaoka, Y. A Report Card to Grade Helicobacter Pylori Therapy. Helicobacter 2007, 12, 275–278. [Google Scholar] [CrossRef]
- Arslan, N.; Yılmaz, Ö.; Demiray-Gürbüz, E. Importance of Antimicrobial Susceptibility Testing for the Management of Eradication in Helicobacter Pylori Infection. World J. Gastroenterol. 2017, 23, 2854–2869. [Google Scholar] [CrossRef]
- Kuo, Y.-T.; Liou, J.-M.; El-Omar, E.M.; Wu, J.-Y.; Leow, A.H.R.; Goh, K.L.; Das, R.; Lu, H.; Lin, J.-T.; Tu, Y.-K.; et al. Primary Antibiotic Resistance in Helicobacter Pylori in the Asia-Pacific Region: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 707–715. [Google Scholar] [CrossRef]
- Mégraud, F. H Pylori Antibiotic Resistance: Prevalence, Importance, and Advances in Testing. Gut 2004, 53, 1374–1384. [Google Scholar] [CrossRef] [Green Version]
- Fischbach, L.; Evans, E.L. Meta-Analysis: The Effect of Antibiotic Resistance Status on the Efficacy of Triple and Quadruple First-Line Therapies for Helicobacter Pylori. Aliment. Pharmacol. Ther. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- De Francesco, V.; Giorgio, F.; Hassan, C.; Manes, G.; Vannella, L.; Panella, C.; Ierardi, E.; Zullo, A. Worldwide H pylori Antibiotic Resistance: A Systematic Review. J. Gastrointest. Liver Dis. 2010, 19, 409–414. [Google Scholar]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y. Study Group participants Helicobacter Pylori Resistance to Antibiotics in Europe and Its Relationship to Antibiotic Consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Morena, F. Systematic Review and Meta-Analysis: Levofloxacin-Based Rescue Regimens after Helicobacter Pylori Treatment Failure. Aliment. Pharmacol. Ther. 2006, 23, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P. Second-Line Rescue Therapy of Helicobacter Pylori Infection. Ther. Adv. Gastroenterol. 2009, 2, 331–356. [Google Scholar] [CrossRef] [Green Version]
- Glocker, E.; Stueger, H.-P.; Kist, M. Quinolone Resistance in Helicobacter Pylori Isolates in Germany. Antimicrob. Agents Chemother. 2007, 51, 346–349. [Google Scholar] [CrossRef] [Green Version]
- Perna, F.; Zullo, A.; Ricci, C.; Hassan, C.; Morini, S.; Vaira, D. Levofloxacin-Based Triple Therapy for Helicobacter Pylori Re-Treatment: Role of Bacterial Resistance. Dig. Liver Dis. 2007, 39, 1001–1005. [Google Scholar] [CrossRef]
- Ahmad, N.; Zakaria, W.R.; Mohamed, R. Analysis of Antibiotic Susceptibility Patterns of Helicobacter Pylori Isolates from Malaysia. Helicobacter 2011, 16, 47–51. [Google Scholar] [CrossRef]
- Nishizawa, T.; Suzuki, H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front. Mol. Biosci. 2014, 1, 19. [Google Scholar] [CrossRef]
| Characteristics | n = 125 n | % |
|---|---|---|
| Gender | ||
| Male | 46 | 36.8 |
| Female | 79 | 63.2 |
| Age (years) | ||
| Mean ± SD | 47.3 ± 14.2 | |
| Range | 17–78 | |
| Endoscopy findings | ||
| Gastritis | 125 | 100 |
| Duodenitis | 30 | 24.0 |
| Gastric ulcer | 16 | 12.8 |
| Duodenal ulcer | 27 | 21.6 |
| Histopathology findings | ||
| Chronic inflammation | 104 | 83.2 |
| Moderate/severe inflammation | 83 | 66.4 |
| Atrophic gastritis | 65 | 52.0 |
| Metaplasia | 12 | 9.6 |
| Dysplasia | 20 | 16.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.B.; Tran, T.N.Q.; Tran, T.Q.A.; Vu, D.L.; Hoang, V.T. Antibiotic Resistance of Helicobacter pylori in Patients with Peptic Ulcer. Medicina 2023, 59, 6. https://doi.org/10.3390/medicina59010006
Vu TB, Tran TNQ, Tran TQA, Vu DL, Hoang VT. Antibiotic Resistance of Helicobacter pylori in Patients with Peptic Ulcer. Medicina. 2023; 59(1):6. https://doi.org/10.3390/medicina59010006
Chicago/Turabian StyleVu, Thanh Binh, Thi Nhu Quynh Tran, Thi Quynh Anh Tran, Dinh Luong Vu, and Van Thuan Hoang. 2023. "Antibiotic Resistance of Helicobacter pylori in Patients with Peptic Ulcer" Medicina 59, no. 1: 6. https://doi.org/10.3390/medicina59010006





