Blood and Salivary Cortisol Variations in Athletes in Relation to Cardiopulmonary Exercise Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Participants and Protocol
2.3. Initial Evaluation
2.4. Resting ECG
2.5. Cardiac Ultrasound
2.6. Cardiopulmonary Exercise Testing
2.7. Cortisol Determinations
2.8. Data Analysis and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reilly, T.; Ekblom, B. The use of recovery methods post-exercise. J. Sports Sci. 2005, 23, 619–627. [Google Scholar] [CrossRef]
- Mohr, M.; Krustrup, P.; Bangsbo, J. Fatigue in soccer: A brief review. J. Sports Sci. 2005, 23, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L.; Jeukendrup, A.E. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004, 34, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Papacosta, E.; Nassis, G.P. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J. Sci. Med. Sport 2011, 14, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Banfi, G. Controlling sources of preanalytical variability in doping samples: Challenges and solutions. Bioanalysis 2013, 5, 1571–1582. [Google Scholar] [CrossRef]
- Hug, M.; Mullis, P.E.; Vogt, M.; Ventura, N.; Hoppeler, H. Training modalities: Over-reaching and over-training in athletes, including a study of the role of hormones. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 191–209. [Google Scholar] [CrossRef]
- Banfi, G.; Dolci, A. Free testosterone/cortisol ratio in soccer: Usefulness of a categorization of values. J. Sports Med. Phys. Fitness 2006, 46, 611–616. [Google Scholar]
- Handziski, Z.; Maleska, V.; Petrovska, S.; Nikolik, S.; Mickoska, E.; Dalip, M.; Kostova, E. The changes of ACTH, cortisol, testosterone and testosterone/cortisol ratio in professional soccer players during a competition half-season. Bratisl. Lek. Listy 2006, 107, 259–263. [Google Scholar]
