Subclinical Target Organ Damage in a Sample of Children with Autosomal Dominant Polycystic Kidney Disease: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Pressure Measurement and Vascular Exams
2.2. Laboratory Exams
2.3. Anthropometric Parameters
2.4. Statistical Analysis
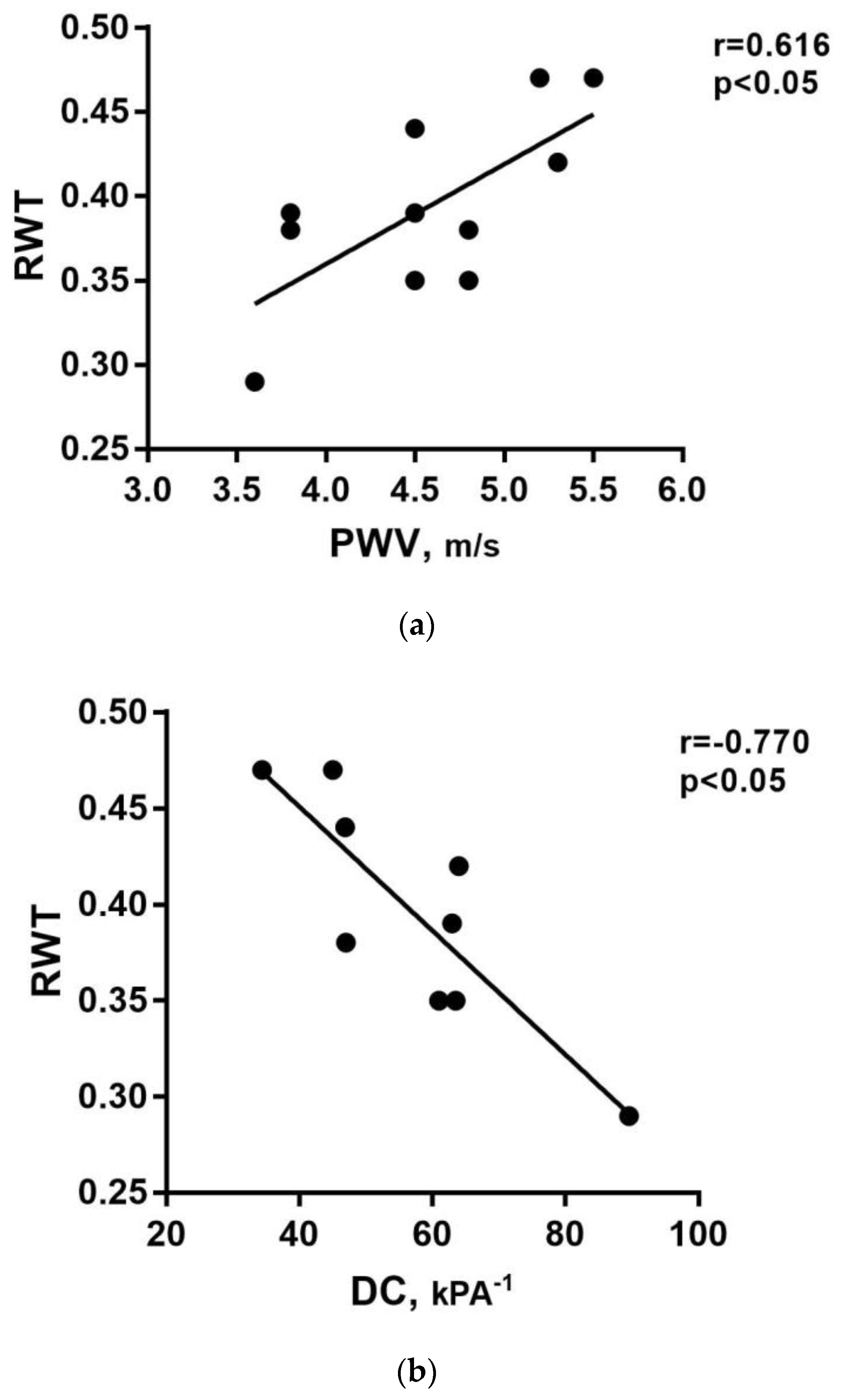
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Srivastava, A.; Patel, N. Autosomal dominant Polycystic kidney disease. Am. Fam. Physician 2014, 90, 303–307. [Google Scholar]
- Harris, P.C.; Torres, V.E. Polycystic Kidney Disease, Autosomal Dominant; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Mochizuki, T.; Wu, G.; Hayashi, T.; Xenophontos, S.L.; Veldhuisen, B.; Saris, J.J.; Reynolds, D.M.; Cai, Y.; Gabow, P.A.; Pierides, A.; et al. PKD2, a Gene for Polycystic Kidney Disease That Encodes an Integral Membrane Protein. Science 1996, 272, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Porath, B.; Gainullin, V.G.; Cornec-Le Gall, E.; Dillinger, E.K.; Heyer, C.M.; Hopp, K.; Edwards, M.E.; Madsen, C.D.; Mauritz, S.R.; Banks, C.J.; et al. Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am. J. Hum. Genet. 2016, 98, 1193–1207. [Google Scholar] [CrossRef]
- Huang, E.; Samaniego-Picota, M.; McCune, T.; Melancon, J.K.; Montgomery, R.A.; Ugarte, R.; Kraus, E.; Womer, K.; Rabb, H.; Watnick, T. DNA Testing for Live Kidney Donors at Risk for Autosomal Dominant Polycystic Kidney Disease. Transplantation 2009, 87, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Gabow, P.A.; Duley, I.; Johnson, A.M. Clinical Profiles of Gross Hematuria in Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 1992, 20, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Johnson, A.M.; Gabow, P.A.; Schrier, R.W. Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1994, 5, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, J.A.A.L.; Neves, R.F.; Eloi, S.R.; Cintra, S.M.; Ajzen, S.A.; Heilberg, I.P. Evaluation of Nephrolithiasis in Autosomal Dominant Polycystic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 838–844. [Google Scholar] [CrossRef]
- Cornec-Le Gall, E.; Audrézet, M.P.; Rousseau, A.; Hourmant, M.; Renaudineau, E.; Charasse, C.; Morin, M.-P.; Moal, M.-C.; Dantal, J.; Wehbe, B.; et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 942–951. [Google Scholar] [CrossRef]
- Chapman, A.B.; Rubinstein, D.; Hughes, R.; Stears, J.C.; Earnest, M.P.; Johnson, A.M.; Gabow, P.A.; Kaehny, W.D. Intracranial Aneurysms in Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 1992, 327, 916–920. [Google Scholar] [CrossRef]
