A C-Arm-Free Minimally Invasive Technique for Spinal Surgery: Cervical and Thoracic Spine
Abstract
:1. Introduction
2. Occiput–Cervical Spine
2.1. Anterior Application
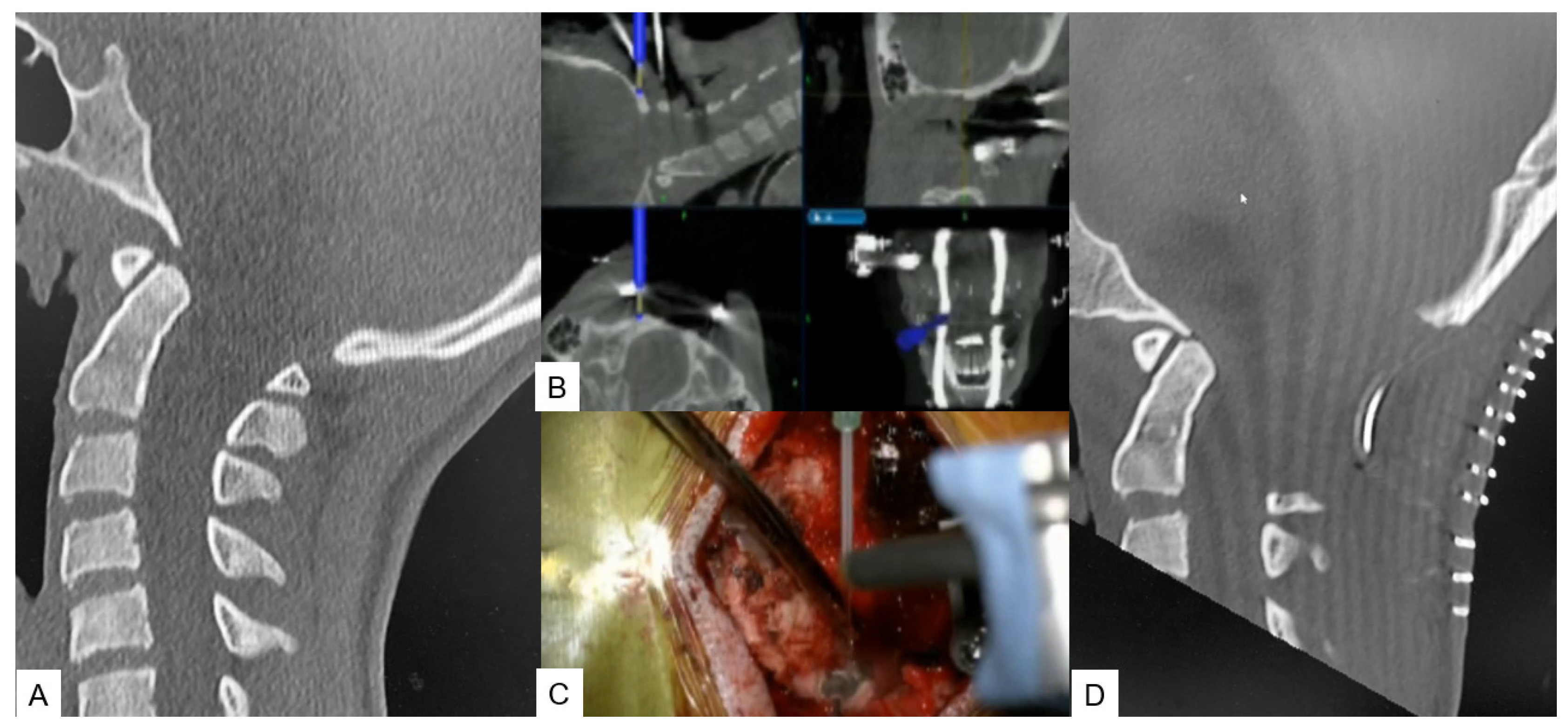
OPLL Resection
2.2. Posterior Application
2.2.1. Posterior Fossa Decompression for Chiari Malformation
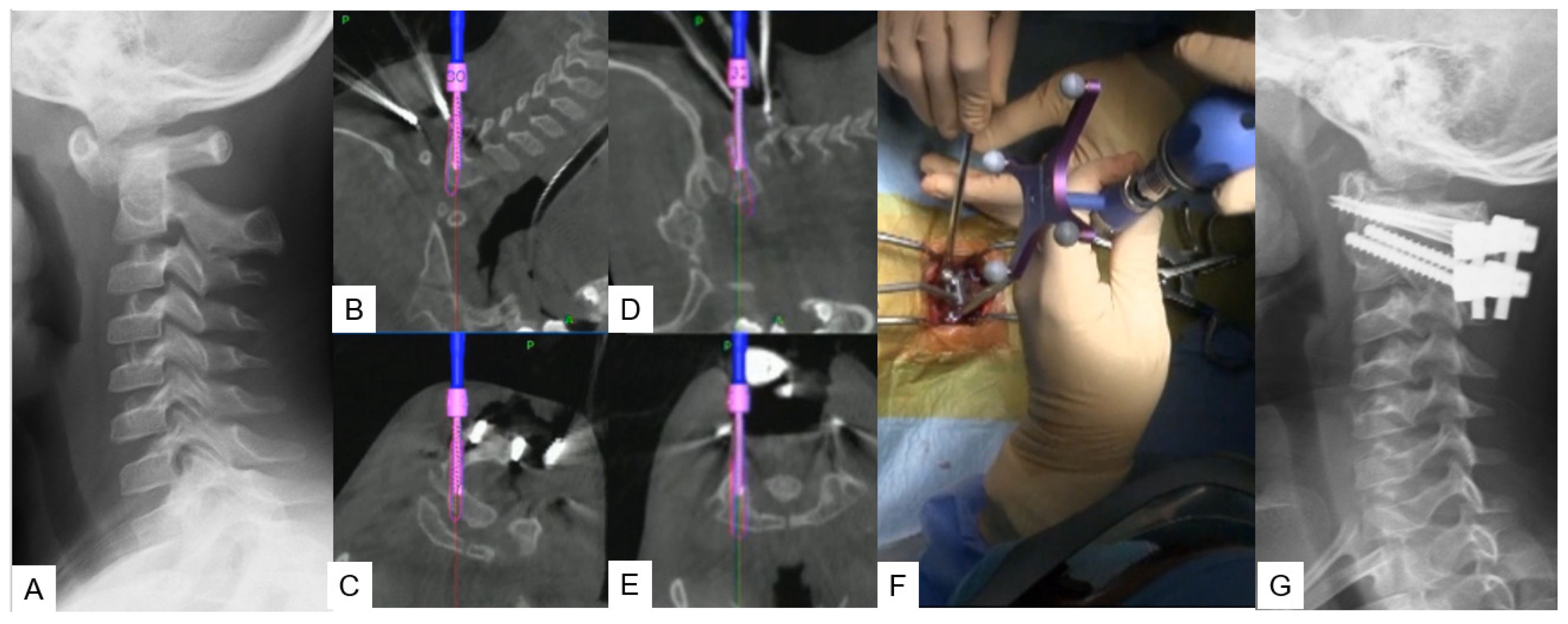
2.2.2. Modified Goel Technique
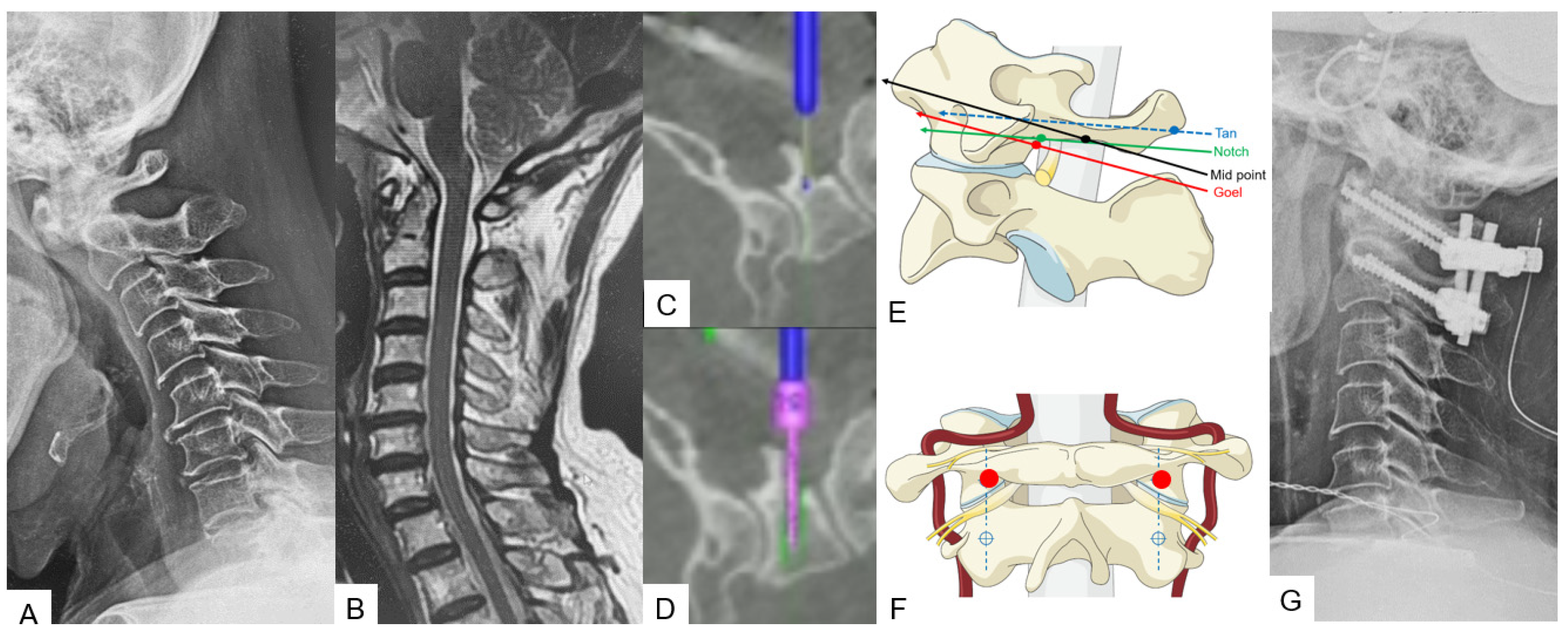
2.2.3. Midpoint Technique for C1 Lateral Mass Screw (LMS) Placement
2.2.4. Minimally Invasive Cervical Pedicle Screw Fixation (MICEPS)
3. Thoracic Spine
3.1. Anterior Application
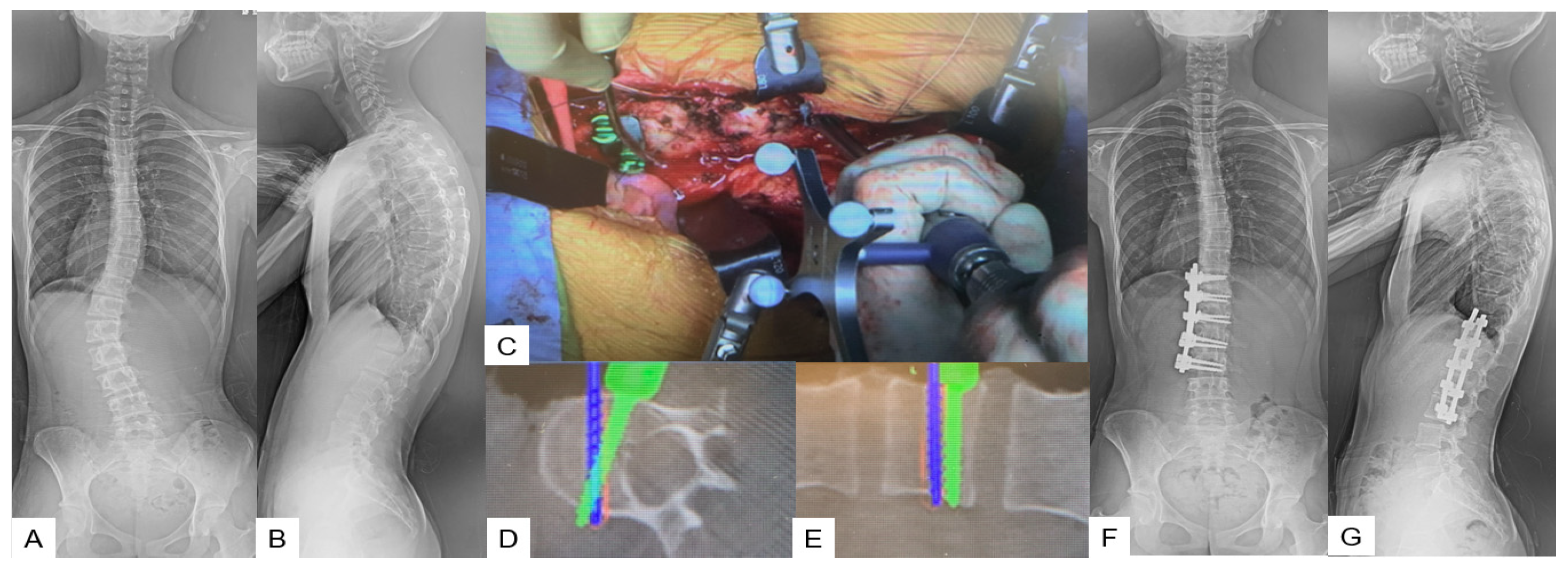
Anterior Correction for Lenke Type 5
3.2. Posterior Application
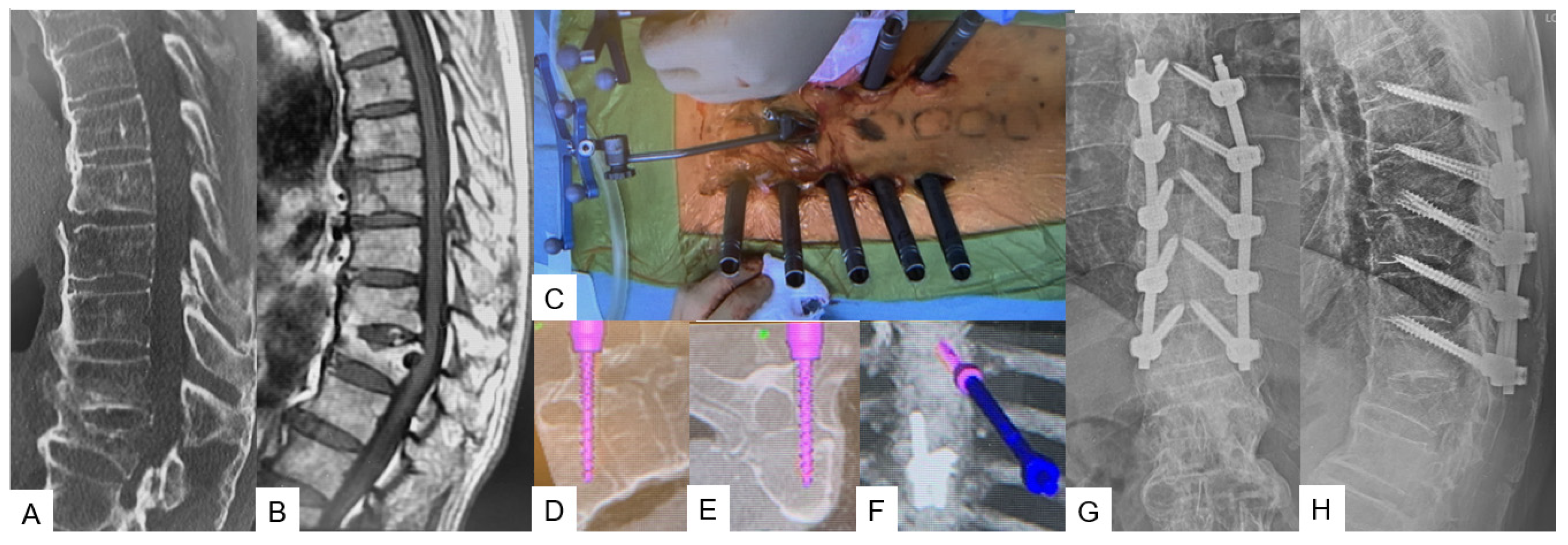
Transdiscal Screw for DISH (Diffuse Idiopathic Skeletal Hyperostosis) Fracture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momin, A.A.; Steinmetz, M.P. Evolution of Minimally Invasive Lumbar Spine Surgery. World Neurosurg. 2020, 140, 622–626. [Google Scholar] [CrossRef]
- Patel, P.D.; Canseco, J.A.; Houlihan, N.; Gabay, A.; Grasso, G.; Vaccaro, A.R. Overview of Minimally Invasive Spine Surgery. World Neurosurg. 2020, 142, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Härtl, R.; Lam, K.S.; Wang, J.; Korge, A.; Kandziora, F.; Audigé, L. Worldwide survey on the use of navigation in spine surgery. World Neurosurg. 2013, 79, 162–172. [Google Scholar] [CrossRef]