- Lippi, G.; Mattiuzzi, C. Screening for recreational drugs in sports. Balance between fair competition and private life. Perform. Enhanc. Health 2013, 2, 72–73. [Google Scholar] [CrossRef]
- Thau, L.; Gandhi, J.; Sharma, S. Physiology, Cortisol. 2022 Aug 29. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Raff, H.; Carroll, T. Cushing’s syndrome: From physiological principles to diagnosis and clinical care. J. Physiol. 2015, 593, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.; Michels, N. Addison disease: Early detection and treatment principles. Am. Fam. Physician 2014, 89, 563–568. [Google Scholar] [PubMed]
- Neary, N.; Nieman, L. Adrenal insufficiency: Etiology, diagnosis and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Sanpawithayakul, K.; Grossman, A. Adrenal Insufficiency. 7 November 2022. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar] [PubMed]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef]
- Wojcik, M.; Ruszala, A.; Janus, D.; Starzyk, J.B. Secondary Adrenal Insufficiency due to Intra-articular Glucocorticoid Injections. Indian Pediatr. 2019, 56, 242–243. [Google Scholar] [CrossRef]
- Neary, J.P.; Malbon, L.; McKenzie, D.C. Relationship between serum, saliva and urinary cortisol and its implication during recovery from training. J. Sci. Med. Sport 2002, 5, 108–114. [Google Scholar] [CrossRef]
- Duclos, M.; Guinot, M.; Le Bouc, Y. Cortisol and GH: Odd and controversial ideas. Appl. Physiol. Nutr. Metab. 2007, 32, 895–903. [Google Scholar] [CrossRef]
- Mishica, C.; Kyröläinen, H.; Hynynen, E.; Nummela, A.; Holmberg, H.C.; Linnamo, V. Relationships between Heart Rate Variability, Sleep Duration, Cortisol and Physical Training in Young Athletes. J. Sports Sci. Med. 2021, 20, 778–788. [Google