Osteoarthritis of the Temporomandibular Joint: A Narrative Overview
Abstract
:1. Introduction
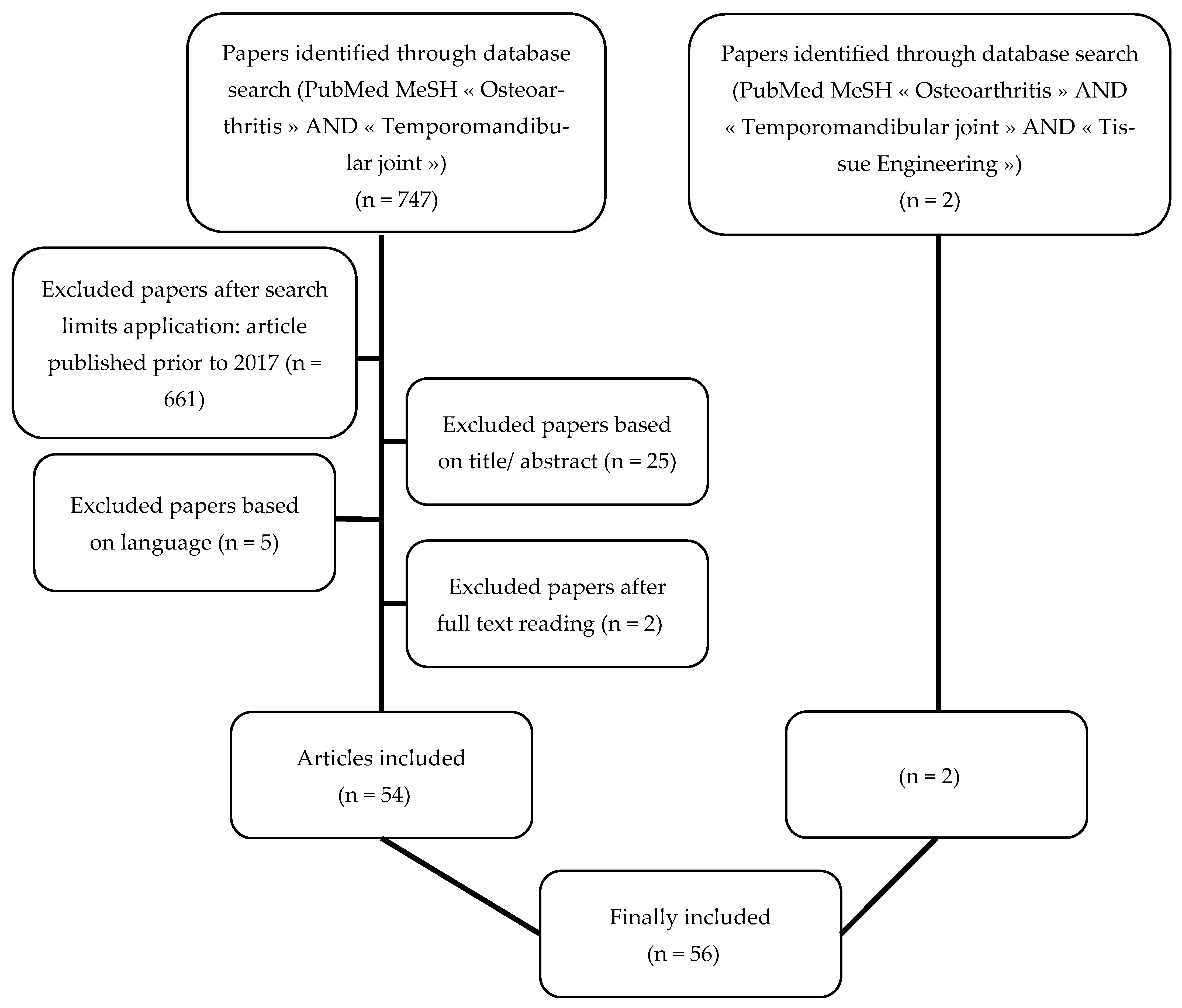
2. Materials and Methods
3. Results
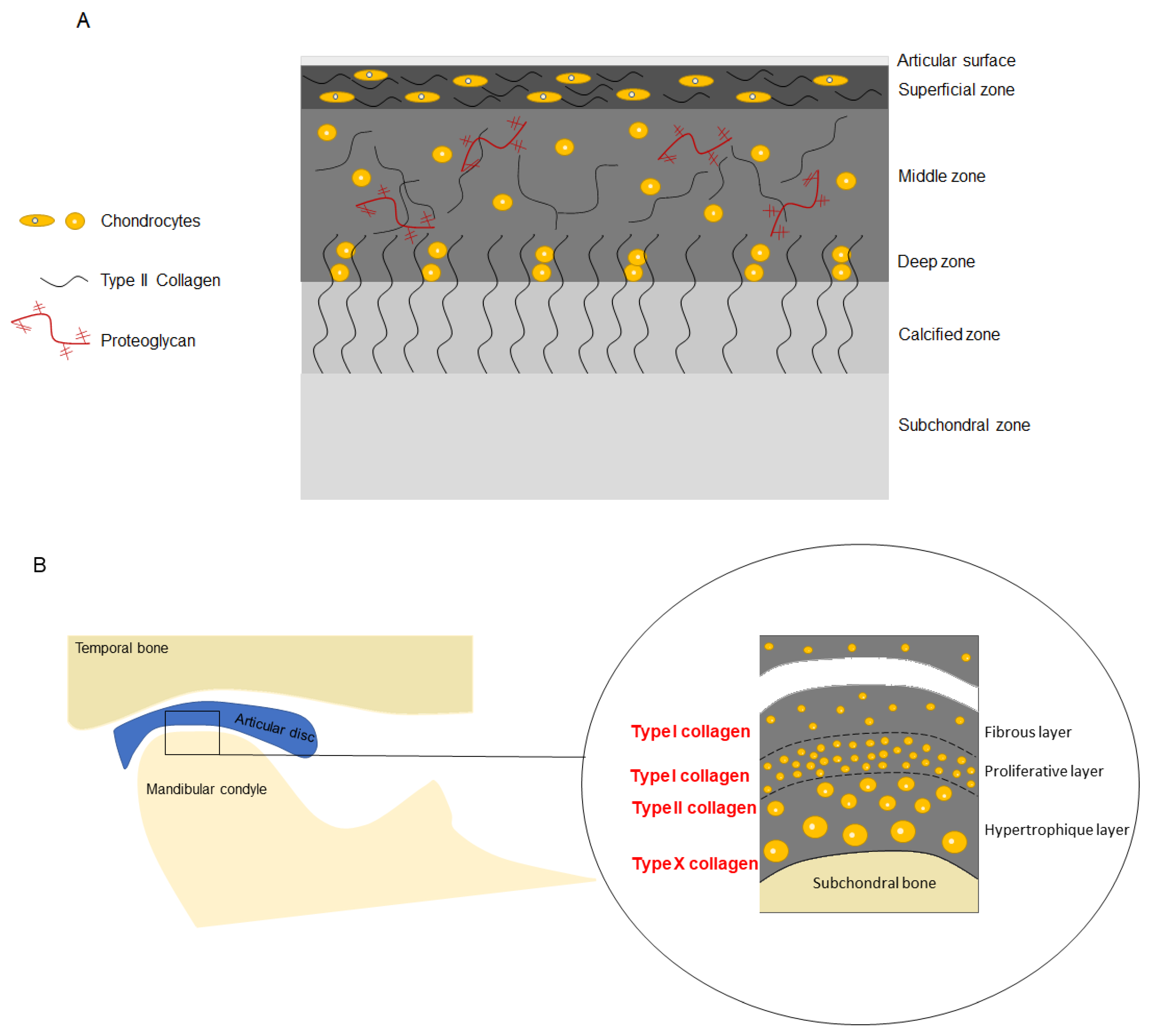
3.1. Histology of the Temporomandibular Joint
3.2. Prevalence
3.3. Etiologies and Risk Factors
3.4. Pathogenesis
3.4.1. Biglycan and Fibromodulin
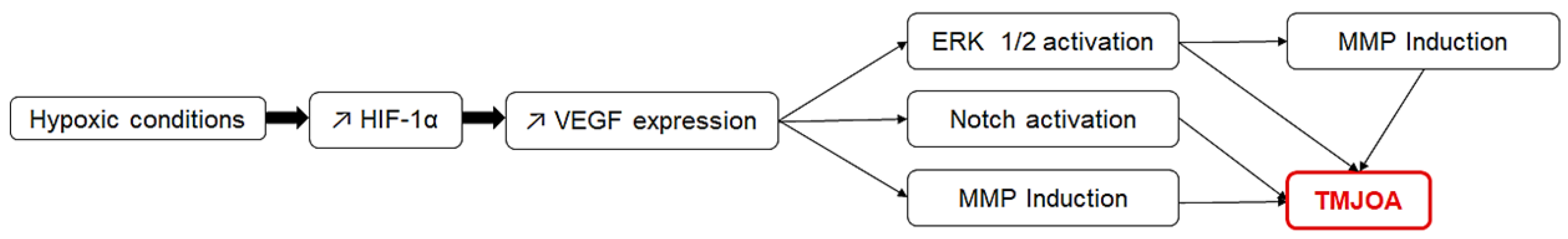
3.4.2. Hypoxia Inducible Factors and Vascular Endothelial Growth Factor
3.4.3. Estrogens
3.4.4. Other Factors
3.5. Clinical Signs
3.6. Diagnostic Tools
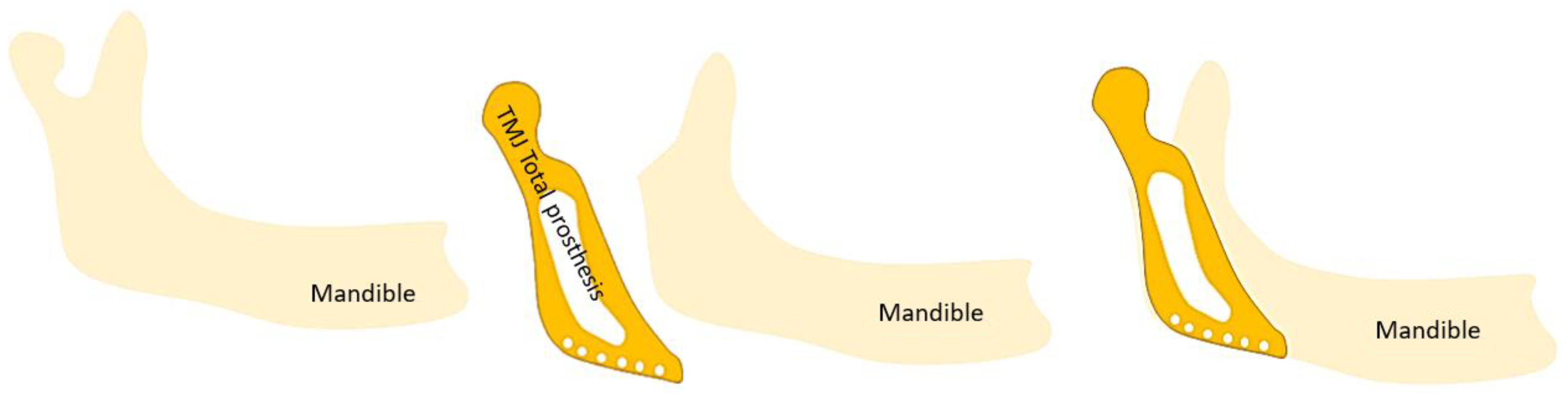
3.7. Treatments
3.8. TMJOA in Adolescents
3.9. Models for the Study of TMJOA
3.10. Contribution of Tissue Engineering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghassemi Nejad, S.; Kobezda, T.; Tar, I.; Szekanecz, Z. Development of temporomandibular joint arthritis: The use of animal models. Jt. Bone Spine 2017, 84, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitzan, D.W.; Svidovsky, J.; Zini, A.; Zadik, Y. Effect of Arthrocentesis on Symptomatic Osteoarthritis of the Temporomandibular Joint and Analysis of the Effect of Preoperative Clinical and Radiologic Features. J. Oral Maxillofac. Surg. 2017, 75, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, J.; Han, J.; Liu, M.; Zhang, Y.; Yap, A.U.-J.; Fu, K.-Y. Degenerative temporomandibular joint changes associated with recent-onset disc displacement without reduction in adolescents and young adults. J. Cranio-Maxillofac. Surg. 2017, 45, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Sperry, M.; Kartha, S.; Winkelstein, B.; Granquist, E. Experimental Methods to Inform Diagnostic Approaches for Painful TMJ Osteoarthritis. J. Dent. Res. 2019, 98, 388–397. [Google Scholar] [CrossRef]
- AbuBakr, N.; Salem, Z.; Ali, Z.; ELAssaly, M. Comparative evaluation of the early effects of the low-level laser therapyversus intra-articular steroids on temporomandibular joint acuteosteoarthritis in rats: A histochemical, molecular and imaging evaluation. Dent. Med. Probl. 2018, 55, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Memis, S.; Candirli, C.; Kerimoglu, G. Short term histopathological effects of GaAlAs laser on experimentally induced TMJ osteoarthritis in rabbits. Braz. Oral Res. 2018, 32, e90. [Google Scholar] [CrossRef]
- Savage, J.; Lababidi, E.; McCullough, M.; Dimitroulis, G. Microbiological investigation of the mandibular condyle in patients with advanced osteoarthritis of the temporomandibular joint. J. Cranio-Maxillofac. Surg. 2019, 47, 1262–1265. [Google Scholar] [CrossRef]