- Willson, M.C.; Ross, J.S. Postoperative spine complications. Neuroimaging Clin. N. Am. 2014, 24, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.K.; Hansen, C.R.; Sahm, L.J.; Kearney, P.M.; Doherty, E.; Bradley, C.P. Economic impact of medication error: A systematic review. Pharmacoepidemiol. Drug Saf. 2017, 26, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Afsar, A. Learning Curve and Minimally Invasive Spine Surgery. World Neurosurg. 2018, 119, 472–478. [Google Scholar] [CrossRef]
- Simony, A.; Hansen, E.J.; Christensen, S.B.; Carreon, L.Y.; Andersen, M.O. Incidence of cancer in adolescent idiopathic scoliosis patients treated 25 years previously. Eur. Spine J. 2016, 25, 3366–3370. [Google Scholar] [CrossRef]
- Cristante, A.F.; Barbieri, F.; da Silva, A.A.R.; Dellamano, J.C. Radiation Exposure during Spine Surgery Using C-Arm Fluoroscopy. Acta Ortop. Bras. 2019, 27, 46–49. [Google Scholar] [CrossRef]
- Croci, D.M.; Nguyen, S.; Streitmatter, S.W.; Sherrod, B.A.; Hardy, J.; Cole, K.L.; Gamblin, A.S.; Bisson, E.F.; Mazur, M.D.; Dailey, A.T. O-Arm Accuracy and Radiation Exposure in Adult Deformity Surgery. World Neurosurg. 2023, 171, e440–e446. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Koski, T.R.; Ganju, A.; Liu, J.C. Approach-related complications after decompression for cervical ossification of the posterior longitudinal ligament. Neurosurg Focus. 2011, 30, E12. [Google Scholar] [CrossRef]