Scholar] [CrossRef]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Chatard, J.C.; Atlaoui, D.; Lac, G.; Duclos, M.; Hooper, S.; Mackinnon, L. Cortisol, DHEA, performance and training in elite swimmers. Int. J. Sports Med. 2002, 23, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hedelin, R.; Kenttä, G.; Wiklund, U.; Bjerle, P.; Henriksson-Larsén, K. Short-term overtraining: Effects on performance, circulatory responses, and heart rate variability. Med. Sci. Sports Exerc. 2000, 32, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, R.P.; Swart, J.; Noakes, T.D.; Lambert, M.I. A novel submaximal cycle test to monitor fatigue and predict cycling performance. Br. J. Sports Med. 2011, 45, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary Exercise Testing: What Is its Value. J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef] [PubMed]
- Tran, D. Cardiopulmonary Exercise Testing. Methods Mol. Biol. 2018, 1735, 285–295. [Google Scholar]
- Arena, R.; Canada, J.M.; Popovic, D.; Trankle, C.R.; Del Buono, M.G.; Lucas, A.; Abbate, A. Cardiopulmonary exercise testing—Refining the clinical perspective by combining assessments. Expert Rev. Cardiovasc. Ther. 2020, 18, 563–576. [Google Scholar] [CrossRef]
- Mezzani, A. Cardiopulmonary Exercise Testing: Basics of Methodology and Measurements. Ann. Am. Thorac. Soc. 2017, 14, S3–S11. [Google Scholar] [CrossRef]
- Foster, C. Is There Risk in Exercise Testing of Athletes. Int. J. Sports Physiol. Perform. 2017, 12, 849–850. [Google Scholar] [CrossRef]
- Gibson, M.E.; Gray, K. Exercise Testing. Curr. Sports Med. Rep. 2019, 18, 349–350. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Löllgen, H.; Leyk, D. Exercise Testing in Sports Medicine. Dtsch. Arztebl. Int. 2018, 115, 409–416. [Google Scholar] [CrossRef]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Panhuyzen-Goedkoop, N.M.; Jørstad, H.T.; Smeets, J.L.R.M. A new consensus document