- Hui, T.; Zhou, Y.; Wang, T.; Li, J.; Zhang, S.; Liao, L.; Gu, J.; Ye, L.; Zhao, L.; Chen, D. Activation of β-catenin signaling in aggrecan-expressing cells in temporomandibular joint causes osteoarthritis-like defects. Int. J. Oral Sci. 2018, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Cascone, P.; Gennaro, P.; Gabriele, G.; Chisci, G.; Mitro, V.; De Caris, F.; Iannetti, G. Temporomandibular Synovial Chondromatosis with Numerous Nodules. J. Craniofacial Surg. 2014, 25, 1114–1115. [Google Scholar] [CrossRef]
- Abrahamsson Ak Kristensen, M.; Arvidsson, L.Z.; Kvien, T.K.; Larheim, T.A.; Haugen, I.K. Frequency of temporomandibular joint osteoarthritis and related symptoms in a hand osteoarthritis cohort. Osteoarthr. Cartil. 2017, 25, 654–657. [Google Scholar] [CrossRef]
- Yotsuya, M.; Iriarte-Diaz, J.; Reed, D.A. Temporomandibular Joint Hypofunction Secondary to Unilateral Partial Discectomy Attenuates Degeneration in Murine Mandibular Condylar Cartilage. Bull. Tokyo Dent. Coll. 2020, 61, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, P.; Jiang, C.; Ji, P.; Wang, M.; Xu, J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 2019, 10, 216. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Kadota-Watanabe, C.; Ogawa, T.; Moriyama, K. Combination of estrogen deficiency and excessive mechanical stress aggravates temporomandibular joint osteoarthritis in vivo. Arch. Oral Biol. 2019, 102, 39–46. [Google Scholar] [CrossRef]
- Sritara, S.; Tsutsumi, M.; Fukino, K.; Matsumoto, Y.; Ono, T.; Akita, K. Evaluating the morphological features of the lateral pterygoid insertion into the medial surface of the condylar process. Clin. Exp. Dent. Res. 2020, 7, 219–225. [Google Scholar] [CrossRef]
- Suh, M.S.; Park, S.H.; Kim, Y.K.; Yun, P.Y.; Lee, W.W. 18 F-NaF PET/CT for the evaluation of temporomandibular joint disorder. Clin. Radiol. 2018, 73, 414.e7–414.e13. [Google Scholar] [CrossRef] [PubMed]
- Sonnesen, L.; Odont, D.; Petersson, A.; Odont, D.; Wiese, M.; Jensen, K.E. Osseous osteoarthritic-like changes and joint mobility of the temporomandibular joints and upper cervical spine: Is there a relation? Oral Maxillofac. Radiol. 2017, 123, 7. [Google Scholar] [CrossRef] [PubMed]
- Balon, P.; Vesnaver, A.; Kansky, A.; Kočar, M.; Prodnik, L. Treatment of end stage temporomandibular joint disorder using a temporomandibular joint total prosthesis: The Slovenian experience. J. Cranio-Maxillofac. Surg. 2019, 47, 60–65. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Qu, X.; Wang, Z.; Zheng, J.; Xie, X.; Ma, G.; Zhang, Z.; Ma, X. Evaluation of trabecular structure changes in osteoarthritis of the temporomandibular joint with cone beam computed tomography imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 315–322. [Google Scholar] [CrossRef]
- Lee, P.P.; Stanton, A.R.; E Schumacher, A.; Truelove, E.; Hollender, L.G. Osteoarthritis of the temporomandibular joint and increase of the horizontal condylar angle: A longitudinal study. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 339–350. [Google Scholar] [CrossRef]