- Gabow, P.A.; Johnson, A.M.; Kaehny, W.D.; Manco-Johnson, M.L.; Duley, I.T.; Everson, G.T. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 1990, 11, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Blumenfeld, J.D.; Chhabra, S.; Dutruel, S.P.; Thimmappa, N.D.; Bobb, W.O.; Donahue, S.; Rennert, H.E.; Tan, A.Y.; Giambrone, A.E.; et al. Pancreatic Cysts in Autosomal Dominant Polycystic Kidney Disease: Prevalence and Association with PKD2 Gene Mutations. Radiology 2016, 280, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Hossack, K.F.; Leddy, C.L.; Johnson, A.M.; Schrier, R.W.; Gabow, P.A. Echocardiographic Findings in Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 1988, 319, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Massella, L.; Mekahli, D.; Paripović, D.; Prikhodina, L.; Godefroid, N.; Niemirska, A.; Ağbaş, A.; Kalicka, K.; Jankauskiene, A.; Mizerska-Wasiak, M.; et al. Prevalence of Hypertension in Children with Early-Stage ADPKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 874–883. [Google Scholar] [CrossRef]
- Griffin, M.D.; Torres, V.E.; Grande, J.P.; Kumar, R. Vascular expression of polycystin. J. Am. Soc. Nephrol. 1997, 8, 616–626. [Google Scholar] [CrossRef]
- Chapman, A.B.; Stepniakowski, K.; Rahbari-Oskoui, F. Hypertension in Autosomal Dominant Polycystic Kidney Disease. Adv. Chronic Kidney Dis. 2010, 17, 153–163. [Google Scholar] [CrossRef]
- Torres, V.E.; Donovan, K.A.; Scicli, G.; Holley, K.E.; Thibodeau, S.N.; Carretero, O.A.; Inagami, T.; McAteer, J.A.; Johnson, C.M. Synthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney disease. Kidney Int. 1992, 42, 364–373. [Google Scholar] [CrossRef]
- Fick, G.M.; Johnson, A.M.; Hammond, W.S.; Gabow, P.A. Causes of death in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1995, 5, 2048–2056. [Google Scholar] [CrossRef]
- Perrone, R.D.; Ruthazer, R.; Terrin, N.C. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: Contribution of extrarenal complications to mortality. Am. J. Kidney Dis. 2001, 38, 777–784. [Google Scholar] [CrossRef]
- Rahman, E.; Niaz, F.A.; Al-Suwaida, A.; Nahrir, S.; Bashir, M.; Rahman, H.; Hammad, D. Analysis of causes of mortality in patients with autosomal dominant polycystic kidney disease: A single center study. Saudi J. Kidney Dis. Transplant. 2009, 20, 806–810. [Google Scholar]
- Chung, C.-M.; Lin, Y.-S.; Chang, S.-T.; Cheng, H.-W.; Yang, T.-Y.; Hsiao, J.-F.; Pan, K.-L.; Hsu, J.-T.; Chu, C.-M. Arterial Stiffness Is the Independent Factor of Left Ventricular Hypertrophy Determined by Electrocardiogram. Am. J. Med. Sci. 2012, 344, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lekakis, J.; Papadopoulos, C.; Triantafyllidi, H.; Paraskevaidis, I.; Georgoula, G.; Tzortzis, S.; Revela, I.; Kremastinos, D.T. Incremental Value of Pulse Wave Velocity in the Determination of Coronary Microcirculatory Dysfunction in Never-treated Patients with Essential Hypertension. Am. J. Hypertens. 