Recent Advances and Adaptive Strategies in Image Guidance for Cervical Cancer Radiotherapy
Abstract
:1. Introduction
2. Imaging Modalities in Radiotherapy Planning
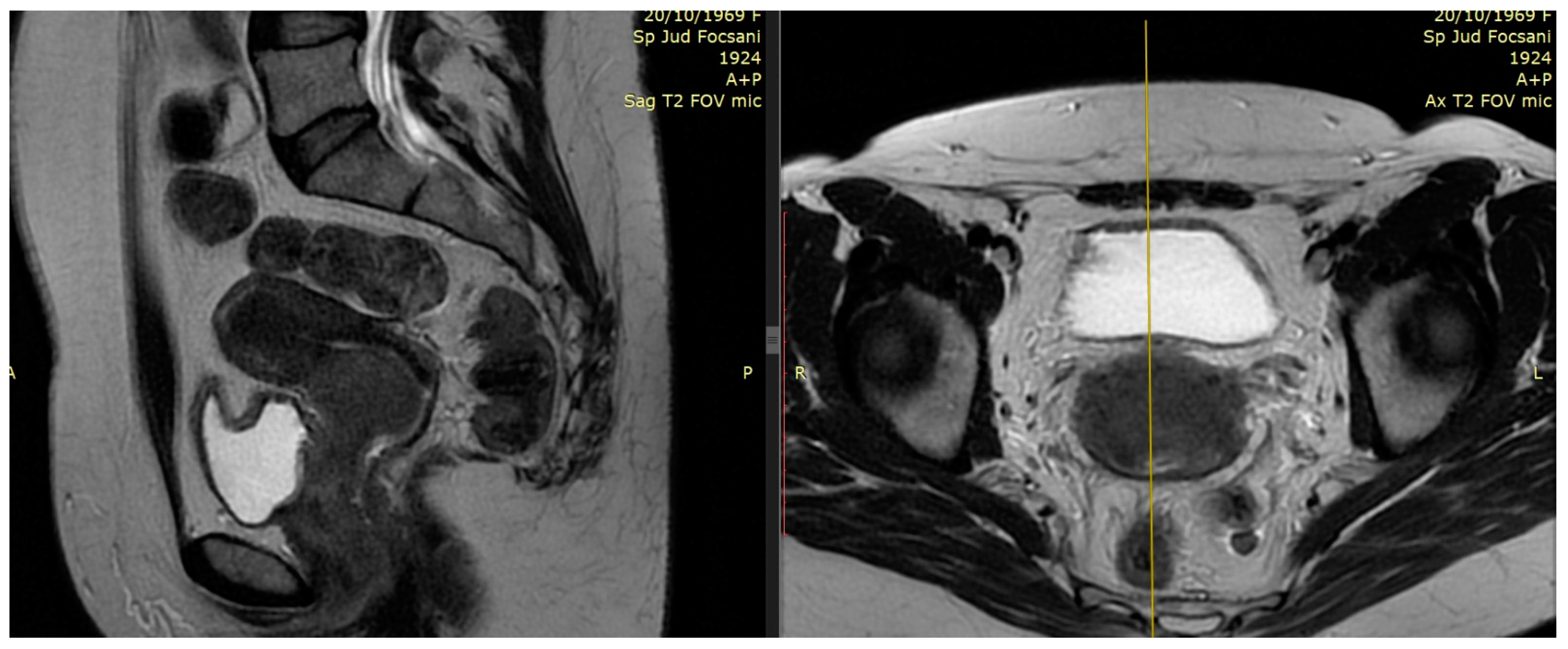
2.1. The Role of MRI
2.2. The Role of PET–CT
3. Advances in External Beam Radiotherapy
3.1. From 2D to 3D Conformal Radiotherapy
3.2. Intensity-Modulated Radiotherapy (IMRT) Volumetric Intensity-Modulated Arcs (VMAT)
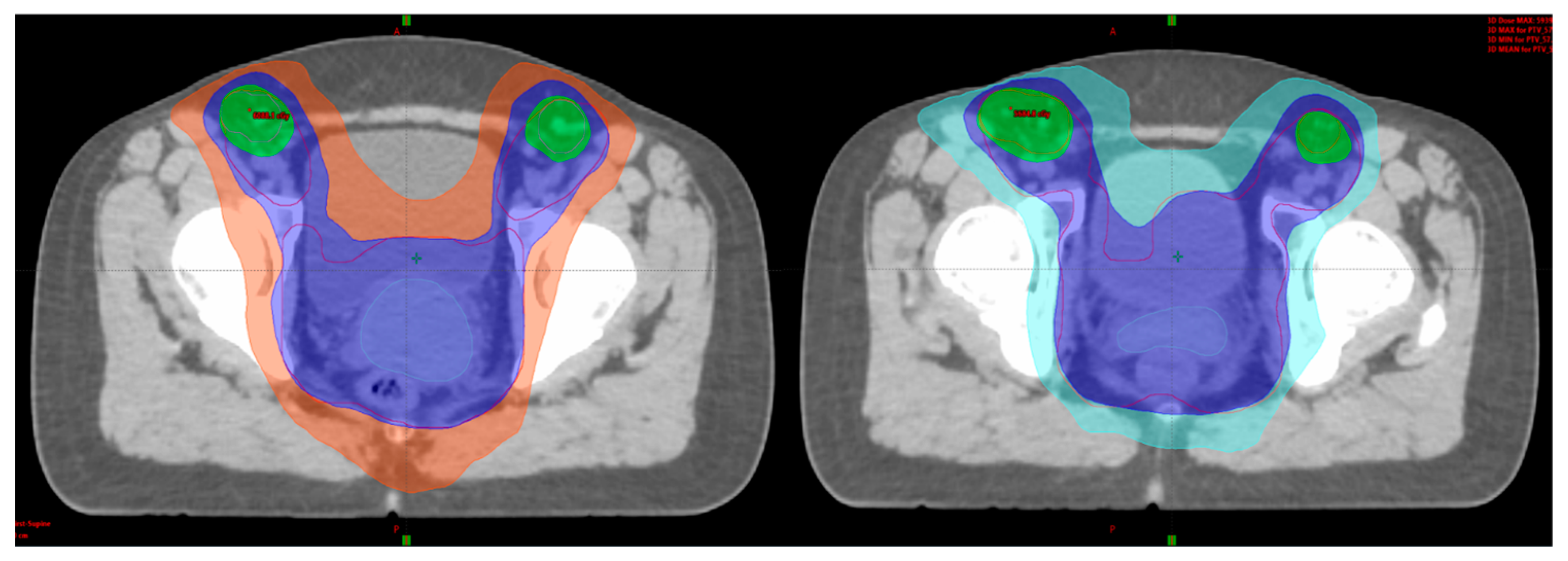
3.3. Adaptive External Beam Radiotherapy
4. Advances in Brachytherapy
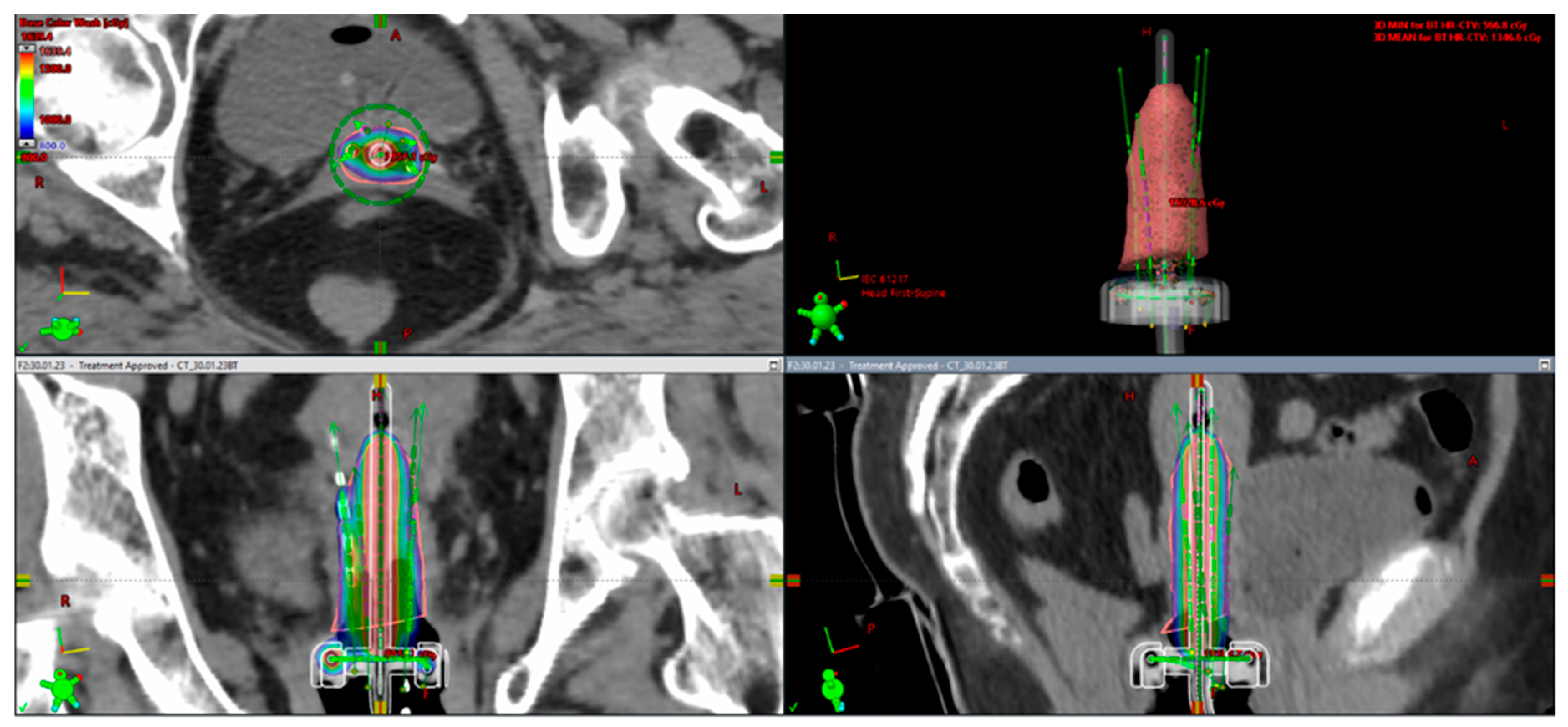
4.1. From 2D Brachytherapy (2D-BT) to 3D Image-Guided Adaptive Brachytherapy (3D-IGABT)
4.2. Adaptive Brachytherapy
5. New Modalities of Radiation Techniques
5.1. MRI-LINAC Using Adaptive Radiotherapy
5.2. 3D Printing in Cervical Cancer Brachytherapy
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | apparent diffusion coefficients |
| BT | brachytherapy |
| CBCT | cone beam computed tomography |
| CI | confidence interval |
| CRT | chemo-radiotherapy |
| CT | computed tomography |
| CTV | clinical target volume |
| DCE MRI | dynamic contrast-enhanced MRI |
| DW-MRI | diffusion-weighted MRI |