- Walewski, L.Â.; Tolentino, E.D.S.; Yamashita, F.C.; Iwaki, L.C.V.; Da Silva, M.C. Cone beam computed tomography study of osteoarthritic alterations in the osseous components of temporomandibular joints in asymptomatic patients according to skeletal pattern, gender, and age. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 70–77. [Google Scholar] [CrossRef]
- Boutault, F.; Cavallier, Z.; Lauwers, F.; Prevost, A. Temporomandibular joint arthroplasty for osteoarthrosis: A series of 24 patients that received a uni- or bilateral inter-positional silicone sheet. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrand, S.; Ingstad, H.K.; Møystad, A.; Bjørnland, T. Long-term effectiveness of arthrocentesis with and without hyaluronic acid injection for treatment of temporomandibular joint osteoarthritis. J. Oral Sci. 2019, 61, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.-H.; Yang, I.-H.; Hyun, H.-K.; Lee, J.-Y. Dental and skeletal maturation in female adolescents with temporomandibular joint osteoarthritis. J. Oral Rehabil. 2017, 44, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Lee, J.K.; Kang, J. Skeletal maturation and predicted adult height in adolescents with temporomandibular joint osteoarthritis. J. Oral Rehabil. 2019, 46, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Liu, Y.; Wang, S.; Yang, X.; Shi, Z.; Liang, X. Glucosamine oral administration as an adjunct to hyaluronic acid injection in treating temporomandibular joint osteoarthritis. Oral Dis. 2018, 24, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Dumbuya, A.; Gomes, A.F.; Marchini, L.; Zeng, E.; Comnick, C.L.; Melo, S.L.S. Bone changes in the temporomandibular joints of older adults: A cone-beam computed tomography study. Spéc. Care Dent. 2020, 40, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Hutami, I.R.; Tanaka, E. Potential Role of Rebamipide in Osteoclast Differentiation and Mandibu- lar Condylar Cartilage Homeostasis. Curr. Rheumatol. Rev. 2018, 14, 62–69. [Google Scholar] [CrossRef]
- Melo, G.; Casett, E.; Stuginski-Barbosa, J.; Guerra, E.N.D.S.; Fernandes, D.A.; Porporatti, A.L.; Flores-Mir, C.; Canto, G.D.L. Effects of glucosamine supplements on painful temporomandibular joint osteoarthritis: A systematic review. J. Oral Rehabil. 2018, 45, 414–422. [Google Scholar] [CrossRef]
- Roberts, W.E.; Stocum, D.L. Part II: Temporomandibular Joint (TMJ)—Regeneration, Degeneration, and Adaptation. Curr. Osteoporos. Rep. 2018, 16, 369–379. [Google Scholar] [CrossRef]
- Salash, J.R.; Hossameldin, R.H.; Almarza, A.J.; Chou, J.C.; McCain, J.P.; Mercuri, L.G.; Wolford, L.M.; Detamore, M.S. Potential Indications for Tissue Engineering in Temporomandibular Joint Surgery. J. Oral Maxillofac. Surg. 2016, 74, 705–711. [Google Scholar] [CrossRef]