- Tanaka, M.; Suthar, H.; Fujiwara, Y.; Oda, Y.; Uotani, K.; Arataki, S.; Yamauchi, T.; Misawa, H. Intraoperative O-arm navigation guided anterior cervical surgery; A technical note and case series. Interdiscip. Neurosurg. 2021, 26, 101288. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yamaura, I.; Kurosa, Y.; Nakai, O.; Shindo, S.; Shinomiya, K. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine 2001, 26, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Onari, K.; Akiyama, N.; Kondo, S.; Toguchi, A.; Mihara, H.; Tsuchiya, T. Long-term follow-up results of anterior interbody fusion applied for cervical myelopathy due to ossification of the posterior longitudinal ligament. Spine 2001, 26, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, F.; Izgi, N.; Kapíjcíjoğlu Sencer, S. Morphometric measurements of the cranium in patients with Chiari type I malformation and comparison with the normal population. Acta Neurochir. 2002, 144, 165–171. [Google Scholar] [CrossRef]
- Menezes, A.H. Primary craniovertebral anomalies and the hindbrain herniation syndrome (Chiari I): Data base analysis. Pediatr. Neurosurg. 1995, 23, 260–269. [Google Scholar] [CrossRef]
- Hiremath, S.B.; Fitsiori, A.; Boto, J.; Torres, C.; Zakhari, N.; Dietemann, J.L.; Meling, T.R.; Vargas, M.I. The Perplexity Surrounding Chiari Malformations—Are We Any Wiser Now? Am. J. Neuroradiol. 