| DFS | disease free survival |
| DRR | digitally reconstructed radiograph |
| EBRT | external beam radiotherapy |
| EMBRACE | European study on MRI-guided brachytherapy in locally advanced cervical cancer |
| EQD2 | biologically equivalent dose in 2 Gy fractions |
| FDG | fluorodeoxyglucose |
| FIGO | International Federation of Gynaecology and Obstetrics |
| GEC- | The Groupe Européen de Curiethérapie and the European society for ESTRO radiotherapy & oncology |
| GOG | Gynecologic Oncology Group |
| GTV | gross tumor volume |
| HDR | high dose rate |
| HR CTV | high risk clinical target volume |
| ICRU | International Comission on Radiation Units and Measurements |
| IGABT | image-guided adaptive brachytherapy |
| IGRT | image-guided radiotherapy |
| IMRT | intensity modulated radiation therapy |
| IR CTV | intermediate risk clinical target volume |
| ITV | internal target volume |
| LACC | locally advanced cervical cancer |
| LC | local control |
| LDR | low dose rate |
| LINAC | linear accelerator |
| LN | lymph node |
| MLC | multileaf collimator |
| MRI | magnetic resonance imaging |
| OARs | organs at risk |
| OS | overall survival |
| OTT | overall treatment time |
| PA | paraaortic |
| PDR | pulsed dose rate |
| PET | positron emission tomography |
| PTV | planning target volume |
| RTOG | Radiation Therapy Oncology Group |
| SIB | simultaneous integrated boost |
| SWOG | Southwest Oncology Group |
| T | Tesla |
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, E191–E203. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Canadian Cancer Statistics Advisory Committee. CMAJ 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Otter, S.; Whitaker, S.; Chatterjee, J.; Stewart, A. The human papillomavirus as a common pathogen in oropharyngeal, anal, and cervical cancers. Clin. Oncol. 2019, 31, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Benard, V.B.; Watson, M.; Saraiya, M.; Harewood, R.; Townsend, J.S.; Stroup, A.M.; Weir, H.K.; Allemani, C. Cervical cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017, 123 (Suppl. 24), 5119–5137. [Google Scholar] [CrossRef]
- Han, K.; Milosevic, M.; Fyles, A.; Pintilie, M.; Viswanathan, A.N. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 111–119. [Google Scholar] [CrossRef]
- Cervical Cancer Version 1.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 22 August 2023).