- Ackland, D.; Robinson, D.; Lee, P.V.S.; Dimitroulis, G. Design and clinical outcome of a novel 3D-printed prosthetic joint replacement for the human temporomandibular joint. Clin. Biomech. 2018, 56, 52–60. [Google Scholar] [CrossRef]
- Nojima, K.; Nagata, M.; Ootake, T.; Nishii, Y.; Yakushiji, T.; Narita, M.; Takano, N.; Sueishi, K. Surgical Orthodontic Treatment Involving Mandibular Premolar Extraction in Patient with Mandibular Retrusion Associated with Temporomandibular Joint Osteoarthritis. Bull. Tokyo Dent. Coll. 2018, 60, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Taleuan, A.; Kamal, U.; Aouinti, L.; ElAlami, M.N. Arthrotic ankylosis of the temporomandibular joint. Pan Afr. Med J. 2019, 32, 151. [Google Scholar]
- Yokota, S.; Chosa, N.; Kyakumoto, S.; Kimura, H.; Ibi, M.; Kamo, M.; Satoh, K.; Ishisaki, A. ROCK/actin/MRTF signaling promotes the fibrogenic phenotype of fibroblast-like synoviocytes derived from the temporomandibular joint. Int. J. Mol. Med. 2017, 39, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Alshenibr, W.; Tashkandi, M.M.; Alsaqer, S.F.; Alkheriji, Y.; Wise, A.; Fulzele, S.; Mehra, P.; Goldring, M.B.; Gerstenfeld, L.C.; Bais, M.V. Anabolic role of lysyl oxidase like-2 in cartilage of knee and temporomandibular joints with osteoarthritis. Arthritis Res. Ther. 2017, 19, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhang, M.; Liu, Q.; Zhang, H.; Zhang, J.; Lu, L.; Xie, M.; Chen, D.; Wang, M. Inhibition of Ihh Reverses Temporomandibular Joint Osteoarthritis via a PTH1R Signaling Dependent Mechanism. Int. J. Mol. Sci. 2019, 20, 3797. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Li, W.; Li, J.; Fang, W.; Ke, J.; Li, B.; Cheng, Y.; Wei, L. Elevated leptin levels in temporomandibular joint osteoarthritis promote proinflammatory cytokine IL -6 expression in synovial fibroblasts. J. Oral Pathol. Med. 2019, 48, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Lu, L.; Wan, X.; Zhang, J.; Zhang, H.; Liu, X.; Huang, X.; Xiao, G.; Wang, M. Matrix replenishing by BMSCs is beneficial for osteoarthritic temporomandibular joint cartilage. Osteoarthr. Cartil. 2017, 25, 1551–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vapniarsky, N.; Huwe, L.W.; Arzi, B.; Houghton, M.K.; Wong, M.E.; Wilson, J.W.; Hatcher, D.C.; Hu, J.C.; Athanasiou, K.A. Tissue engineering toward temporomandibular joint disc regeneration. Sci. Transl. Med. 2018, 10, eaaq1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mino-Oka, A.; Izawa, T.; Shinohara, T.; Mori, H.; Yasue, A.; Tomita, S.; Tanaka, E. Roles of hypoxia inducible factor-1α in the temporomandibular joint. Arch. Oral Biol. 2017, 73, 274–281. [Google Scholar] [CrossRef]