2008, 21, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of Clinical Cardiovascular Events with Carotid Intima-Media Thickness. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality with Arterial Stiffness. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Farmer, H.; Cadnapaphornchai, M.A.; Gitomer, B.; Chonchol, M. Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2017, 32, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Karava, V.; Benzouid, C.; Hogan, J.; Dossier, C.; Denjean, A.P.; Deschênes, G. Early cardiovascular manifestations in children and adolescents with autosomal dominant polycystic kidney disease: A single center study. Pediatr. Nephrol. 2018, 33, 1513–1521. [Google Scholar] [CrossRef]
- Wühl, E.; Witte, K.; Soergel, M.; Mehls, O.; Schaefer, F.; German Working Group on Pediatric Hypertension. Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. J. Hypertens. 2002, 20, 1995–2007. [Google Scholar] [CrossRef] [PubMed]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114 (Suppl. S2), 555–576. [Google Scholar] [CrossRef]
- Pozza, R.D.; Ehringer-Schetitska, D.; Fritsch, P.; Jokinen, E.; Petropoulos, A.; Oberhoffer, R. Intima media thickness measurement in children: A statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis 2015, 238, 380–387. [Google Scholar] [CrossRef]
- Bianchini, E.; Bozec, E.; Gemignani, V.; Faita, F.; Giannarelli, C.; Ghiadoni, L.; Demi, M.; Boutouyrie, P.; Laurent, S. Assessment of carotid stiffness and intima-media thickness from ultrasound data: Comparison between two methods. J. Ultrasound Med. 2010, 29, 1169–1175. [Google Scholar] [CrossRef]
- Doyon, A.; Kracht, D.; Bayazit, A.K.; Deveci, M.; Duzova, A.; Krmar, R.T.; Litwin, M.; Niemirska, A.; Oguz, B.; Schmidt, B.M.W.; et al. Carotid artery intima-media thickness and distensibility in children and adolescents: Reference values and role of body dimensions. Hypertension 2013, 62, 550–556. [Google Scholar] [CrossRef]
- Elmenhorst, J.; Hulpke-Wette, M.; Barta, C.; Pozza, R.D.; Springer, S.; Oberhoffer, R. Percentiles for central blood pressure and pulse wave velocity in children and adolescents recorded with an oscillometric device. Atherosclerosis 2015, 238, 9–16. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Daniels, S.R.; Koren, M.J.; Meyer, R.A.; Laragh, J.H. Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J. Am. Coll. Cardiol. 1995, 25, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Khoury, P.R.; Mitsnefes, M.; Daniels, S.R.; Kimball, T.R. Age-Specific Reference Intervals for Indexed Left Ventricular Mass in Children. J. Am. Soc. Echocardiogr. 2009, 22, 709–714. [Google Scholar] [CrossRef]