- Vale, C.; Tierney, J.F.; Stewart, L.A.; Brady, M.; Dinshaw, K.; Jakobsen, A. Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C.; Clarke-Pearson, D.L.; Liao, S.Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.A., III; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Mundt, A.J.; Lujan, A.E.; Rotmensch, J.; Waggoner, S.E.; Yamada, S.D.; Fleming, G.; Roeske, J.C. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1330–1337. [Google Scholar] [CrossRef]
- Mundt, A.J.; Mell, L.K.; Roeske, J.C. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1354–1360. [Google Scholar] [CrossRef]
- Chen, M.F.; Tseng, C.J.; Tseng, C.C.; Kuo, Y.C.; Yu, C.Y.; Chen, W.C. Clinical outcome in posthysterectomy cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy: Comparison with conventional radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1438–1444. [Google Scholar] [CrossRef]
- Beriwal, S.; Gan, G.N.; Heron, D.E.; Selvaraj, R.N.; Kim, H.; Lalonde, R.; Kelley, J.L.; Edwards, R.P. Early clinical outcome with concurrent chemotherapy and extended-field, intensity-modulated radiotherapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 166–171. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC—ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef]
- Lee, S.I.; Atri, M. 2018 FIGO staging system for uterine cervical cancer: Enter cross-sectional imaging. Radiology 2019, 292, 15–24. [Google Scholar] [CrossRef]
- Dhoot, N.M.; Kumar, V.; Shinagare, A.; Kataki, A.C.; Barmon, D.; Bhuyan, U. Evaluation of carcinoma cervix using magnetic resonance imaging: Correlation with clinical FIGO staging and impact on management. J. Med. Imaging Radiat. Oncol. 2012, 56, 58–65. [Google Scholar] [CrossRef]
- Bipat, S.; Glas, A.S.; van der Velden, J.; Zwinderman, A.H.; Bossuyt, P.M.; Stoker, J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma. Gynecol. Oncol. 2003, 91, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, E.M.; Raaymakers, B.W.; van der Heide, U.A.; van de Bunt, L.; JurgenliemkSchulz, I.M.; Lagendijk, J.J. On line MRI guidance for healthy tissue sparing in patients with cervical cancer. Radiother. Oncol. 2008, 88, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, J.C.; Lang, S.; Kirisits, C.; Fidarova, E.F.; Berger, D.; Georg, P.; Dörr, W.; Pötter, R. Dosevolume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Balleyguier, C.; Sala, E.; Da Cunha, T.; Bergman, A.; Brkljacic, B.; Danza, F.; Forstner, R.; Hamm, B.; Kubik-Huch, R.; Lopez, C.; et al. Staging of uterine cervical cancer with MRI: Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2011, 21, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Fields, E.C.; Weiss, E. A practical review of magnetic resonance imaging for the evaluation and management of cervical cancer. Radiat. Oncol. 2016, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Sekiya, Y.; Araki, H.; Sakai, M.; Togawa, T.; Narita, Y.; Akiyama, Y.; Kimura, S.; Ito, H. Evaluation of the therapeutic effect of radiotherapy on cervical cancer using magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 639–644. [Google Scholar] [CrossRef]
- Vincens, E.; Balleyguier, C.; Rey, A.; Uzan, C.; Zareski, E.; Gouy, S.; Pautier, P.; Duvillard, P.; Haie-Meder, C.; Morice, P. Accuracy of magnetic resonance imaging in predicting residual disease in patients treated for stage IB2/II cervical carcinoma with chemoradiation therapy: Correlation of radiologic findings with surgico-pathologic results. Cancer 2008, 113, 2158–2165. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, C.K.; Park, S.Y.; Simonetti, A.W.; Kim, E.; Park, B.K.; Huh, S.J. Assessment of early response to concurrent chemoradiotherapy in cervical cancer: Value of diffusion-weighted and dynamic contrast-enhanced MR imaging. Magn. Reson. Imaging 2014, 32, 993–1000. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Kulasingam, S.L.; Matchar, D.B.; Myers, E.R. FDG—PET for management of cervical and ovarian cancer. Gynecol. Oncol. 2005, 97, 183–191. [Google Scholar] [CrossRef]