- Tolba, Y.M.; Omar, S.S.; Nagui, D.A.; Nawwar, M.A. Effect of high molecular weight hyaluronic acid in treatment of osteoarthritic temporomandibular joints of rats. Arch. Oral Biol. 2020, 110, 104618. [Google Scholar] [CrossRef] [PubMed]
- He, D.; An, Y.; Li, Y.; Wang, J.; Wu, G.; Chen, L.; Zhu, G. RNA sequencing reveals target genes of temporomandibular joint osteoarthritis in rats after the treatment of low-intensity pulsed ultrasound. Gene 2018, 672, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jiang, Y.; Bi, R.; Jiang, N.; Zhu, S. Inhibition of notch signaling pathway temporally postpones the cartilage degradation progress of temporomandibular joint arthritis in mice. J. Cranio-Maxillofac. Surg. 2018, 46, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Sun, D.; Mu, T.; Chu, Y.; Miao, H.; Zhang, M.; Yang, H.; Liu, Q.; Lu, L.; Xing, X.; et al. Differential effects of high-physiological oestrogen on the degeneration of mandibular condylar cartilage and subchondral bone. Bone 2018, 111, 9–22. [Google Scholar] [CrossRef]
- Yotsuya, M.; Bertagna, A.E.; Hasan, N.; Bicknell, S.; Sato, T.; Reed, D.A. Neuron/Glial Antigen 2-Type VI Collagen Interactions During Murine Temporomandibular Joint Osteoarthritis. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liao, L.; Zhu, J.; Wan, X.; Xie, M.; Zhang, H.; Zhang, M.; Lu, L.; Yang, H.; Jing, D.; et al. Osteochondral Interface Stiffening in Mandibular Condylar Osteoarthritis. J. Dent. Res. 2018, 97, 563–570. [Google Scholar] [CrossRef]
- Chu, W.C.; Zhang, S.; Sng, T.J.; Ong, Y.J.; Tan, W.-L.; Ang, V.Y.; Foldager, C.B.; Toh, W.S. Distribution of pericellular matrix molecules in the temporomandibular joint and their chondroprotective effects against inflammation. Int. J. Oral Sci. 2017, 9, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Xu, G.; Gu, Z.; Wu, H. Hedgehog signal expression in articular cartilage of rat temporomandibular joint and association with adjuvant-induced osteoarthritis. J. Oral Pathol. Med. 2017, 46, 284–291. [Google Scholar] [CrossRef]
- Shirakura, M.; Kram, V.; Robinson, J.; Sikka, S.; Kilts, T.M.; Wadhwa, S.; Young, M.F. Extracellular Matrix Mediates BMP-2 in a Model of Temporomandibular Joint Osteoarthritis. Cells Tissues Organs 2017, 204, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, P.; Doyran, B.; Li, Q.; Han, B.; Bechtold, T.E.; Koyama, E.; Lu, X.L.; Han, L. Biomechanical properties of murine TMJ articular disc and condyle cartilage via AFM-nanoindentation. J. Biomech. 2017, 60, 134–141. [Google Scholar] [CrossRef]