- De Simone, G.; Daniels, S.R.; Kimball, T.R.; Roman, M.J.; Romano, C.; Chinali, M.; Galderisi, M.; Devereux, R.B. Evaluation of concentric left ventricular geometry in humans: Evidence for age-related systematic underestimation. Hypertension 2005, 45, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ganau, A.; Devereux, R.B.; Roman, M.J.; de Simone, G.; Pickering, T.G.; Saba, P.S.; Vargiu, P.; Simongini, I.; Laragh, J.H. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J. Am. Coll. Cardiol. 1992, 19, 1550–1558. [Google Scholar] [CrossRef]
- Barlow, S.E.; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. S4), S164–S192. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef]
- Cadnapaphornchai, M.A.; McFann, K.; Strain, J.D.; Masoumi, A.; Schrier, R.W. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008, 74, 1192–1196. [Google Scholar] [CrossRef]
- Zeier, M.; Geberth, S.; Schmidt, K.G.; Mandelbaum, A.; Ritz, E. Elevated blood pressure profile and left ventricular mass in children and young adults with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1993, 3, 1451–1457. [Google Scholar] [CrossRef]
- Saggar-Malik, A.K.; Missouris, C.G.; Gill, J.S.; Singer, D.R.J.; Markandu, N.D.; MacGregor, G.A. Left ventricular mass in normotensive subjects with autosomal dominant polycystic kidney disease. BMJ 1994, 309, 1617–1618. [Google Scholar] [CrossRef]
- Chapman, A.B.; Johnson, A.M.; Rainguet, S.; Hossack, K.; Gabow, P.; Schrier, R.W. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1997, 8, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Jin, X.; Ye, C.; Chen, J.; Mei, C. Carotid vascular remodelling in patients with autosomal dominant polycystic kidney disease. Nephrology 2009, 14, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Oflaz, H.; Uslu, B.; Cimen, A.O.; Elitok, A.; Kasikcioglu, E.; Alisir, S.; Tufan, F.; Namli, S.; Uysal, M.; et al. Coronary Flow Velocity Reserve and Carotid Intima Media Thickness in Patients with Autosomal Dominant Polycystic Kidney Disease: From Impaired Tubules to Impaired Carotid and Coronary Arteries. Clin. J. Am. Soc. Nephrol. 2008, 3, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Kocaman, O.; Oflaz, H.; Yekeler, E.; Dursun, M.; Erdogan, D.; Demirel, S.; Alisir, S.; Turgut, F.; Mercanoglu, F.; Ecder, T. Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2004, 43, 854–860. [Google Scholar] [CrossRef]
- Ecder, T.; Schrier, R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. 2009, 5, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Johnson, A.M.; Mcfann, K.; Chapman, A.B. The role of parental hypertension in the frequency and age of diagnosis of hypertension in offspring with autosomal-dominant polycystic kidney disease. Kidney Int. 2003, 64, 1792–1799. [Google Scholar] [CrossRef]