on electrocardiographic interpretation in athletes: Does it help to prevent sudden cardiac death in athletes. Neth. Heart J. 2018, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Glaab, T.; Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 2022, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Capostagno, B.; Lambert, M.I.; Lamberts, R.P. A Systematic Review of Submaximal Cycle Tests to Predict, Monitor, and Optimize Cycling Performance. Int. J. Sports Physiol. Perform. 2016, 11, 707–714. [Google Scholar] [CrossRef]
- Basset, F.A.; Boulay, M.R. Treadmill and Cycle Ergometer Tests are Interchangeable to Monitor Triathletes Annual Training. J. Sports Sci. Med. 2003, 2, 110–116. [Google Scholar]
- Wood, P. Salivary steroid assays—Research or routine. Ann. Clin. Biochem. 2009, 46, 183–196. [Google Scholar] [CrossRef]
- Lippi, G.; De Vita, F.; Salvagno, G.L.; Gelati, M.; Montagnana, M.; Guidi, G.C. Measurement of morning saliva cortisol in athletes. Clin. Biochem. 2009, 42, 904–906. [Google Scholar] [CrossRef]
- Lane, A.R.; Hackney, A.C. Relationship between salivary and serum testosterone levels in response to different exercise intensities. Hormones (Athens) 2015, 14, 258–264. [Google Scholar] [CrossRef]
- Cadore, E.; Lhullier, F.; Brentano, M.; Silva, E.; Ambrosini, M.; Spinelli, R.; Silva, R.; Kruel, L. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J. Sports Sci. 2008, 26, 1067–1072. [Google Scholar] [CrossRef]
- VanBruggen, M.D.; Hackney, A.C.; McMurray, R.G.; Ondrak, K.S. The relationship between serum and salivary cortisol levels in response to different intensities of exercise. Int. J. Sports Physiol. Perform. 2011, 6, 396–407. [Google Scholar] [CrossRef]
