Abstract
Background and Objectives: Alzheimer’s disease (AD) stands as a pervasive neurodegenerative ailment of global concern, necessitating a relentless pursuit of remedies. This study aims to furnish a comprehensive exposition, delving into the intricate mechanistic actions of medicinal herbs and phytochemicals. Furthermore, we assess the potential of these compounds in inhibiting human acetylcholinesterase through molecular docking, presenting encouraging avenues for AD therapeutics. Materials and Methods: Our approach entailed a systematic exploration of phytochemicals like curcumin, gedunin, quercetin, resveratrol, nobiletin, fisetin, and berberine, targeting their capability as human acetylcholinesterase (AChE) inhibitors, leveraging the PubChem database. Diverse bioinformatics techniques were harnessed to scrutinize molecular docking, ADMET (absorption, distribution, metabolism, excretion, and toxicity), and adherence to Lipinski’s rule of five. Results: Results notably underscored the substantial binding affinities of all ligands with specific amino acid residues within AChE. Remarkably, gedunin exhibited a superior binding affinity (−8.7 kcal/mol) compared to the reference standard. Conclusions: These outcomes accentuate the potential of these seven compounds as viable candidates for oral medication in AD treatment. Notably, both resveratrol and berberine demonstrated the capacity to traverse the blood-brain barrier (BBB), signaling their aptitude for central nervous system targeting. Consequently, these seven molecules are considered orally druggable, potentially surpassing the efficacy of the conventional drug, donepezil, in managing neurodegenerative disorders.
1. Introduction
Alzheimer’s disease (AD), a prevalent neurodegenerative disorder, is commonly known as senile dementia and is characterized by cognitive decline and memory loss [1,2]. It is the most prevalent form of dementia in the aging population [3], leading to irreversible damage in various brain regions [4]. The global number of AD patients was estimated to be nearly 50 million in 2018, with projections suggesting that this number could reach 150 million by 2050 [5]. While AD is recognized as a complex disease influenced by both environmental and genetic factors, including gender and family history, its exact etiology remains uncertain. Despite well-identified histopathological features within the brain, the biochemical mechanisms of AD are not yet fully understood [6]. The prevailing amyloid hypothesis has garnered substantial support, suggesting that AD is a progressive and irreversible neurodegenerative disease characterized by the aggregation of microtubule-associated protein tau into neurofibrillary tangles (NFTs) within neurons and the extraneuronal deposition of amyloid β (Aβ) protein as senile plaques. Aβ is formed through the sequential proteolytic cleavage of amyloid precursor protein (APP) by γ- and β-secretases in the amyloidogenic pathway [2,7]. Accumulation of Aβ results in the enlargement of senile amyloid plaques, starting from allocortical and limbic regions and eventually impacting the hippocampus and entorhinal cortex [8,9]. The presence of Aβ disrupts synaptic signaling pathways, resulting in the impairment of behavior and memory through the destruction of dendritic spines and alterations in synaptic morphology [10,11]. Imaging methods depend on detecting amyloid deposits as part of the distinctive diagnosis process for AD [12,13]. Contemporary research is primarily centered on Aβ as a target for creating therapeutic medications, such as anti-Aβ antibodies and vaccines [11,14]. Nevertheless, options for treating AD symptoms remain restricted, and there is a notable absence of effective therapies to decelerate disease progression. Researchers are currently exploring traditional medicinal plants, historically used to address memory-related disorders, as potential sources for novel treatments for AD [15].
One of the major contributors to AD is the decreased levels of the neurotransmitter acetylcholine (ACh) at synapses in the human cerebral cortex, leading to impaired cognitive functions and memory loss [16]. Reactive oxygen species (ROS), generated during metabolic processes, contribute to oxidative stress, which is implicated in the development of degenerative diseases like AD [17]. Natural antioxidants, such as flavonoids and polyphenols, function by neutralizing ROS, especially free radicals, by providing them with an extra electron. This process helps stabilize these highly reactive molecules, thus reducing oxidative damage in the brain [18].