2020, 41, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sharma, S.; Fujiwara, Y.; Arataki, S.; Omori, T.; Kanamaru, A.; Kodama, Y.; Saad, H.; Yamauchi, T. Accurate Posterior Fossa Decompression Technique for Chiari Malformation Type I and a Syringomyelia With Navigation: A Technical Note. Int. J. Spine Surg. 2023, 17, 8483. [Google Scholar] [CrossRef]
- Limonadi, F.M.; Selden, N.R. Dura-splitting dewithression of the craniocervical junction: Reduced operative time, hospital stay, and cost with equivalent early outcome. J. Neurosurg. 2004, 101, 184–188. [Google Scholar]
- Burgess, L.C.; Wainwright, T.W. What Is the Evidence for Early Mobilisation in Elective Spine Surgery? A Narrative Review. Healthcare 2019, 7, 92. [Google Scholar] [CrossRef]
- Tanaka, M.; Sonawane, S.; Fujiwara, Y.; Uotani, K.; Arataki, S.; Yamauchi, T.; Ye, Y.; Misawa, H. C-arm Free O-arm Navigated Posterior Atlantoaxial Fixation in Down Syndrome: A Technical Note. Acta Med. Okayama 2022, 76, 71–78. [Google Scholar]
- Guo, J.; Lu, W.; Ji, X.; Ren, X.; Tang, X.; Zhao, Z.; Hu, H.; Song, T.; Du, Y.; Li, J.; et al. Surgical treatment of atlantoaxial subluxation by intraoperative skull traction and C1-C2 fixation. BMC Musculoskelet. Disord. 2020, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.D.; Hsu, W.K. Vertebral artery injuries in cervical spine surgery. Surg. Neurol. Int. 2013, 4 (Suppl. S5), S362–S367. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kanemura, T.; Yoshida, G.; Matsumoto, A.; Ito, Z.; Tauchi, R.; Muramoto, A.; Ohno, S.; Nishimura, Y. Intraoperative, full-rotation, three-dimensional image (O-arm)-based navigation system for cervical pedicle screw insertion. J. Neurosurg. Spine 2011, 15, 472–478. [Google Scholar] [CrossRef]
- Yukawa, Y.; Kato, F.; Ito, K.; Horie, Y.; Hida, T.; Nakashima, H.; Machino, M. Placement and complications of cervical pedicle screws in 144 cervical trauma patients using pedicle axis view techniques by fluoroscope. Eur. Spine J. 2009, 18, 1293–1299. [Google Scholar] [CrossRef]
- Ketenci, I.E.; Yanik, H.S.; Ulusoy, A.; Demiroz, S.; Erdem, S. Lowest Instrumented Vertebrae Selection for Posterior Fusion of Lenke 5C Adolescent Idiopathic Scoliosis: Can We Stop the Fusion One Level Proximal to Lower-end Vertebra? Indian J. Orthop. 2018, 52, 657–664. [Google Scholar] [CrossRef]
- Wang, Z.W.; Shen, Y.Q.; Wu, Y.; Li, J.; Liu, Z.; Xu, J.K.; Chen, Q.X.; Chen, W.S.; Chen, L.W.; Zhang, N.; et al. Anterior Selective Lumbar Fusion Saving More Distal Fusion Segments Compared with Posterior Approach in the Treatment of Adolescent Idiopathic Scoliosis with Lenke Type 5: A Cohort Study with More Than 8-Year Follow-up. Orthop. Surg. 2021, 13, 2327–2334. [Google Scholar] [CrossRef]
- Hirase, T.; Ling, J.F.; Haghshenas, V.; Thirumavalavan, J.; Dong, D.; Hanson, D.S.; Marco, R.A.W. Anterior versus posterior spinal fusion for Lenke type 5 adolescent idiopathic scoliosis: A systematic review and meta-analysis of comparative studies. Spine Deform. 