- Hynynen, E.; Uusitalo, A.; Konttinen, N.; Rusko, H. Heart rate variability during night sleep and after awakening in overtrained athletes. Med. Sci. Sports Exerc. 2006, 38, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, M.F.; Rice, T.; Rosmond, R.; Borecki, I.B.; An, P.; Gagnon, J.; Leon, A.S.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C.; et al. A genetic study of cortisol measured before and after endurance training: The HERITAGE Family Study. Metabolism 2002, 51, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Jürimäe, J.; Mäestu, J.; Purge, P.; Jürimäe, T. Changes in stress and recovery after heavy training in rowers. J. Sci. Med. Sport 2004, 7, 335–339. [Google Scholar] [CrossRef]
- Iellamo, F.; Pigozzi, F.; Parisi, A.; Di Salvo, V.; Vago, T.; Norbiato, G.; Lucini, D.; Pagani, M. The stress of competition dissociates neural and cortisol homeostasis in elite athletes. J. Sports Med. Phys. Fitness 2003, 43, 539–545. [Google Scholar] [PubMed]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Kölling, S.; Wiewelhove, T.; Raeder, C.; Endler, S.; Ferrauti, A.; Meyer, T.; Kellmann, M. Sleep monitoring of a six-day microcycle in strength and high-intensity training. Eur. J. Sport. Sci. 2016, 16, 507–515. [Google Scholar] [CrossRef]
- Lehmann, M.; Foster, C.; Keul, J. Overtraining in endurance athletes: A brief review. Med. Sci. Sports Exerc. 1993, 25, 854–862. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Costill, D.L.; Flynn, M.G.; Mitchell, J.B.; Fink, W.J.; Neufer, P.D.; Houmard, J.A. Physiological responses to successive days of intense training in competitive swimmers. Med. Sci. Sports Exerc. 1988, 20, 255–259. [Google Scholar] [CrossRef]
- Lippi, G.; Dipalo, M.; Buonocore, R.; Gnocchi, C.; Aloe, R.; Delsignore, R. Analytical Evaluation of Free Testosterone and Cortisol Immunoassays in Saliva as a Reliable Alternative to Serum in Sports Medicine. J. Clin. Lab. Anal. 2016, 30, 732–735. [Google Scholar] [CrossRef]