Multiple studies have demonstrated that the neurotransmitter acetylcholine (ACh) levels decrease due to reduced choline (Ch) and acetyltransferase activity, leading researchers to focus on cholinesterase (ChE) inhibitors as a symptomatic treatment option. By inhibiting the cholinesterase enzymes, including butyrylcholinesterase (BChE) and acetylcholinesterase (AChE), it may be possible to restore ACh levels, thereby improving cholinergic transmission and reducing amyloid aggregation, which could potentially benefit individuals with dementia and Alzheimer’s disease [3,19]. Consequently, these studies have revealed the AChE inhibitory effects of active natural compounds found in plant extracts. Several plants contain secondary metabolites with antioxidant and anti-AChE properties, which can slow the progression of Alzheimer’s disease by inhibiting AChE activity and protecting neurons from oxidative damage [4,5,20,21]. Numerous phytochemicals and herbs have been investigated for their therapeutic potential in Alzheimer’s disease, and significant progress has been made in this area. To compile this article, we conducted an extensive search of the Scopus and MEDLINE (PubMed) databases, utilizing relevant terms such as phytomedicine, herb, phytochemical, and Alzheimer’s. Our objective is to provide a comprehensive summary of the current understanding of the effects of phytochemicals and herbs contained in their extract molecules which exert a neuroprotective effect, which plays a fundamental role in the action of plant extracts against Alzheimer’s disease. Moreover, this research aimed to elucidate the binding affinity and interaction between acetylcholinesterase and various compounds through the use of molecular docking. The utilization of these widely available and easily accessible plants as sources of valuable phytochemicals for the pharmaceutical industry is an area of great interest [3,4,5,19,20,21].
2. Medicinal Herbs against Alzheimer’s
Phytotherapy, also known as Herbalism in Western medicine, is a specialized branch of medicine that harnesses the therapeutic potential of plants and natural active ingredients for the treatment of diseases or as agents to promote overall health [22]. In the context of Alzheimer’s disease, herbs have emerged as potential candidates for therapeutic intervention and may hold promise for the development of effective natural anti-Alzheimer’s drugs in the future [23].
2.1. Rosa damascena Herrm.
R. damascena is part of the family Rosaceae [24], a plant used in traditional medicine [25]. It contains bioactive secondary metabolites, including anthocyanins, with pelargonidin 3-glucoside as the predominant compound, constituting 80.6% of all anthocyanins [26]. Cerezo et al. demonstrated that pelargonidin 3-glucoside possesses antioxidant activity [27]. It contains phenolic compounds such as quercetin and kaempferol [26], which have a beneficial effect on the central nervous system [28,29]. In rats, Esfandiary et al. showed that R. damascena extract (300, 600, 1200 mg/kg p.o for 1 month) reversed behavioral deficits caused by Aβ in AD [30]. It increased the transcription factor CREBR, the expression of the neurotrophic factor, and the both the total volume of hippocampus and the absolute volume (CA1, DG), which were altered under the influence of Aβ [31].
2.2. Picrasma quassioides (D.Don) Benn.
Picrasma quassioides belongs to the family Simaroubaceae, mostly distributed in the temperate to tropical areas of East Asia, including China and Japan [32]. P. quassioides possesses many medicinal properties, including anti-inflammatory [33], antimalarial, antihypertensive, antibacterial, anticancer, antioxidant effects [34,35,36], as well as neuroprotective activity, potency as gastric indigestion and asthma treatments, and as a preventative treatment for osteoporosis [37]. Guo et al. showed that compounds in this plant have exhibited neuroprotective activities. In mice, P. quassioides extract (25, 50, and 100 mg/kg, p.o) inducted a neuroprotective activity in Aβ25-35-stimulated SH-SY5Y and L-glutamate-stimulated PC12 cell models along with improved cognitive abilities and memory in AD mice induced by Aβ [38].
