Is COPD the Determinant Factor for Myocardial Injury and Cardiac Wall Stress in OSA Patients?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Examination
2.3. Polysomnography
2.4. Lung Function and Diagnosis of COPD
2.5. Blood Samples and Measurements
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Cardiovascular Comorbidity in Overlap Syndrome
4.2. NT-proBNP and hs-cTn as Markers of Myocardial Wall Stress and Injury
4.3. Assessment of NT-proBNP and hs-cTn in OSA and COPD Populations
4.4. Associations between hs-cTnT and NT-proBNP and Parameters Related to OSA and COPD
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shawon, M.S.R.; Perret, J.L.; Senaratna, C.V.; Lodge, C.; Hamilton, G.S.; Dharmage, S.C. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Med. Rev. 2017, 32, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Voulgaris, A.; Archontogeorgis, K.; Steiropoulos, P.; Papanas, N. Cardiovascular Disease in Patients with Chronic Obstructive Pulmonary Disease, Obstructive Sleep Apnoea Syndrome and Overlap Syndrome. Curr. Vasc. Pharmacol. 2021, 19, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Czerwaty, K.; Dżaman, K.; Sobczyk, K.M.; Sikorska, K.I. The Overlap Syndrome of Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease: A Systematic Review. Biomedicines 2022, 11, 16. [Google Scholar] [CrossRef]
- Voulgaris, A.; Archontogeorgis, K.; Papanas, N.; Pilitsi, E.; Nena, E.; Xanthoudaki, M.; Mikhailidis, D.P.; Froudarakis, M.E.; Steiropoulos, P. Increased risk for cardiovascular disease in patients with obstructive sleep apnoea syndrome-chronic obstructive pulmonary disease (overlap syndrome). Clin. Respir. J. 2019, 13, 708–715. [Google Scholar] [CrossRef]
- McNicholas, W.T. COPD-OSA Overlap Syndrome. Chest 2017, 152, 1318–1326. [Google Scholar] [CrossRef]
- Voulgaris, A.; Archontogeorgis, K.; Pataka, A.; Flaris, A.N.; Ntolios, P.; Bonsignore, M.R.; Schiza, S.; Steiropoulos, P. Burden of Comorbidities in Patients with OSAS and COPD-OSAS Overlap Syndrome. Medicina 2021, 57, 1201. [Google Scholar] [CrossRef]
- Tang, M.; Long, Y.; Liu, S.; Yue, X.; Shi, T. Prevalence of Cardiovascular Events and Their Risk Factors in Patients With Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea Overlap Syndrome. Front. Cardiovasc. Med. 2021, 8, 694806. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Quek, E.; Alqahtani, J.S.; Hurst, J.R.; Mandal, S. Cardiovascular outcomes in patients with COPD-OSA overlap syndrome: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 63, 101627. [Google Scholar] [CrossRef] [PubMed]