- Akkas, B.E.; Demirel, B.B.; Vural, G.E. Clinical impact of 18F-FDG PET/CT in the pretreatment evaluation of patients with locally advanced cervical carcinoma. Nucl. Med. Commun. 2012, 33, 1081–1088. [Google Scholar] [CrossRef]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Rader, J.S.; Mutch, D.G.; Powell, M.A.; Grigsby, P.W. Lymph node staging by positron emission tomography in cervical cancer. J. Clin. Oncol. 2010, 28, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lin, J.B.; Sun, F.J.; Chen, Y.J.; Chang, C.L.; Jan, Y.T.; Wu, M.H. Safety and efficacy of semi-extended field intensity-modulated radiation therapy and concurrent cisplatin in locally advanced cervical cancer patients: An observational study of 10-year experience. Medicine 2017, 96, e6158. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.S.; Lai, C.H.; Chang, T.C.; Yen, T.C.; Ng, K.K.; Hsueh, S.; Lee, S.P.; Hong, J.-H. A prospective randomized trial to study the impact of pretreatment FDG—PET for cervical cancer patients with MRI-detected positive pelvic but negative paraaortic lymphadenopathy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Chen, W.M.; Chen, M.; Shia, B.C.; Wu, S.Y. Survival effect of pre-RT PET—CT on cervical cancer: Image-guided intensity-modulated radiation therapy era. Front. Oncol. 2023, 13, 1012491. [Google Scholar] [CrossRef]
- Gouy, S.; Morice, P.; Narducci, F.; Uzan, C.; Martinez, A.; Rey, A.; Bentivegna, E.; Pautier, P.; Deandreis, D.; Querleu, D.; et al. Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J. Clin. Oncol. 2013, 31, 3026–3033. [Google Scholar] [CrossRef]
- Lazzari, R.; Cecconi, A.; Jereczek-Fossa, B.A.; Travaini, L.L.; Dell’Acqua, V.; Cattani, F.; Rizzo, S.; Fodor, C.; Landoni, F.; Orecchia, R. The role of [(18)F]FDG—PET/CT in staging and treatment planning for volumetric modulated RapidArc radiotherapy in cervical cancer: Experience of the European Institute of Oncology, Milan, Italy. Ecancermedicalscience 2014, 8, 405. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Choi, H.J.; Ju, W.; Myung, S.K.; Kim, Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: Meta-analysis. Cancer Sci. 2010, 101, 1471–1479. [Google Scholar] [CrossRef]
- Adam, J.A.; van Diepen, P.R.; Mom, C.H.; Stoker, J.; van Eck-Smit, B.L.F.; Bipat, S. [18F] FDG—PET or PET/CT in the evaluation of pelvic and para-aortic lymph nodes in patients with locally advanced cervical cancer: A systematic review of the literature. Gynecol. Oncol. 2020, 159, 588–596. [Google Scholar] [CrossRef]
- Nomden, C.N.; Pötter, R.; de Leeuw, A.A.C.; Tanderup, K.; Lindegaard, J.C.; Schmid, M.P.; Fortin, I.; Haie-Meder, C.; Mahantshetty, U.; Hoskin, P.; et al. EMBRACE Collaborative Group. Nodal failure after chemo-radiation and MRI guided brachytherapy in cervical cancer: Patterns of failure in the EMBRACE study cohort. Radiother. Oncol. 2019, 134, 185–190. [Google Scholar] [CrossRef]
- Ramlov, A.; Kroon, P.S.; Jürgenliemk-Schulz, I.M.; De Leeuw, A.A.; Gormsen, L.C.; Fokdal, L.U.; Tanderup, K.; Lindegaard, J.C. Impact of radiation dose and standardized uptake value of (18)FDG PET on nodal control in locally advanced cervical cancer. Acta Oncol. 2015, 54, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Bonin, S.R.; Lanciano, R.M.; Corn, B.W.; Hogan, W.M.; Hartz, W.H.; Hanks, G.E. Bony landmarks are not an adequate substitute for lymphangiography in defining pelvic lymph node location for the treatment of cervical cancer with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 167–172. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiation Units and Measurements. ICRU Report 50 Prescribing, Recording, and Reporting Photon Beam Therapy; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1993; Volume 21. [Google Scholar]
- International Commission on Radiation Units and Measurements. ICRU Report 62 Prescribing, Recording, and Reporting Photon Beam Therapy; Supplement to ICRU Report 50; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1999; Volume 21. [Google Scholar]