- Zhou, Y.; Shu, B.; Xie, R.; Huang, J.; Zheng, L.; Zhou, X.; Xiao, G.; Zhao, L.; Chen, D. Deletion of Axin1 in condylar chondrocytes leads to osteoarthritis-like phenotype in temporomandibular joint via activation of β-catenin and FGF signaling. J. Cell. Physiol. 2019, 234, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, G.; Zhu, T.; Chen, H.; Zhu, Y.; Han, F.; Zhao, H. VEGF promotes cartilage angiogenesis by phospho-ERK1/2 activation of Dll4 signaling in temporomandibular joint osteoarthritis caused by chronic sleep disturbance in Wistar rats. Oncotarget 2017, 8, 17849–17861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, D.A.; Yotsuya, M.; Gubareva, P.; Toth, P.; Bertagna, A. Two-photon fluorescence and second harmonic generation characterization of extracellular matrix remodeling in post-injury murine temporomandibular joint osteoarthritis. PLoS ONE 2019, 14, e0214072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, J.; Zhou, S.; Luo, F.; Tan, Q.; Sun, X.; Ni, Z.; Chen, H.; Du, X.; Xie, Y.; et al. Loss of Fgfr1 in chondrocytes inhibits osteoarthritis by promoting autophagic activity in temporomandibular joint. J. Biol. Chem. 2018, 293, 8761–8774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Alcântara Camejo, F.; Azevedo, M.; Ambros, V.; Caporal, K.S.T.; Doetzer, A.D.; Almeida, L.E.; Olandoski, M.; Noronha, L.; Trevilatto, P.C. Interleukin-6 expression in disc derangement of human temporomandibular joint and association with osteoarthrosis. J. Cranio-Maxillofac. Surg. 2017, 45, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Monasterio, G.; Castillo, F.; Rojas, L.; Cafferata, E.A.; Alvarez, C.; Carvajal, P.; Núñez, C.; Flores, G.; Díaz, W.; Vernal, R. Th1/Th17/Th22 immune response and their association with joint pain, imagenological bone loss, RANKL expression and osteoclast activity in temporomandibular joint osteoarthritis: A preliminary report. J. Oral Rehabil. 2018, 45, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Q.; Yang, H.; Shi, L.; Wang, P.; Xie, M.; Liu, J.; Xu, X.; Liu, X.; Yu, S.; et al. Early growth response 1 reduction in peripheral blood involving condylar subchondral bone loss. Oral Dis. 2019, 25, 1759–1768. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Lin, M.-T.; Chang, H.-P. Effectiveness of platelet-rich plasma injection in patients with temporomandibular joint osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 106–116. [Google Scholar] [CrossRef]
- Stocum, D.L.; Roberts, W.E. Part I: Development and Physiology of the Temporomandibular Joint. Curr. Osteoporos. Rep. 2018, 16, 360–368. [Google Scholar] [CrossRef]
- Palukuru, U.P.; McGoverin, C.M.; Pleshko, N. Assessment of hyaline cartilage matrix composition using near infrared spectroscopy. Matrix Biol. 2014, 38, 3–11. [Google Scholar] [CrossRef]