- Einvik, G.; Røsjø, H.; Randby, A.; Namtvedt, S.K.; Hrubos-Strøm, H.; Brynildsen, J.; Somers, V.K.; Omland, T. Severity of Obstructive Sleep Apnea is Associated with Cardiac Troponin I Concentrations in a Community-based Sample: Data from the Akershus Sleep Apnea Project. Sleep 2014, 37, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Hoiseth, A.D.; Neukamm, A.; Karlsson, B.D.; Omland, T.; Brekke, P.H.; Soyseth, V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011, 66, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Labaki, W.W.; Xia, M.; Murray, S.; Curtis, J.L.; Barr, R.G.; Bhatt, S.P.; Bleecker, E.R.; Hansel, N.N.; Cooper, C.B.; Dransfield, M.T.; et al. NT-proBNP in stable COPD and future exacerbation risk: Analysis of the SPIROMICS cohort. Respir. Med. 2018, 140, 87–93. [Google Scholar] [CrossRef]
- Tasci, S.; Manka, R.; Scholtyssek, S.; Lentini, S.; Troatz, C.; Stoffel-Wagner, B.; Lüderitz, B. NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clin. Res. Cardiol. 2006, 95, 23–30. [Google Scholar] [CrossRef]
- Hübner, R.-H.; El Mokhtari, N.E.; Freitag, S.; Rausche, T.; Göder, R.; Tiroke, A.; Lins, M.; Simon, R.; Bewig, B. NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir. Med. 2008, 102, 134–142. [Google Scholar] [CrossRef][Green Version]
- Strehmel, R.; Valo, M.; Teupe, C. Natriuretic Peptide and High-Sensitive Troponin T Concentrations Correlate with Effectiveness of Short-Term CPAP in Patients with Obstructive Sleep Apnea and Coronary Artery Disease. Clin. Med. Insights Circ. Respir. Pulm. Med. 2016, 10, 33–39. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Tsara, V.; Serasli, E.; Amfilochiou, A.; Constantinidis, T.; Christaki, P. Greek Version of the Epworth Sleepiness Scale. Sleep Breath. 2004, 8, 91–95. [Google Scholar] [CrossRef]
- Mancia Chairperson, G.; Kreutz Co-Chair, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J. Hypertens. 2023. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Iwanaga, Y.; Nishi, I.; Furuichi, S.; Noguchi, T.; Sase, K.; Kihara, Y.; Goto, Y.; Nonogi, H. B-Type Natriuretic Peptide Strongly Reflects Diastolic Wall Stress in Patients With Chronic Heart Failure: Comparison Between Systolic and Diastolic Heart Failure. J. Am. Coll. Cardiol. 2006, 47, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Hall, C. Essential biochemistry and physiology of (NT-pro)BNP. Eur. J. Heart Fail. 2004, 6, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Braun, S.; Niemöller, K.; Mehilli, J.; von Beckerath, N.; von Beckerath, O.; Vogt, W.; Schömig, A.; Kastrati, A. Prognostic Value of N-Terminal Pro–Brain Natriuretic Peptide in Patients with Chronic Stable Angina. Circulation 2005, 112, 2102–2107. [Google Scholar] [CrossRef]