2.3. Actinidia arguta (Siebold & Zucc.) Planch. ex Miq.
Actinidia arguta, a plant belonging to the Actinidiaceae family, has been traditionally used in Korea for treating inflammatory and gastrointestinal diseases [39]. Moreover, research has revealed several beneficial properties associated with A. arguta, including anti-inflammatory, antiapoptotic, antioxidant, and anti-allergic effects [40,41]. It is also known to contain numerous antioxidants such as catechins, anthocyanin, carotenoids, chlorophyll, and vitamin C [42,43]. It contains phenolic compounds such as quercetin and kaempferol [44], which have a beneficial effect on the central nervous system [28,29]. In a study conducted on mice by Su Ha et al., it was found that the extract of A. arguta at different doses (5, 10, and 20 mg/kg, orally) significantly improved memory and learning deficits induced by Aβ (amyloid-beta). Additionally, the extract exhibited beneficial effects on cholinergic functions and protected antioxidant systems. This was achieved by reducing AChE activity, increasing ACh levels, lowering oxidized glutathione (GSH)/total GSH ratio and malondialdehyde (MDA) levels, and increasing superoxide dismutase (SOD) levels in the brain. Furthermore, A. arguta extract was found to prevent mitochondrial dysfunction by normalizing levels of apoptotic signaling molecules, including phosphorylated tau (p-tau), cytochrome c, and phosphorylated Akt (p-Akt) in the brain tissues [45].
2.4. Alpinia galanga (L.)
Alpinia galanga (Zingiberaceae) is generally dispersed in India. It is an aromatic perennial that resembles a rhizome, is traditionally used as a nerve tonic and stimulant. It is still used as a repellent, digestive aid, aphrodisiac, stomach tonic, anti-inflammatory, and antiseptic [46]. Many molecules are isolated and tested for biological activity, including terpenyl ester (2-endohydroxy-1,8-cineole) for antibacterial activity and antimicrobial [47], essential oils with hypoglycemic activity [48], antifungal activity [49], and in vitro cholinesterase enzyme inhibition [50]. It contains phenolic compounds such as quercetin and kaempferol [51], which have a beneficial effect on the central nervous system [28,29]. In mice, Singh et al. showed that Alpinia galanga extract (200 and 400 mg/kg, oral administration) induced increases in Na+/K+-ATPase, free radical scavenging property and improved brain membrane integrity [46].
2.5. Piper nigrum L.
P. nigrum is a common plant in the Piperaceae family. It is cultivated in Pakistan, South America, Africa, and the southwestern Indian highlands. P. nigrum’s main alkaloid component is piperine. The spiciness and pungency of black pepper is due to the alkaloidal elements found in the fruit. It is used as a spice all over the world, and since it contains pharmacological characteristics, it is utilized in ancient medicinal systems like Ayurveda and Unani medicine to treat diseases, pain, fever, and inflammation [52]. It contains limonene [53], a molecule with neuroprotective effects [54]. In rats, Hritcu et al., 2014, showed that P. nigrum extract (50 and 100 mg/kg, p.o) inhibiting oxidative stress in the hippocampus, which ameliorates β-amyloid (1–42)-induced spatial memory impairment [55].
2.6. Rheum ribes L.
R. ribes is a plant that is part of the family of Polygonaceae, which is generally used in traditional remedies due to its many biological activities, including antibacterial and antioxidant [56]. It contains curcumin [57], a molecule with neuroprotective effects [58]. Yildirim et al. showed that R. ribes extract increased antioxidant enzyme activities and diminished blood glucose levels in STZ-induced diabetic rats [59]. Zahedia et al. showed that R. ribes extract (250 and 500 mg/kg, p.o) ameliorates memory deficits generated by bilateral NBM lesions in rats [60].
2.7. Markhamia tomentosa (Benth.) K. Schum. ex Engl.
M. tomentosa, a plant belonging to the Bignoniaceae family [61], has been found to be safe for oral administration in rats, as demonstrated by Ibrahim et al. This indicates a lack of toxicity on renal and hepatic function parameters [62]. The extract of M. tomentosa has been reported to possess various beneficial properties, including antioxidant, antimicrobial, anti-inflammatory, antiulcer, and analgesic activities [63,64,65,66]. It contains catechin [67], a molecule with neuroprotective effects [68]. In a separate study conducted by Ionita et al. on rats, the oral administration of M. tomentosa extract at doses of 50 and 200 mg/kg resulted in improved memory performance in behavioral tests. Additionally, the extract exhibited anti-acetylcholinesterase activity and reduced oxidative stress in the rat hippocampus [67].