- Armiento, A.; Stoddart, M.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Matzat, S.J.; Kogan, F.; Fong, G.W.; Gold, G.E. Imaging Strategies for Assessing Cartilage Composition in Osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, A.; Chan, P.M.B.; Wen, C. Do immune cells lead the way in subchondral bone disturbance in osteoarthritis? Prog. Biophys. Mol. Biol. 2019, 148, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, B.; Zhu, Y.; Zhao, H.; Ma, C. HIF-1-VEGF-Notch mediates angiogenesis in temporomandibular joint osteoarthritis. Am. J. Transl. Res. 2019, 11, 2969–2982. [Google Scholar]
- Luo, P.; Feng, C.; Jiang, C.; Ren, X.; Gou, L.; Ji, P.; Xu, J. IL-37b alleviates inflammation in the temporomandibular joint cartilage via IL-1R8 pathway. Cell Prolif. 2019, 52, e12692. [Google Scholar] [CrossRef]
- Sun, S.; Bay-Jensen, A.-C.; A Karsdal, M.; Siebuhr, A.S.; Zheng, Q.; Maksymowych, W.P.; Christiansen, T.G.; Henriksen, K. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet. Disord. 2014, 15, 93. [Google Scholar] [CrossRef] [Green Version]
- Martín-Granizo, R. Simple and secure intra-articular infiltration during arthroscopy of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 2018, 56, 763–765. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Ma, J.; Shen, B.; Pei, F.; Kraus, V. Effectiveness of low-level laser therapy in patients with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Induction of osteoarthritis by injecting monosodium iodoacetate into the patellofemoral joint of an experimental rat model. PLoS ONE 2018, 13, e0196625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuang, F.; Zhu, J.; Song, K.; Hou, S.; Liu, Y.; Zhang, C.; Tang, J. Establishment of a Rat Model of Adjuvant-Induced Osteoarthritis of the Lumbar Facet Joint. Cell Biophys. 2014, 70, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F.; Ravindran, S.; Huang, C.-C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2020, 10, 1569. [Google Scholar] [CrossRef] [PubMed]
- Fabre, H.; Ducret, M.; Degoul, O.; Rodriguez, J.; Perrier-Groult, E.; Aubert-Foucher, E.; Pasdeloup, M.; Auxenfans, C.; McGuckin, C.; Forraz, N.; et al. Characterization of Different Sources of Human MSCs Expanded in Serum-Free Conditions with Quantification of Chondrogenic Induction in 3D. Stem Cells Int. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Maumus, M.; Pers, Y.M.; Ruiz, M.; Jorgensen, C.; Noël, D. Mesenchymal stem cells and regenerative medicine: Future perspectives in osteoarthritis. Médecine/Sciences 2018, 34, 1092–1099. [Google Scholar] [CrossRef]
- Freyria, A.M.; Courtes, S.; Mallein-Gerin, F. Differentiation of adult human mesenchymal stem cells: Chondrogenic effetc of BMP-2. Pathol. Biol. 2008, 56, 326–333. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [Green Version]