- Gabow, P.A.; Chapman, A.B.; Johnson, A.M.; Tangel, D.J.; Duley, I.T.; Kaehny, W.D.; Manco-Johnson, M.; Schrier, R.W. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1990, 38, 1177–1180. [Google Scholar] [CrossRef]
- Fick, G.M.; Duley, I.T.; Johnson, A.M.; Strain, J.D.; Manco-Johnson, M.L.; Gabow, P.A. The spectrum of autosomal dominant polycystic kidney disease in children. J. Am. Soc. Nephrol. 1994, 4, 1654–1660. [Google Scholar] [CrossRef]
- Chapman, A.B.; Johnson, A.; Gabow, P.A.; Schrier, R.W. The Renin–Angiotensin–Aldosterone System and Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 1990, 323, 1091–1096. [Google Scholar] [CrossRef]
- Harrap, S.B.; Davies, D.L.; Macnicol, A.M.; Dominiczak, A.F.; Fraser, R.; Wright, A.F.; Watson, M.L.; Briggs, J.D. Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int. 1991, 40, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.H.H.T.; Ligtenberg, G.; Oey, P.L.; Koomans, H.A.; Blankestijn, P.J. Sympathetic Activity Is Increased in Polycystic Kidney Disease and Is Associated with Hypertension. J. Am. Soc. Nephrol. 2001, 12, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Giusti, R.; Neri, M.; Angelini, D.; Carlini, A.; Fiorini, I.; Bigongiari, P.; Antonelli, A. Plasma Concentration of Endothelin and Arterial Pressure in Patients with ADPKD. In Autosomal Dominant Polycystic Kidney Disease; Karger Publishers: Basel, Switzerland, 1995; Volume 115, pp. 118–121. [Google Scholar] [CrossRef]
- Hocher, B.; Zart, R.; Schwarz, A.; Vogt, V.; Braun, C.; Thöne-Reineke, C.; Braun, N.; Neumayer, H.H.; Koppenhagen, K.; Bauer, C.; et al. Renal endothelin system in polycystic kidney disease. J. Am. Soc. Nephrol. 1998, 9, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Munemura, C.; Uemasu, J.; Kawasaki, H. Epidermal Growth Factor and Endothelin in Cyst Fluid from Autosomal Dominant Polycystic Kidney Disease Cases: Possible Evidence of Heterogeneity in Cystogenesis. Am. J. Kidney Dis. 1994, 24, 561–568. [Google Scholar] [CrossRef]
- Wang, D.; Iversen, J.; Strandgaard, S. Endothelium-Dependent Relaxation of Small Resistance Vessels Is Impaired in Patients with Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2000, 11, 1371–1376. [Google Scholar] [CrossRef]
- Wang, D.; Iversen, J.; Wilcox, C.S.; Strandgaard, S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003, 64, 1381–1388. [Google Scholar] [CrossRef]
- Torres, V.E.; Cai, Y.; Chen, X.; Wu, G.Q.; Geng, L.; Cleghorn, K.A.; Johnson, C.M.; Somlo, S. Vascular Expression of Polycystin-2. J. Am. Soc. Nephrol. 2001, 12, 1–9. [Google Scholar] [CrossRef]
- Ecder, T. Reversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 1999, 14, 1113–1116. [Google Scholar] [CrossRef]
- Schrier, R.; McFann, K.; Johnson, A.; Chapman, A.; Edelstein, C.; Brosnahan, G.; Ecder, T.; Tison, L. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: Results of a seven-year prospective randomized study. J. Am. Soc. Nephrol. 2002, 13, 1733–1739. [Google Scholar] [CrossRef]