2.8. Cassia obtusifolia L.
C. obtusifolia, a plant belonging to the Leguminosae family, is commonly used in traditional Chinese medicine. The seeds of this plant, known as Juemingzi in Chinese, have a history of being utilized to address various conditions, such as red and watery eyes, dizziness, and headaches [69]. Researchers have extensively investigated the chemical composition of these seeds, leading to the isolation of numerous anthraquinones [70]. It contains emodin [71], a molecule with neuroprotective effects [72]. In a study involving mice, Kim et al. demonstrated that the oral administration of C. obtusifolia extract at doses of 25, 50, and 100 mg/kg resulted in the alleviation of memory impairment induced by scopolamine. Additionally, the extract inhibited acetylcholinesterase, leading to an improvement in the cholinergic function of the nervous system [73].
3. Phytochemicals against Alzheimer’s Disease
Curcumin, resveratrol, nobiletin, berberines, limonoid, galantamine, and quercetin are a group of naturally occurring phytochemicals derived from various plants. Extensive research has demonstrated their potential to reduce the risk of various debilitating conditions, including neurodegenerative diseases, cardiovascular diseases, depression, and diabetes. Notably, these phytochemicals exhibit neuroprotective properties, suggesting their capacity to mitigate the symptoms associated with Alzheimer’s disease.
3.1. Curcumin
Curcumin is a dietary polyphenol derived from Curcuma longa L. [74], a spice commonly used in India, Southeast Asia, and China for its aromatic, coloring, and preservative properties [75]. Extensive research has elucidated the molecular mechanisms underlying the therapeutic effects of curcumin, including its anticancer, antioxidant, anti-inflammatory, antidiabetic, immunomodulatory, lipid-regulating, hepatoprotective, antiarthritic, antidepressant, and anti-Alzheimer’s properties [74,76]. Despite its broad range of effects, curcumin possesses intrinsic physicochemical characteristics such as photodegradation, low bioavailability, poor water solubility, short half-life, and chemical instability [77,78].
The anti-Alzheimer’s effects of curcumin have been demonstrated in various murine models. In mice, intraperitoneal administration of curcumin at a dosage of 50 mg/kg resulted in a reduction of Aβ plaque burden in the dentate gyrus of the hippocampus and prefrontal cortex (PFC). Moreover, a significant decrease in pyknotic or tangle-like neurons was observed in regions such as CA1 and CA3 of the hippocampus and the PFC, along with a decrease in the expression of Iba-1 and GFAP in the PFC [79]. Ray et al. reported that intraperitoneal injection of curcumin at a dosage of 25 mg/kg led to a decrease in H2O2 levels and an increase in glutathione (GSH) levels in the brains of athymic mice, suggesting a favorable intracellular redox environment. Additionally, the increased ratio of free to oxidized glutathione (GSH: GSSH) indicated an improved redox status compared to controls [80]. In rats, Doaa et al. demonstrated that oral administration of curcumin at a dosage of 80 mg/kg reduced β-amyloid accumulation in the hippocampus and ameliorated cognitive deficits [81]. Han-chang et al. found that intraperitoneal injection of curcumin at a dosage of 10 mg/kg reduced oxidative stress, improved active avoidance and locomotor activity, and reduced neurodegeneration in experimental models [82].
3.2. Gedunin
Gedunin is a principal limonoid found primarily in the seeds of many genera of the family Meliaceae. Many biological activities have been attributed to gedunin, such as antimalarial, anticancer, antiallergic, neuroprotective, antibacterial, anti-inflammatory, and insecticidal effects [83,84,85]. Thom et al. showed that gedunin inhibited the activation of NF-κB induced by Aβ1–42, thereby reducing the levels of nitric oxide (NO) and interleukin-1 beta (IL-1β), pro-inflammatory molecules. Furthermore, gedunin inhibits neuroinflammation by activating nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream targets, including γ-glutamylcysteine synthetase, heme oxygenase 1, and NADPH quinone dehydrogenase 1, which are involved in quenching reactive oxygen and nitrogen species (NO) generated by NF-κB activation [86].