- Maisel, A.S.; Clopton, P.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; E Hollander, J.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; et al. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: Results from the Breathing Not Properly (BNP) multinational study. Am. Heart J. 2004, 147, 1078–1084. [Google Scholar] [CrossRef]
- Keller, T.; Zeller, T.; Peetz, D.; Tzikas, S.; Roth, A.; Czyz, E.; Bickel, C.; Baldus, S.; Warnholtz, A.; Fröhlich, M.; et al. Sensitive Troponin I Assay in Early Diagnosis of Acute Myocardial Infarction. N. Engl. J. Med. 2009, 361, 868–877. [Google Scholar] [CrossRef]
- Zeller, T.; Tunstall-Pedoe, H.; Saarela, O.; Ojeda, F.; Schnabel, R.B.; Tuovinen, T.; Woodward, M.; Struthers, A.; Hughes, M.; Kee, F.; et al. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: The MORGAM Biomarker Project Scottish Cohort. Eur. Heart J. 2014, 35, 271–281. [Google Scholar] [CrossRef]
- Adamson, P.D.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.; Celli, B.R.; Cowans, N.J.; Crim, C.; Dixon, I.J.; Martinez, F.J.; Newby, D.E.; et al. Cardiac Troponin I and Cardiovascular Risk in Patients with Chronic Obstructive Pulmonary Disease. J. Am. Coll. Cardiol. 2018, 72, 1126–1137. [Google Scholar] [CrossRef]
- Waschki, B.; Alter, P.; Zeller, T.; Magnussen, C.; Neumann, J.T.; Twerenbold, R.; Sinning, C.; Herr, C.; Kahnert, K.; Fähndrich, S.; et al. High-sensitivity troponin I and all-cause mortality in patients with stable COPD: An analysis of the COSYCONET study. Eur. Respir. J. 2019, 55, 1901314. [Google Scholar] [CrossRef] [PubMed]
- Roca, G.Q.; Redline, S.; Punjabi, N.; Claggett, B.; Ballantyne, C.M.; Solomon, S.D.; Shah, A.M. Sleep Apnea Is Associated with Subclinical Myocardial Injury in the Community. The ARIC-SHHS Study. Am. J. Respir. Crit. Care Med. 2013, 188, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lei, T.; Yu, H.; Zhang, L.; Feng, Z.; Shuai, T.; Guo, H.; Liu, J. NT-proBNP in Different Patient Groups of COPD: A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Z.; Huang, K.; Li, G.; Luo, J.; Xu, Y.; Chen, P.; Chen, J.; Wang, L. The Clinical Value of N-Terminal Pro B-Type Natriuretic Peptide in Evaluating Obstructive Sleep Apnea in Patients With Coronary Artery Disease. Sleep Med. 2019, 15, 1403–1409. [Google Scholar] [CrossRef]
- Due-Andersen, R.; Pedersen-Bjergaard, U.; Høi-Hansen, T.; Olsen, N.V.; Kistorp, C.; Faber, J.; Boomsma, F.; Thorsteinsson, B. NT-pro-BNP during hypoglycemia and hypoxemia in normal subjects: Impact of renin-angiotensin system activity. J. Appl. Physiol. 2008, 104, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Raymond, I.; A Groenning, B.; Hildebrandt, P.R.; Nilsson, J.C.; Baumann, M.; Trawinski, J.; Pedersen, F. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart 2003, 89, 745–751. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The Intersection Between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Vigen, R.; Maddox, T.M.; Allen, L.A. Aging of the United States Population: Impact on Heart Failure. Curr. Heart Fail. Rep. 2012, 9, 369–374. [Google Scholar] [CrossRef]