- Ponce, P.; Del Arco, A.; Loprinzi, P. Physical Activity versus Psychological Stress: Effects on Salivary Cortisol and Working Memory Performance. Medicina 2019, 55, 119. [Google Scholar] [CrossRef] [PubMed]
- Moyers, S.A.; Hagger, M.S. Physical activity and cortisol regulation: A meta-analysis. Biol. Psychol. 2023, 179, 108548. [Google Scholar] [CrossRef] [PubMed]

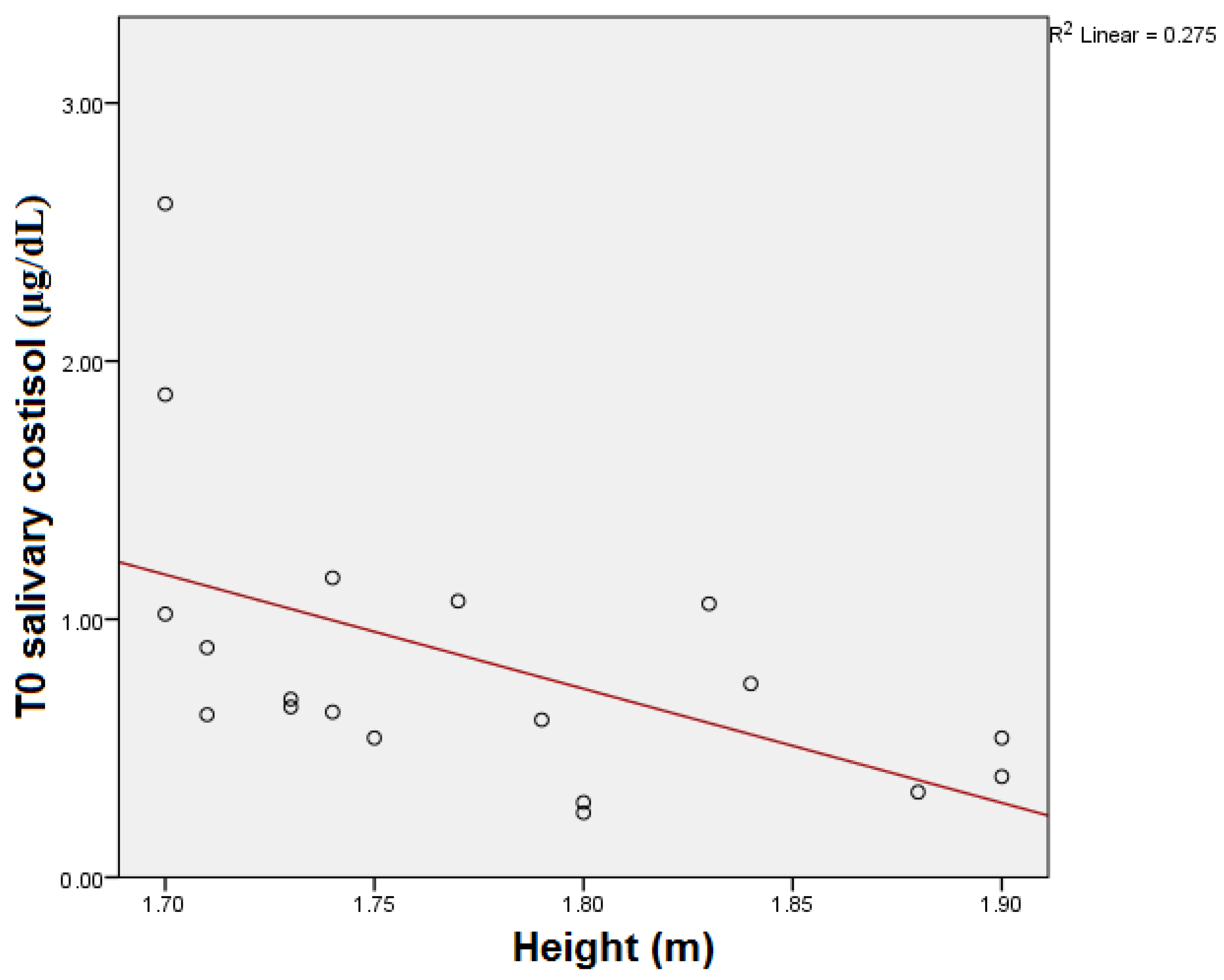
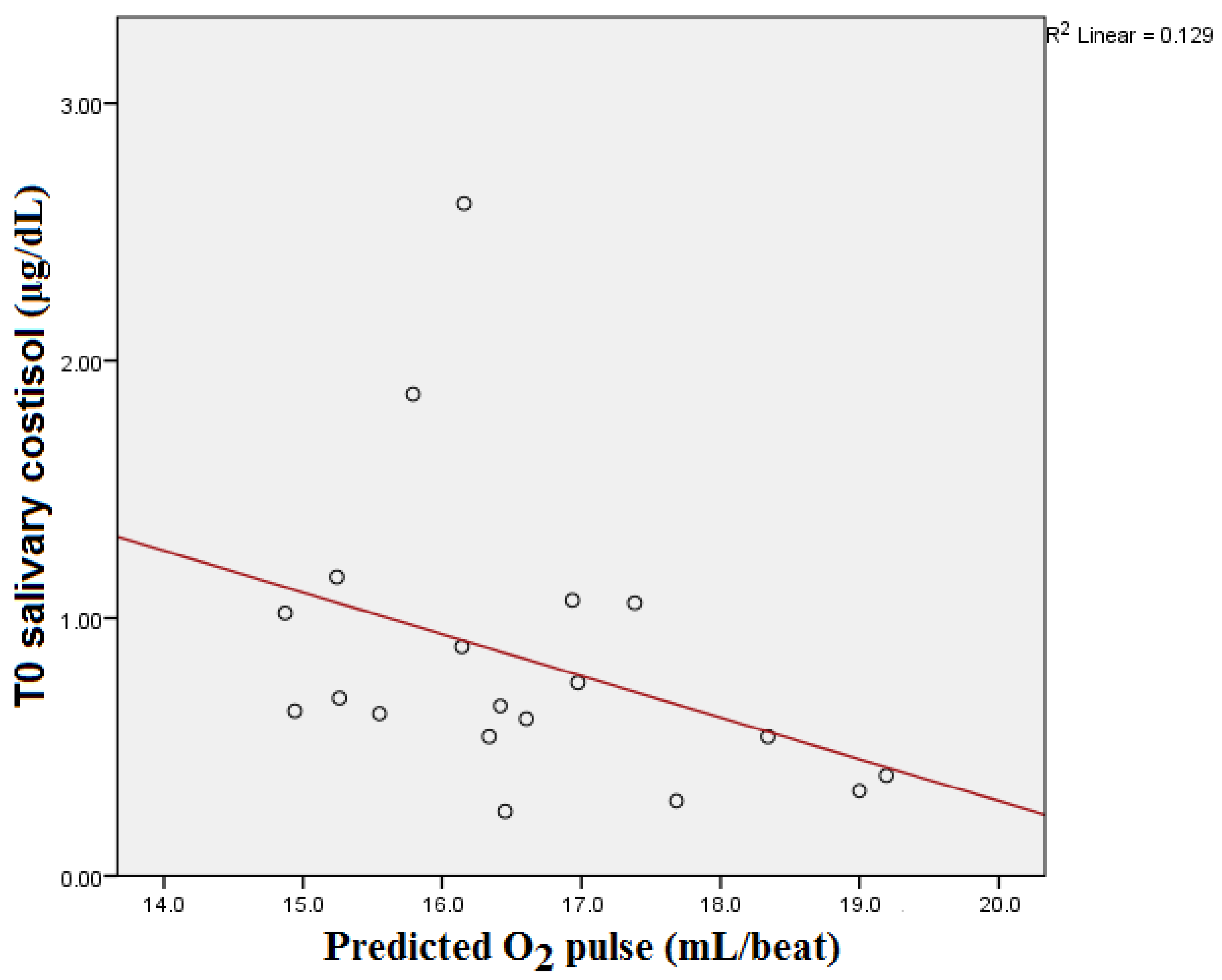
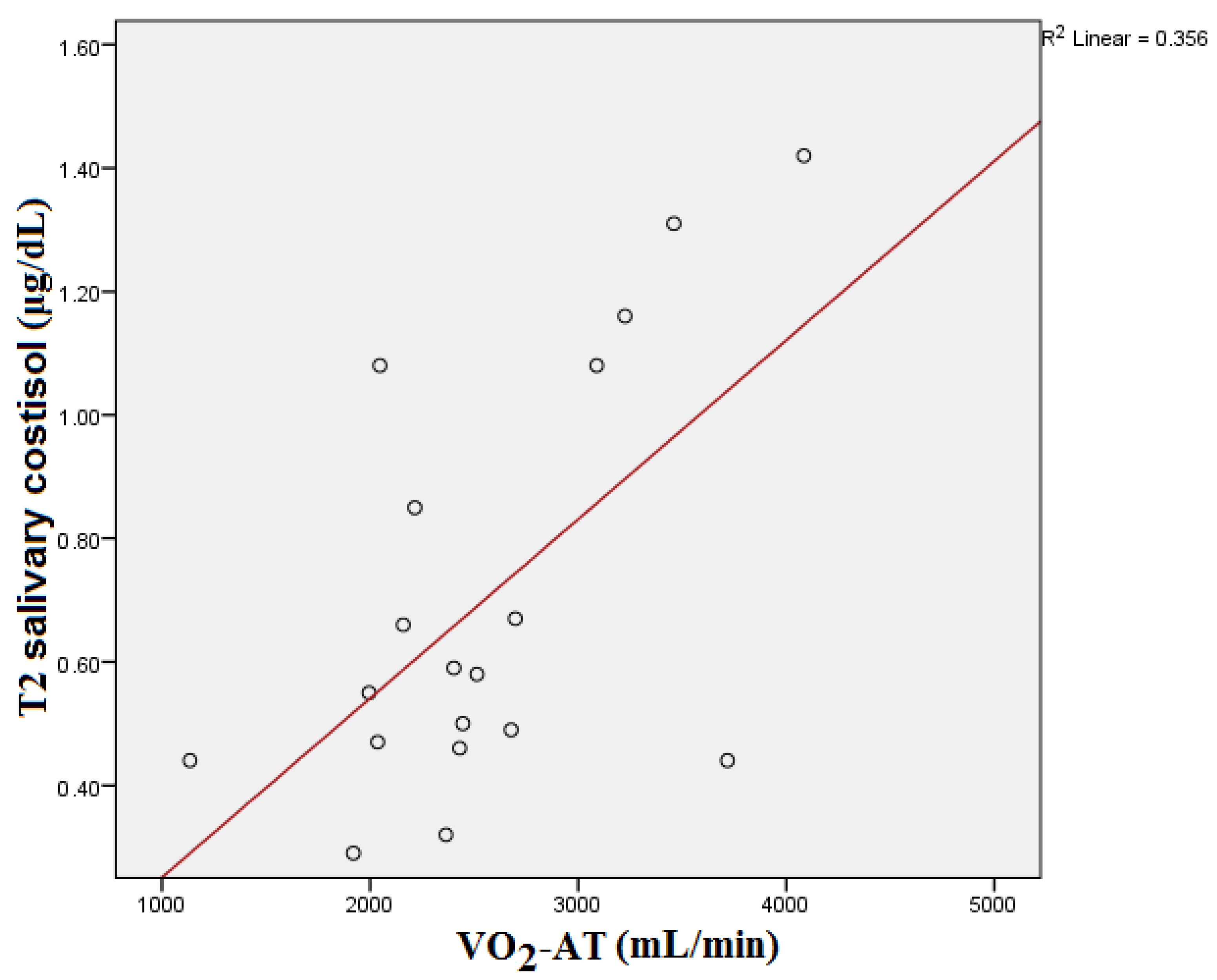
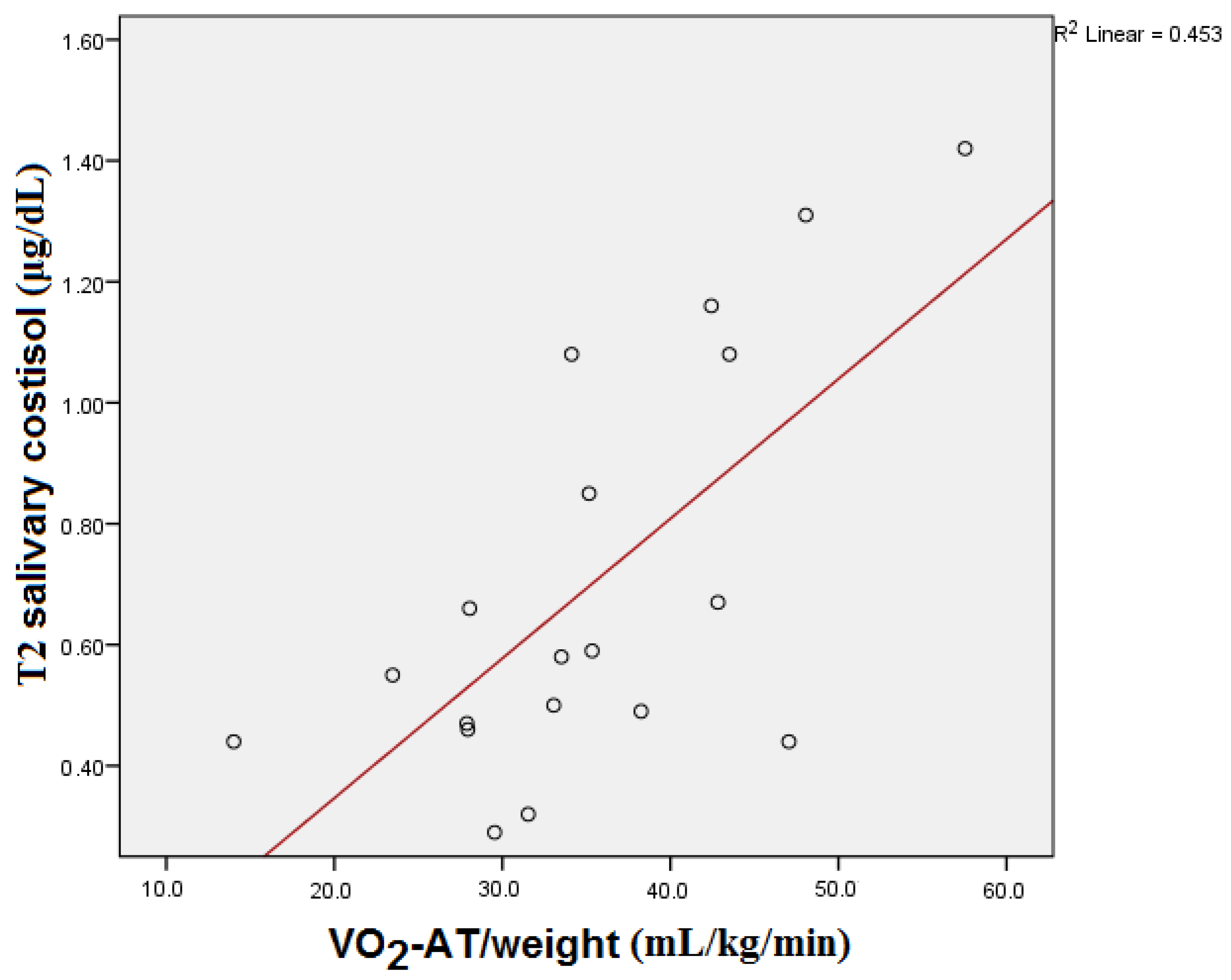
| Parameter | Median | Interquartile Range |
|---|---|---|
| VO2 max (mL/min) | 3753 | 795 |
| VO2 max body weight (mL/min/kg) | 50.06 | 11.1 |
| %VO2 max | 109.00 | 25.00 |
| VO2@AT (mL/min) | 2431.00 | 1042.00 |
| VO2@AT body weight (mL/min/kg) | 34.11 | 14.77 |
| RER | 1.11 | 0.11 |
| VE/VCO2 | 22.35 | 5.35 |
| ΔVO2/ΔWR (mL/min/Watt) | 13.2 | 2.34 |
| WR max (Watt) | 248.00 | 37 |
| %WR max | 86.00 | 7.00 |
| O2 pulse (mL/beat) | 21.7 | 5.2 |
| %O2 pulse | 134.32 | 29.71 |
| HR max (bpm) | 167 | 16 |
| %HR max | 83 | 8 |
| HR_rez (bpm) | 107 | 14 |
| SBP max (mmHg) | 220.0 | 30.0 |
| DBP max (mmHg) | 85.0 | 5.0 |
| Parameter | Median | Interquartile Range |
|---|---|---|
| T0 serum cortisol (ng/mL) | 303.01 | 110.83 |
| T1 serum cortisol (ng/mL) | 279.92 | 207.52 |
| T0 saliva cortisol (ug/dL) | 0.66 | 0.52 |
| T2 saliva cortisol (ug/dL) | 0.58 | 0.62 |
| T3 saliva cortisol (ug/dL) | 0.55 | 0.22 |
| T0 Serum Cortisol (ng/mL) | T1 Serum Cortisol (ng/mL) | |||
|---|---|---|---|---|
| r | p | r | p | |
| VO2 max (mL/min) | 0.009 | 0.972 | −0.251 | 0.300 |
| VO2 max/weight (mL/kg/min) | 0.151 | 0.538 | −0.163 | 0.505 |
| VO2 max % predicted | 0.087 | 0.723 | −0.237 | 0.328 |
| VO2-AT (mL/min) | 0.416 | 0.077 | 0.312 | 0.193 |
| VO2-AT/weight (mL/kg/min) | 0.460 | 0.048 | 0.372 | 0.117 |