2022, 10, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fujiwara, Y.; Uotani, K.; Kamath, V.; Yamauchi, T.; Ikuma, H. Percutaneous transdiscal pedicle screw fixation for osteoporotic vertebral fracture: A technical note. Interdiscip. Neurosurg. 2021, 26, 00903. [Google Scholar] [CrossRef]
- Lall, R.R.; Smith, Z.A.; Wong, A.P.; Miller, D.; Fessler, R.G. Minimally invasive thoracic corpectomy: Surgical strategies for malignancy, trauma, and complex spinal pathologies. Minim. Invasive Surg. 2012, 2012, 213791. [Google Scholar] [CrossRef]
- Vozenin-Brotons, M.C. Tissue toxicity induced by ionizing radiation to the normal intestine: Understanding the pathophysiological mechanisms to improve the medical management. World J. Gastroenterol. 2007, 13, 3031–3032. [Google Scholar] [CrossRef] [PubMed]
- Masse, F.X. Dosimetry Report of the Medtronic O-Arm System. O-Arm ImagingSystem, Version 3.1; Medtronic: Littleton, MA, USA, 2009; pp. 4–6. [Google Scholar]
- Jones, D.P.; Robertson, P.A.; Lunt, B.; Jackson, S.A. Radiation exposure during fluoroscopically assisted pedicle screw insertion in the lumbar spine. Spine 2000, 25, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fujiwara, Y.; Uotani, K.; Kadiri, V.; Yamauchi, T. C-Arm-Free Minimally Invasive Cervical Pedicle Screw Fixation (MICEPS): A Technical Note. Acta Med. Okayama 2020, 74, 551–556. [Google Scholar] [PubMed]


| Advantage | Disadvantage |