- Gerstner, N.; Wachter, S.; Knocke, T.H.; Fellner, C.; Wambersie, A.; Pötter, R. The benefit of Beam’s eye view-based 3D treatment planning for cervical cancer. Radiother. Oncol. 1999, 51, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small Jr, W.; Thompson, S.; et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Tsunoda, A.T.; Martus, P.; Vieira, M.; Affonso Junior, R.J.; Nunes, J.; Budach, V.; Hatel, H.; Mustea, A.; Sehouli, J.; et al. Surgical versus clinical staging prior to primary chemoradiation in patients with cervical cancer FIGO stages IIB-IVA: Oncologic results of a prospective randomized international multicenter (Uterus-11) intergroup study. Int. J. Gynecol. Cancer. 2020, 30, 1855–1861. [Google Scholar] [CrossRef]
- Chopra, S.; Dora, T.; Gupta, S.; Kannan, S.; Engineer, R.; Mangaj, A.; Maheshwari, A.; Shylasree, T.S.; Ghosh, J.; Paul, S.N.; et al. Phase III randomized trial of postoperative adjuvant conventional radiation (3DCRT) versus image-guided intensity-modulated radiotherapy (IG-IMRT) in cervical cancer (PARCER): Final analysis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S1–S2. [Google Scholar] [CrossRef]
- Mell, L.K.; Sirák, I.; Wei, L.; Tarnawski, R.; Mahantshetty, U.; Yashar, C.M.; McHale, M.T.; Xu, R.; Honerkamp-Smith, G.; Carmona, R.; et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: An international multicenter phase II clinical trial (INTERTECC-2). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 536–545. [Google Scholar] [CrossRef]
- Jadon, R.; Pembroke, C.A.; Hanna, C.L.; Palaniappan, N.; Evans, M.; Cleves, A.E.; Staffurth, J. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin. Oncol. R. Coll. Radiol. 2014, 26, 185–196. [Google Scholar] [CrossRef]
- Hoppe, R.T.; Phillips, T.L.; Roach, M. Chapter 12: Image-guided Adaptive Radiotherapy. In Textbook of Radiation Oncology, 3rd ed.; Keall, P.J., Hsu, A., Xing, L., Eds.; Saunders: Philadelphia, PA, USA, 2004. [Google Scholar]
- Verellen, D.; De Ridder, M.; Storme, G. A (short) history of image-guided radiotherapy. Radiother. Oncol. 2008, 86, 4–13. [Google Scholar] [CrossRef]
- Chan, P.; Dinniwell, R.; Haider, M.A.; Cho, Y.B.; Jaffray, D.; Lockwood, G.; Levin, W.; Manchul, L.; Fyles, A.; Milosevic, M. Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: A cinematic-MRI point-of-interest study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1507–1515. [Google Scholar] [CrossRef]
- Bondar, L.; Hoogeman, M.; Mens, J.W.; Dhawtal, G.; de Pree, I.; Ahmad, R.; Quint, S.; Heijmen, B. Toward an individualized target motion management for IMRT of cervical cancer based on model-predicted cervix-uterus shape and position. Radiother. Oncol. 2011, 99, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Shelley, C.E.; Barraclough, L.H.; Nelder, C.L.; Otter, S.J.; Stewart, A.J. Adaptive Radiotherapy in the Management of Cervical Cancer: Review of Strategies and Clinical Implementation. Clin. Oncol. R. Coll. Radiol. 2021, 33, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, V.; Serban, D.; Costea, D.; Dumitrescu, D.; Bobirca, F.; Geavlete, B.; Bratu, D.G.; Tribus, L.; Serboiu, C.; Alius, C.; et al. Transabdominal Preperitoneal Versus Lichtenstein Procedure for Inguinal Hernia Repair in Adults: A Comparative Evaluation of the Early Postoperative Pain and Outcomes. Cureus 2023, 15, e41886. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Eifel, P.J. Comment on Trends in the utilization of brachytherapy in cervical cancer in the United States. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Tod, M.C.; Meredith, W.J. A dosage system for use in the treatment of cancer of the uterine cervix. Br. J. Radiol. 1938, 11, 809–824. [Google Scholar] [CrossRef]
- Tod, M.C.; Meredith, W.J. Treatment of cancer of the cervix uteri, a revised Manchester method. Br. J. Radiol. 1953, 26, 252–257. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Erickson, B.; Gaffney, D.K.; Beriwal, S.; Bhatia, S.K.; Lee Burnett, O.; D’Souza, D.P.; Patil, N.; Haddock, M.G.; Jhingran, A.; et al. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 320–328. [Google Scholar] [CrossRef]
- Haie-Meder, C.; Pötter, R.; Van Limbergen, E.; Briot, E.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Hellebust, T.P.; Kirisits, C.; Lang, S.; et al. Recommendations from Gynaecological (GYN) GEC—ESTRO Working Group (I): Concepts and terms in 3D image-based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother. Oncol. 2005, 74, 235–245. [Google Scholar] [CrossRef]