- Acri, T.M.; Shin, K.; Seol, D.; Laird, N.Z.; Song, I.; Geary, S.M.; Chakka, J.L.; Martin, J.A.; Salem, A.K. Tissue Engineering for the Temporomandibular Joint. Adv. Heal. Mater. 2019, 8, e1801236. [Google Scholar] [CrossRef]
- Ahmad, M.; Hollender, L.; Anderson, Q.; Kartha, K.; Ohrbach, R.; Truelove, E.L.; John, M.T.; Schiffman, E.L. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): Development of image analysis criteria and examiner reliability for image analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, 844–860. [Google Scholar] [CrossRef] [Green Version]
- Badel, T.; Lovko, S.K.; Podoreski, D.; Pavcin, I.S.; Kern, J. Anxiety, splint treatment and clinical characteristics of patients with osteoarthritis of temporomandibular joint and dental students--a pilot study. Med. Glas. Off. Publ. Med Assoc. Zenica-Doboj Canton Bosnia Herzeg. 2011, 8, 60–63. [Google Scholar]
- Fonseca-Rodrigues, D.; Rodrigues, A.; Martins, T.; Pinto, J.; Amorim, D.; Almeida, A.; Pinto-Ribeiro, F. Correlation between pain severity and levels of anxiety and depression in osteoarthritis patients: A systematic review and meta-analysis. Rheumatology 2021, 61, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Aluko, Y.; Myint, P.K.; Smith, T. Prevalence of depressive symptoms and anxiety in osteoarthritis: A systematic review and meta-analysis. Age Ageing 2016, 45, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.J.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism physiology and pathology: An overview for clinicians. J. Oral Rehabilitation 2008, 35, 476–494. [Google Scholar] [CrossRef]
- Reduce, Refine, Replace. Nat. Immunol. 2010, 11, 971. [CrossRef]
| Type of Article | Study First Author, Year |
|---|---|
| Observational Studies | |
| Review | |
| Case study | |
| In vitro studies |
|
| Combination of in vitro and in vivo studies | |
| In vivo studies on animal models |
|
| In vivo study on human samples | |
| In vivo study on human samples and on rats |
|
| Systematic review and meta-analysis |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mélou, C.; Pellen-Mussi, P.; Jeanne, S.; Novella, A.; Tricot-Doleux, S.; Chauvel-Lebret, D. Osteoarthritis of the Temporomandibular Joint: A Narrative Overview. Medicina 2023, 59, 8. https://doi.org/10.3390/medicina59010008
Mélou C, Pellen-Mussi P, Jeanne S, Novella A, Tricot-Doleux S, Chauvel-Lebret D. Osteoarthritis of the Temporomandibular Joint: A Narrative Overview. Medicina. 2023; 59(1):8. https://doi.org/10.3390/medicina59010008
Chicago/Turabian StyleMélou, Caroline, Pascal Pellen-Mussi, Sylvie Jeanne, Agnès Novella, Sylvie Tricot-Doleux, and Dominique Chauvel-Lebret. 2023. "Osteoarthritis of the Temporomandibular Joint: A Narrative Overview" Medicina 59, no. 1: 8. https://doi.org/10.3390/medicina59010008