|---|---|
| Zero exposure of OR staff to radiation | Absence of dynamic change in a perioperative image |
| Excellent accuracy | Steep learning curve |
| Less surgical time | System malfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Zygogiannnis, K.; Sake, N.; Arataki, S.; Fujiwara, Y.; Taoka, T.; de Moraes Modesto, T.H.; Chatzikomninos, I. A C-Arm-Free Minimally Invasive Technique for Spinal Surgery: Cervical and Thoracic Spine. Medicina 2023, 59, 1779. https://doi.org/10.3390/medicina59101779
Tanaka M, Zygogiannnis K, Sake N, Arataki S, Fujiwara Y, Taoka T, de Moraes Modesto TH, Chatzikomninos I. A C-Arm-Free Minimally Invasive Technique for Spinal Surgery: Cervical and Thoracic Spine. Medicina. 2023; 59(10):1779. https://doi.org/10.3390/medicina59101779
Chicago/Turabian StyleTanaka, Masato, Konstantinos Zygogiannnis, Naveen Sake, Shinya Arataki, Yoshihiro Fujiwara, Takuya Taoka, Thiago Henrique de Moraes Modesto, and Ioannis Chatzikomninos. 2023. "A C-Arm-Free Minimally Invasive Technique for Spinal Surgery: Cervical and Thoracic Spine" Medicina 59, no. 10: 1779. https://doi.org/10.3390/medicina59101779
APA StyleTanaka, M., Zygogiannnis, K., Sake, N., Arataki, S., Fujiwara, Y., Taoka, T., de Moraes Modesto, T. H., & Chatzikomninos, I. (2023). A C-Arm-Free Minimally Invasive Technique for Spinal Surgery: Cervical and Thoracic Spine. Medicina, 59(10), 1779. https://doi.org/10.3390/medicina59101779