- Tanindi, A.; Cemri, M. Troponin elevation in conditions other than acute coronary syndromes. Vasc. Health Risk Manag. 2011, 7, 597–603. [Google Scholar] [CrossRef]
- McNicholas, W.; Kent, B.D.; Mitchell, P.D. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 199–208. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Preiss, D.; Hayward, C.; Shah, A.S.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Padmanabhan, S.; Welsh, C.; et al. Cardiac Troponin T and Troponin I in the General Population. Circulation 2019, 139, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S. The Role of Smoking in the Mechanisms of Development of Chronic Obstructive Pulmonary Disease and Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 8725. [Google Scholar] [CrossRef] [PubMed]
- Carratù, P.; Di Ciaula, A.; Dragonieri, S.; Ranieri, T.; Ciccone, M.M.; Portincasa, P.; Resta, O. Relationships between Obstructive Sleep Apnea Syndrome and cardiovascular risk in a naïve population of southern Italy. Int. J. Clin. Pract. 2021, 75, e14952. [Google Scholar] [CrossRef]
- Kang, K.; Park, J.-M.; Ryu, W.-S.; Jeong, S.-W.; Kim, D.-E.; Park, H.-K.; Cho, Y.-J.; Hong, K.-S.; Lee, K.B.; Park, T.H.; et al. Body mass index and waist circumference as predictors of recurrent vascular events after a recent ischemic stroke. J. Stroke Cerebrovasc. Dis. 2023, 32, 107221. [Google Scholar] [CrossRef]

| OSA Patients (n = 53) | OS Patients (n = 53) | p | |
|---|---|---|---|
| Gender (males/females) | 44/9 | 49/4 | 0.139 |
| Age (years) | 50 (41.5–60) | 62 (54.5–70) | <0.001 |
| Neck circumference (cm) | 44 (42–46) | 46.5 (44–50) | <0.001 |
| Waist circumference (cm) | 118 (110–126) | 132 (120–136) | <0.001 |
| Hip circumference (cm) | 115 (108–123) | 119 (111–128) | 0.156 |
| WHR | 0.88 (0.82–0.97) | 0.90 (0.87–1.01) | 0.158 |
| BMI (kg/m2) | 35.3 (31.1–39.2) | 37 (34.5–41.5) | 0.050 |
| Tobacco smoking | 62.3% | 86.8% | 0.004 |
| Alcohol consumption | 64.2% | 73.6% | 0.294 |
| OSA Patients (n = 53) | OS Patients (n = 53) | p | |
|---|---|---|---|
| TST (min) | 337.3 (301.3–362.1) | 317.8 (281.5–351.5) | 0.132 |
| N1 (%) | 5.2 (3.5–9.5) | 9.9 (4.5–18.2) | 0.011 |
| N2 (%) | 78.8 (72.3–89) | 71.2 (63.1–85.9) | 0.004 |
| N3 (%) | 4.3 (0–10.7) | 7.4 (0–15.5) | 0.119 |
| REM (%) | 4.9 (1.7–9.5) | 4 (0.8–11.5) | 0.824 |