| RER | −0.063 | 0.799 | −0.209 | 0.391 |
| VE/VCO2 | −0.179 | 0.464 | 0.168 | 0.491 |
| ΔVO2/ΔWR (mL/min/Watt) | −0.028 | 0.909 | −0.289 | 0.229 |
| WR (Watt) | 0.101 | 0.679 | −0.230 | 0.344 |
| WR % predicted | 0.156 | 0.524 | −0.333 | 0.164 |
| Predicted O2 pulse | −0.340 | 0.154 | −0.102 | 0.679 |
| O2 pulse (mL/beat) | −0.024 | 0.923 | −0.281 | 0.244 |
| O2 pulse % predicted | 0.082 | 0.737 | −0.249 | 0.304 |
| HR max (bpm) | 0.163 | 0.504 | 0.076 | 0.756 |
| HR max % predicted | 0.177 | 0.468 | 0.079 | 0.747 |
| SBP max (mmHg) | −0.026 | 0.916 | 0.351 | 0.141 |
| DBP max (mmHg) | −0.288 | 0.231 | 0.018 | 0.942 |
| T0 Salivary Cortisol (µg/dL) | T2 Salivary Cortisol (µg/dL) | T3 Salivary Cortisol (µg/dL) | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| VO2 max (mL/min) | 0.017 | 0.946 | −0.161 | 0.511 | −0.178 | 0.465 |
| VO2 max/weight (mL/kg/min) | 0.206 | 0.397 | −0.055 | 0.822 | −0.119 | 0.629 |
| VO2 max % predicted | 0.197 | 0.418 | −0.098 | 0.689 | −0.099 | 0.686 |
| VO2-AT (mL/min) | 0.265 | 0.273 | 0.500 | 0.029 | 0.209 | 0.391 |
| VO2-AT/weight (mL/kg/min) | 0.330 | 0.168 | 0.616 | 0.005 | 0.344 | 0.149 |
| RER | 0.014 | 0.956 | −0.349 | 0.143 | −0.156 | 0.523 |
| VE/VCO2 | −0.193 | 0.428 | 0.162 | 0.506 | 0.348 | 0.145 |
| ΔVO2/ΔWR (mL/min/Watt) | 0.060 | 0.808 | −0.138 | 0.574 | −0.143 | 0.559 |
| WR (Watt) | 0.016 | 0.948 | −0.098 | 0.691 | −0.151 | 0.537 |
| WR % predicted | 0.145 | 0.553 | −0.063 | 0.799 | −0.173 | 0.479 |
| Predicted O2 pulse | −0.493 | 0.032 | −0.261 | 0.281 | −0.154 | 0.530 |
| O2 pulse (mL/beat) | 0.134 | 0.584 | −0.129 | 0.598 | −0.205 | 0.401 |
| O2 pulse % predicted | 0.293 | 0.223 | −0.023 | 0.926 | −0.112 | 0.647 |
| HR max (bpm) | −0.077 | 0.754 | −0.045 | 0.855 | 0.048 | 0.844 |
| HR max % predicted | −0.065 | 0.792 | −0.041 | 0.868 | 0.035 | 0.886 |