- Jafar, T.H.; Stark, P.C.; Schmid, C.H.; Strandgaard, S.; Kamper, A.-L.; Maschio, G.; Becker, G.; Perrone, R.D.; Levey, A.S.; ACE Inhibition in Progressive Renal Disease (AIPRD) Study Group. The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005, 67, 265–271. [Google Scholar] [CrossRef]
- Ecder, T.; Chapman, A.B.; Brosnahan, G.M.; Edelstein, C.L.; Johnson, A.M.; Schrier, R.W. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2000, 35, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.A.; Breuning, M.H.; Duiser, R.; van Es, L.A.; Westendorp, R.G.J. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2003, 18, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, R.; Poliak, R.; Stiasny, B.; Schmieder, R.E.; Schulze, B.D. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2007, 23, 573–579. [Google Scholar] [CrossRef] [PubMed]
| Female | Male | High BP at ABPM/OBP | Normal BP at ABPM/OBP | |||
|---|---|---|---|---|---|---|
| (n = 4) | (n = 7) | (n = 5) | (n = 6) | |||
| Mean ± SD | Mean ± SD | p-Value | Mean ± SD | Mean ± SD | p-Value | |
| Age, years | 8.8 ± 3.4 | 9.9 ± 3.3 | 0.612 | 8.6 ± 2.6 | 10.2 ± 3.8 | 0.453 |
| BMI, kg/m2 | 19.8 ± 2.8 | 18.4 ± 3.8 | 0.544 | 18.1 ± 3.0 | 19.7 ± 3.8 | 0.473 |
| Percentile BMI for age | 84 ± 12.6 | 52.8 ± 27.4 | 0.030 | 65.2 ± 34.9 | 63.3 ± 22.6 | 0.913 |
| Office SBP, mmHg | 110.0 ± 13.6 | 105.3 ± 14.5 | 0.610 | 107.8 ± 11.1 | 106.3 ± 16.6 | 0.870 |
| Percentile SBP, mmHg | 76.6 ± 23.1 | 59.5 ± 28 | 0.330 | 72.0 ± 19.7 | 60.4 ± 32.0 | 0.500 |
| Office DBP, mmHg | 70.3 ± 6.2 | 59.4 ± 8.2 | 0.039 | 67.6 ± 7.0 | 59.8 ± 9.5 | 0.166 |
| Percentile DBP, mmHg | 77.2 ± 13.9 | 39.9 ± 13 | 0.005 | 62.7 ± 24.8 | 45.7 ± 19.5 | 0.233 |
| 24 h SBP, mmHg | 116 ± 3.6 | 119.2 ± 6.1 | 0.384 | 119.6 ± 7.2 | 116.2 ± 1.8 | 0.337 |
| Percentile 24 h-SBP | 83.8 ± 16.9 | 82.2 ± 15.9 | 0.880 | 88.0 ± 21.2 | 81.1 ± 4.9 | 0.302 |
| 24 h DBP, mmHg | 66.5 ± 3.3 | 67.8 ± 3.4 | 0.559 | 67.8 ± 4.1 | 66.8 ± 2.6 | 0.656 |
| Percentile 24 h-DBP | 53.7 ± 27.3 | 54.9 ± 22.8 | 0.941 | 58.9 ± 28.0 | 49.9 ± 19.2 | 0.573 |
| cIMT, mm | 0.48 ± 0.05 | 0.49 ± 0.05 | 0.675 | 0.48 ± 0.05 | 0.49 ± 0.04 | 0.702 |
| Percentile c-IMT for height | 97.1 ± 3.4 | 96.5 ± 5.4 | 0.849 | 97.0 ± 3.0 | 96.4 ± 6.0 | 0.848 |
| cDC, kPA-1 | 52.3 ± 9.2 | 59.6 ± 18.9 | 0.556 | 54.6 ± 9.4 | 51.2 ± 20.7 | 0.701 |
| Percentile cDC for height | 26.7 ± 14 | 44.7 ± 32 | 0.411 | 35.0 ± 17.5 | 45.3 ± 36.0 | 0.349 |
| PWV, m/s | 4.4 ± 0.4 | 4.7 ± 0.7 | 0.525 | 4.6 ± 0.61 | 4.5 ± 0.71 | 0.835 |
| Percentile PWV for height | 31.8 ± 17.2 | 59.5 ± 31.9 | 0.156 | 44.4 ± 19.3 | 52.5 ± 39.2 | 0.694 |