| AHI (events/hour) | 36.5 (19.1–66.8) | 35.5 (18–53.5) | 0.587 |
| Aver SpO2 (%) | 93.3 (91–94.5) | 91.1 (87.9–93.2) | 0.002 |
| Min SpO2 (%) | 72 (65–84) | 75 (65.5–80) | 0.695 |
| T < 90% (%) | 9 (0.8–27.9) | 22.7 (6.9–50) | 0.003 |
| Arousal index | 1 (0–4) | 5 (1–7.8) | 0.001 |
| Sleep efficiency (%) | 89.1 (79–93.3) | 84 (74.6–89.7) | 0.027 |
| ESS score | 10 (6.5–13) | 11(6–16) | 0.432 |
| OSA Patients (n = 53) | OS Patients (n = 53) | p | |
|---|---|---|---|
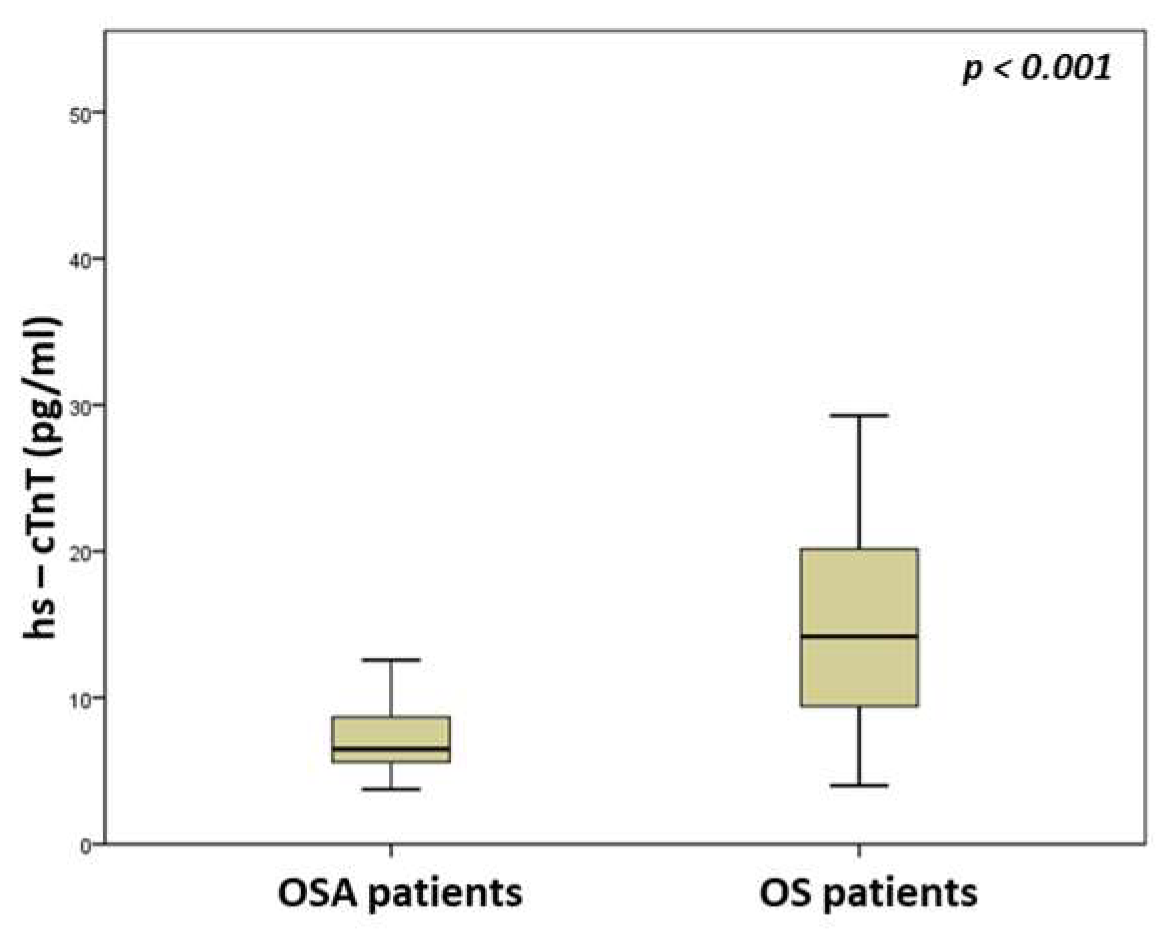
| hs-cTnT (pg/mL) | 6.5 (5.6–8.7) | 14.2 (9.1–20.2) | <0.001 |
| NT-proBNP (pg/mL) | 19.2 (8.3–35.4) | 93.1 (37.9–182.5) | <0.001 |
| CRP (mg/dl) | 0.49 (0.15–0.81) | 0.48 (0.16–0.81) | 0.246 |
| Glucose (mg/dL) | 111 (97–141) | 112 (93.5–133.8) | 0.703 |
| Creatinine (mg/dL) | 0.9 (0.8–1) | 0.9 (0.85–1.1) | 0.206 |
| SGOT (U/L) | 20 (18–25) | 20 (17–23.8) | 0.780 |
| SGPT (U/L) | 26 (19–34.5) | 22 (18–27) | 0.131 |
| Cholesterol (mg/dL) | 201 (185.5–226) | 189 (162–224) | 0.199 |
| Triglycerides (mg/dL) | 181 (133.5–206) | 156 (118–216.5) | 0.200 |
| LDL-C (mg/dL) | 119.5 (99.3–144.8) | 104.6 (80.4–141.6) | 0.123 |
| HDL-C (mg/dL) | 46 (40–54) | 46 (40.5–51) | 0.914 |
| FEV1 (% predicted) | 100 (88.1–115) | 65.1 (49.6–77.4) | <0.001 |
| FVC (% predicted) | 97 (87.8–109.5) | 77 (61.2–90.1) | <0.001 |
| FEV1/FVC (%) | 85.7 (81.5–89) | 68.9 (63.1–69.7) | <0.001 |
| pH | 7.43 (7.42–7.44) | 7.42 (7.41–7.44) | 0.072 |
| pO2 (mmHg) | 80 (72.5–87) | 68 (61–78) | <0.001 |
| pCO2 (mmHg) | 42 (40–43) | 46 (42–51) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voulgaris, A.; Archontogeorgis, K.; Apessos, I.; Paxinou, N.; Nena, E.; Steiropoulos, P. Is COPD the Determinant Factor for Myocardial Injury and Cardiac Wall Stress in OSA Patients? Medicina 2023, 59, 1759. https://doi.org/10.3390/medicina59101759
Voulgaris A, Archontogeorgis K, Apessos I, Paxinou N, Nena E, Steiropoulos P. Is COPD the Determinant Factor for Myocardial Injury and Cardiac Wall Stress in OSA Patients? Medicina. 2023; 59(10):1759. https://doi.org/10.3390/medicina59101759
Chicago/Turabian StyleVoulgaris, Athanasios, Kostas Archontogeorgis, Ioulianos Apessos, Nikoleta Paxinou, Evangelia Nena, and Paschalis Steiropoulos. 2023. "Is COPD the Determinant Factor for Myocardial Injury and Cardiac Wall Stress in OSA Patients?" Medicina 59, no. 10: 1759. https://doi.org/10.3390/medicina59101759
APA StyleVoulgaris, A., Archontogeorgis, K., Apessos, I., Paxinou, N., Nena, E., & Steiropoulos, P. (2023). Is COPD the Determinant Factor for Myocardial Injury and Cardiac Wall Stress in OSA Patients? Medicina, 59(10), 1759. https://doi.org/10.3390/medicina59101759