- Pötter, R.; Haie-Meder, C.; Van Limbergen, E.; Barillot, I.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Erickson, B.; Lang, S.; Nulens, A.; et al. Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy. Radiother. Oncol. 2006, 78, 67–77. [Google Scholar] [CrossRef]
- Rijkmans, E.C.; Nout, R.A.; Rutten, I.H.; Ketelaars, M.; Neelis, K.J.; Laman, M.S.; Coen, V.L.; Gaarenstroom, K.N.; Kroep, J.R.; Creutzberg, C.L. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol. Oncol. 2014, 135, 231–238. [Google Scholar] [CrossRef]
- Lin, A.J.; Kidd, E.; Dehdashti, F.; Siegel, B.A.; Mutic, S.; Thaker, P.H.; Massad, L.S.; Powell, M.A.; Mutch, D.G.; Markovina, S.; et al. Intensity Modulated Radiation Therapy and Image-Guided Adapted Brachytherapy for Cervix Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Dimopoulos, J.; Kirisits, C.; Berger, D.; Pötter, R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef]
- Schmid, M.; Haie-Meder, C.; Mahanshetty, U.; Jürgenliemk-Schulz, I.M.; Segedin, B.; Hoskin, P.; Pötter, R. OC-0055: Local failures after radiochemotherapy and MR-image-guided brachytherapy in cervical cancer patients. Radiother. Oncol. 2017, 123, S26. [Google Scholar] [CrossRef]
- Fortin, I.; Tanderup, K.; Haie-Meder, C.; Lindegaard, J.C.; Mahantshetty, U.; Segedin, B.; Jürgenliemk-Schulz, I.M.; Hoskin, P.; Kirisits, C.; Potter, R.; et al. Image-guided brachytherapy in cervical cancer: A comparison between intracavitary and combined intracavitary/interstitial brachytherapy in regard to doses to HR CTV, OARs and late morbidity—Early results from the Embrace study in 999 patients. Brachytherapy 2016, 15, S21. [Google Scholar] [CrossRef]
- Anghel, B. PO24: High Dose-Rate Tandem and Ovoid 3D CT Based Brachytherapy in Cervical Cancer: Initial Single Center Experience. Brachytherapy 2021, 20, S66–S67. [Google Scholar] [CrossRef]
- Charra-Brunaud, C.; Harter, V.; Delannes, M.; Haie-Meder, C.; Quetin, P.; Kerr, C.; Castelain, B.; Thomas, L.; Peiffert, D. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother. Oncol. 2012, 102, 305–313. [Google Scholar] [CrossRef]
- Kirisits, C.; Lang, S.; Dimopoulos, J.; Oechs, K.; Georg, D.; Potter, R. Uncertainties when using only one MRI-based treatment plan for subsequent high-dose-rate tandem and ring applications in brachytherapy of cervix cancer. Radiother. Oncol. 2006, 81, 269–275. [Google Scholar] [CrossRef]
- Beriwal, S.; Kim, H.; Coon, D.; Mogus, R.; Heron, D.E.; Huq, M.S. Single magnetic resonance imaging vs magnetic resonance imaging/computed tomography planning in cervical cancer brachytherapy. Clin. Oncol. R. Coll. Radiol. 2009, 21, 483–487. [Google Scholar] [CrossRef]
- Kim, H.; Rajagopalan, M.S.; Beriwal, S.; Huq, M.S.; Smith, K.J. Cost-effectiveness analysis of 3D image-guided brachytherapy compared with 2D brachytherapy in the treatment of locally advanced cervical cancer. Brachytherapy 2015, 14, 29–36. [Google Scholar] [CrossRef]
- Tan, L.T.; Tanderup, K.; Kirisits, C.; Mahantshetty, U.; Swamidas, J.; Jürgenliemk-Schulz, I.; Lindegaard, J.; de Leeuw, A.; Nesvacil, N.; Assenholt, M.; et al. Education and training for image-guided adaptive brachytherapy for cervix cancer-The (GEC)-ESTRO/EMBRACE perspective. Brachytherapy 2020, 19, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, J.C.; Kirisits, C.; Petric, P.; Georg, P.; Lang, S.; Berger, D.; Potter, R. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Clinical feasibility and preliminary results. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 83–90. [Google Scholar] [CrossRef] [PubMed]
- De Brabandere, M.; Mousa, A.G.; Nulens, A.; Swinnen, A.; Van Limbergen, E. Potential of dose optimisation in MRI-based PDR brachytherapy of cervix carcinoma. Radiother. Oncol. 2008, 88, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, J.C.; Tanderup, K.; Nielsen, S.K.; Haack, S.; Gelineck, J. MRI-guided 3D optimization significantly improves DVH parameters of pulsed-dose-rate brachytherapy in locally advanced cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 756–764. [Google Scholar] [CrossRef]