| RWT | 0.40 ± 0.03 | 0.39 ± 0.07 | 0.869 | 0.39 ± 0.05 | 0.39 ± 0.07 | 0.985 |
| LVM/BSA, g/m2 | 25.8 ± 4.8 | 33.9 ± 9.9 | 0.102 | 27.4 ± 6.1 | 34.0 ± 10.6 | 0.249 |
| HDL cholesterol, mg/dL | 54.3 ± 8.8 | 55.1 ± 8.0 | 0.867 | 52.2 ± 8.9 | 57.0 ± 6.9 | 0.340 |
| LDL cholesterol, mg/dL | 73.0 ± 12.9 | 57.3 ± 18.4 | 0.171 | 74.7 ± 13.3 | 53.3 ± 15.5 | 0.036 |
| Triglycerides, mg/dL | 86.3 ± 17.6 | 76.3 ± 64.7 | 0.774 | 72.4 ± 24.8 | 86.2 ± 0.09 | 0.681 |
| Uric Acid (mg/dL) | 3.8 ± 1.0 | 3.0 ± 0.7 | 0.147 | 3.2 ± 1.2 | 3.4 ± 0.6 | 0.832 |
| Albumin/creatinine, mg/mmoL creat.) | 1.8 ± 1.9 | 0.56 ± 0.15 | 0.143 | 0.17 ± 2.0 | 0.62 ± 0.23 | 0.176 |
| Schwartz eGFR, ml/min | 110.8 ± 9.9 | 109.0 ± 16.0 | 0.849 | 119 ± 13.4 | 101.8 ± 7.1 | 0.026 |
| Cystatin-c, mg/L | 0.85 ± 0.05 | 0.87 ± 0.10 | 0.707 | 0.86 ± 0.09 | 0.86 ± 0.09 | 0.921 |
| Proteinuria, g/die | 0.07 ± 0.04 | 0.11 ± 0.06 | 0.067 | 75.6 ± 3.4 | 72.6 ± 4.7 | 0.277 |
| Total Population (n = 11) | |
|---|---|
| n (%) | |
| Percentile BMI for age < 95th | 8 (72.7%) |
| 90th ≤ Percentile BMI for age < 95th | 3 (27.3%) |
| Percentile BMI for age ≥ 95th | 0 |
| Percentile Office SBP and DBP < 95th | 9 (81.8%) |
| Percentile Office SBP or DBP ≥ 95th | 2 (18.2%) |
| Percentile ABPM SBP and DBP < 95th | 5 (55.6%) |
| Percentile ABPM SBP or DBP ≥ 95th | 4 (44.4%) |
| Percentile cIMT for height < 95th | 2 (20%) |
| Percentile cIMT for height ≥ 95th | 8 (80%) |
| Percentile cDC for height ≥ 5th | 8 (100%) |
| Percentile cDC for height < 5th | 0 |
| Percentile PWV for height < 95th | 9 (100%) |
| Percentile PWV for height ≥ 95th | 0 |
| RWT < 0.375 | 3 (27.3%) |
| RWT ≥ 0.375 | 8 (72.7%) |
| Percentile LVM < 95th | 10 (90.9%) |
| Percentile LVM ≥ 95th | 1 (9.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, S.; Marcon, D.; Branz, L.; Tagetti, A.; Monamì, G.; Giontella, A.; Malesani, F.; Pecoraro, L.; Minuz, P.; Brugnara, M.; et al. Subclinical Target Organ Damage in a Sample of Children with Autosomal Dominant Polycystic Kidney Disease: A Pilot Study. Medicina 2023, 59, 1777. https://doi.org/10.3390/medicina59101777
Romano S, Marcon D, Branz L, Tagetti A, Monamì G, Giontella A, Malesani F, Pecoraro L, Minuz P, Brugnara M, et al. Subclinical Target Organ Damage in a Sample of Children with Autosomal Dominant Polycystic Kidney Disease: A Pilot Study. Medicina. 2023; 59(10):1777. https://doi.org/10.3390/medicina59101777
Chicago/Turabian StyleRomano, Simone, Denise Marcon, Lorella Branz, Angela Tagetti, Giada Monamì, Alice Giontella, Francesca Malesani, Luca Pecoraro, Pietro Minuz, Milena Brugnara, and et al. 2023. "Subclinical Target Organ Damage in a Sample of Children with Autosomal Dominant Polycystic Kidney Disease: A Pilot Study" Medicina 59, no. 10: 1777. https://doi.org/10.3390/medicina59101777