| SBP max (mmHg) | 0.026 | 0.915 | 0.159 | 0.515 | 0.146 | 0.551 |
| DBP max (mmHg) | −0.178 | 0.466 | −0.074 | 0.764 | 0.054 | 0.828 |
| T0 Serum Cortisol (ng/mL) | T1 Serum Cortisol (ng/mL) | |||
|---|---|---|---|---|
| r | p | r | p | |
| T0 salivary cortisol (µg/dL) | 0.720 | 0.002 | 0.534 | 0.036 |
| T2 salivary cortisol (µg/dL) | 0.569 | 0.022 | 0.872 | <0.001 |
| T3 salivary cortisol (µg/dL) | 0.303 | 0.414 | 0.765 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honceriu, C.; Roca, M.; Costache, A.D.; Abălașei, B.; Popescu, L.; Puni, A.R.; Maștaleru, A.; Oancea, A.; Drugescu, A.; Adam, C.; et al. Blood and Salivary Cortisol Variations in Athletes in Relation to Cardiopulmonary Exercise Testing. Medicina 2023, 59, 1726. https://doi.org/10.3390/medicina59101726
Honceriu C, Roca M, Costache AD, Abălașei B, Popescu L, Puni AR, Maștaleru A, Oancea A, Drugescu A, Adam C, et al. Blood and Salivary Cortisol Variations in Athletes in Relation to Cardiopulmonary Exercise Testing. Medicina. 2023; 59(10):1726. https://doi.org/10.3390/medicina59101726
Chicago/Turabian StyleHonceriu, Cezar, Mihai Roca, Alexandru Dan Costache, Beatrice Abălașei, Lucian Popescu, Alexandru Rareș Puni, Alexandra Maștaleru, Andra Oancea, Andrei Drugescu, Cristina Adam, and et al. 2023. "Blood and Salivary Cortisol Variations in Athletes in Relation to Cardiopulmonary Exercise Testing" Medicina 59, no. 10: 1726. https://doi.org/10.3390/medicina59101726
APA StyleHonceriu, C., Roca, M., Costache, A. D., Abălașei, B., Popescu, L., Puni, A. R., Maștaleru, A., Oancea, A., Drugescu, A., Adam, C., Mitu, O., Costache, I.-I., Leon, M. M., Roca, I. C., Mocanu, V., & Mitu, F. (2023). Blood and Salivary Cortisol Variations in Athletes in Relation to Cardiopulmonary Exercise Testing. Medicina, 59(10), 1726. https://doi.org/10.3390/medicina59101726