- Tanderup, K.; Lindegaard, J.C.; Kirisits, C.; Haie-Meder, C.; Kirchheiner, K.; de Leeuw, A.; Jürgenliemk-Schulz, I.; Van Limbergen, E.; Pötter, R. Image Guided Adaptive Brachytherapy in cervix cancer: A new paradigm changing clinical practice and outcome. Radiother. Oncol. 2016, 120, 365–369. [Google Scholar] [CrossRef]
- Sturdza, A.E.; Knoth, J. Image-guided brachytherapy in cervical cancer including fractionation. Int. J. Gynecol. Cancer: Off. J. Int. Gynecol. Cancer Soc. 2022, 32, 273–280. [Google Scholar] [CrossRef]
- Vojtíšek, R. Image guided adaptive brachytherapy of cervical cancer—Practical recommendations. Klin. Onkol. 2023, 36, 96–103. [Google Scholar] [CrossRef]
- Sturdza, A.E.; Pötter, R.; Kossmeier, M.; Kirchheiner, K.; Mahantshetty, U.; Haie-Meder, C.; Lindegaard, J.C.; Jurgenliemk-Schulz, I.; Tan, L.T.; Hoskin, P.; et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with radiochemotherapy including image-guided brachytherapy: A Retro-EMBRACE study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 168–177. [Google Scholar] [CrossRef]
- Dumitru, M.; Berghi, O.N.; Taciuc, I.A.; Vrinceanu, D.; Manole, F.; Costache, A. Could Artificial Intelligence Prevent Intraoperative Anaphylaxis? Reference Review and Proof of Concept. Medicina 2022, 58, 1530. [Google Scholar] [CrossRef]
- Corradini, S.; Alongi, F.; Andratschke, N.; Belka, C.; Boldrini, L.; Cellini, F.; Debus, J.; Guckenberger, M.; Hörner-Rieber, J.; Lagerwaard, F.J.; et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat. Oncol. 2019, 14, 92. [Google Scholar] [CrossRef]
- Ng, J.; Gregucci, F.; Pennell, R.T.; Nagar, H.; Golden, E.B.; Knisely, J.P.S.; Sanfilippo, N.J.; Formenti, S.C. MRI-LINAC: A transformative technology in radiation oncology. Front. Oncol. 2023, 13, 1117874. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.W.; Rammohan, N.; Das, I.J.; Yadav, P. Towards Accurate and Precise Image-Guided Radiotherapy: Clinical Applications of the MR-Linac. J. Clin. Med. 2022, 11, 4044. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Boldrini, L.; Dhont, J.; Fiorino, C.; Green, O.; Güngör, G.; Jornet, N.; Klüter, S.; Landry, G.; Mattiucci, G.C.; et al. Artificial Intelligence in magnetic Resonance guided Radiotherapy: Medical and physical considerations on state of art and future perspectives. Phys. Med. 2021, 85, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Ding, Y.; Sheng, C.; Pi, Y.; Guo, Y.; Wu, A.; Zhang, Z. Application of 3D printing in cervical cancer brachytherapy. J. Radiat. Res. Appl. Sci. 2022, 15, 18–24. [Google Scholar] [CrossRef]
- Marar, M.; Simiele, E.; Niedermayr, T.; Kidd, E.A. Applying 3D-Printed Templates in High-Dose-Rate Brachytherapy for Cervix Cancer: Simplified Needle Insertion for Optimized Dosimetry. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 111–119. [Google Scholar] [CrossRef]
- Webster, A.; Appelt, A.L.; Eminowicz, G. Image-Guided Radiotherapy for Pelvic Cancers: A Review of Current Evidence and Clinical Utilisation. Clin. Oncol. R. Coll. Radiol. 2020, 32, 805–816. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, B.; Serboiu, C.; Marinescu, A.; Taciuc, I.-A.; Bobirca, F.; Stanescu, A.D. Recent Advances and Adaptive Strategies in Image Guidance for Cervical Cancer Radiotherapy. Medicina 2023, 59, 1735. https://doi.org/10.3390/medicina59101735
Anghel B, Serboiu C, Marinescu A, Taciuc I-A, Bobirca F, Stanescu AD. Recent Advances and Adaptive Strategies in Image Guidance for Cervical Cancer Radiotherapy. Medicina. 2023; 59(10):1735. https://doi.org/10.3390/medicina59101735
Chicago/Turabian StyleAnghel, Beatrice, Crenguta Serboiu, Andreea Marinescu, Iulian-Alexandru Taciuc, Florin Bobirca, and Anca Daniela Stanescu. 2023. "Recent Advances and Adaptive Strategies in Image Guidance for Cervical Cancer Radiotherapy" Medicina 59, no. 10: 1735. https://doi.org/10.3390/medicina59101735
APA StyleAnghel, B., Serboiu, C., Marinescu, A., Taciuc, I.-A., Bobirca, F., & Stanescu, A. D. (2023). Recent Advances and Adaptive Strategies in Image Guidance for Cervical Cancer Radiotherapy. Medicina, 59(10), 1735. https://doi.org/10.3390/medicina59101735