3.3. Quercetin
Quercetin, a bioactive compound found in various vegetables and fruits, such as white onion bulbs, blueberries, and cranberries [87,88], exhibits distinct actions in the brain, influencing glucose homeostasis in experimental diabetes and conferring beneficial effects [89]. It possesses a multitude of health benefits, including antioxidant, anti-inflammatory, cardiovascular, anticancer properties, and neuroprotection [90,91,92,93,94,95]. Particularly in Alzheimer’s disease (AD), pretreatment of neuronal cultures obtained from 18-day-old Sprague–Dawley rat fetuses with quercetin significantly attenuated Aβ1–42-induced cytotoxicity at the two lowest doses (5 and 10μM), protein oxidation, lipid peroxidation and apoptosis [96]. In a study conducted by Manouchehr et al., rats treated with quercetin via intraperitoneal injection at doses of 40 and 80 mg/kg exhibited improved spatial memory in an AD model induced by intracerebroventricular administration of streptozotocin (ICV-STZ) [97].
3.4. Resveratrol
Resveratrol, a member of the polyphenol group of phytochemicals, is naturally present in wild fruits such as grapes and blueberries, offering beneficial effects on human health [98,99]. Notably, resveratrol has shown potential in improving cognitive function in dementia and playing a neuroprotective role in the neurodegenerative processes associated with Alzheimer’s disease [100]. It possesses antioxidant properties, making it potentially valuable in combating oxidative stress [101]. Additionally, resveratrol exhibits anticarcinogenic and anti-inflammatory properties [102]. In a study conducted by Yazir et al., rats treated with resveratrol via intraperitoneal injection at doses of 5 or 20 mg/kg for 35 days demonstrated a significant attenuation of scopolamine-induced deficits in spatial memory and emotional learning in chronically stressed rats [103]. Another study by Sharma and Gupta, revealed that intraperitoneal administration of resveratrol at doses of 10 and 20 mg/kg in rats significantly suppressed cognitive impairment induced by intracerebroventricular administration of streptozotocin (ICV-STZ). The resveratrol-treated ICV-STZ rats exhibited increased brain glutathione levels and a modest elevation in brain malondialdehyde levels [104].
3.5. Nobiletin
Nobiletin is an important polymethoxyflavones existing in fruits including oranges, tangerines, and lemons [105]. Diverse pharmacological effects attributed to nobiletin comprise antidiabetic, anti-atherogenic, antioxidant, anticarcinogenic and anti-inflammatory effects [106,107]. In rats, Matsuzaki et al. showed that nobiletin (50 mg/kg, intraperitoneally injected) acts as a protector against Aβ1–40-induced impairment of learning ability [108]. In mice, Akira Nakajima et al. showed that nobiletin (30 mg/kg, intraperitoneally injected for 3 months) ameliorates memory impairment in olfactory-bulbectomized mice and learning, NMDA receptor antagonist-treated mice and amyloid precursor protein transgenic mice. In the hippocampus, nobiletin reduced ROS levels [109].
3.6. Fisetin
Fisetin is a flavonoid found in many commonly consumed foods, such as strawberries [110], and has various biological properties that are beneficial in the treatment of AD. For instance, fisetin protects neurons from oxidative stress-induced death [111], and promotes neuronal differentiation [112,113]. In rats, Das et al. showed that fisetin (20 mg/kg, oral administration for 12 weeks) reduced lipid peroxides and preserved Na+/K+-ATPase activity which was found modified in the epileptic rats and also found to attenuate the seizure related cognitive dysfunctions [114]. In mice, Ashfaq et al. showed that Fisetin (20 mg/kg, intraperitoneally injected for 2 weeks) acts as a neuroprotector against Aβ1–42-induced neurotoxicity. It also induced increased levels of presynaptic (SNAP-25 and SYN) and postsynaptic (SNAP-23, PSD-95, p-GluR1 (Ser 845), pCAMKII (Thr 286) and p-CREB (Ser 133)) proteins which allows reversing synaptic dysfunction induced by Aβ1–42 [115].
3.7. Berberine
Berberine (BBR) is an isoquinoline alkaloid isolated from C. chinensis. Numerous preclinical and clinical studies have shown that BBR is beneficial in diabetes and increased peripheral and central cholinergic nervous system activity [116,117,118,119,120]. Lee et al. showed that berberine (20 mg/kg, i.p. for 14 days) decreased tumor necrosis factor-α, the expression of pro-inflammatory cytokines including interleukin-1β, and cyclooxygenase-2 mRNA in the hippocampus [116]. In rats, Hend et al. showed that berberine (50 mg/kg, orally) significantly improved cognitive behavior and provided protective effects against heavy metal-induced memory impairment [121].
4. Materials and Methods
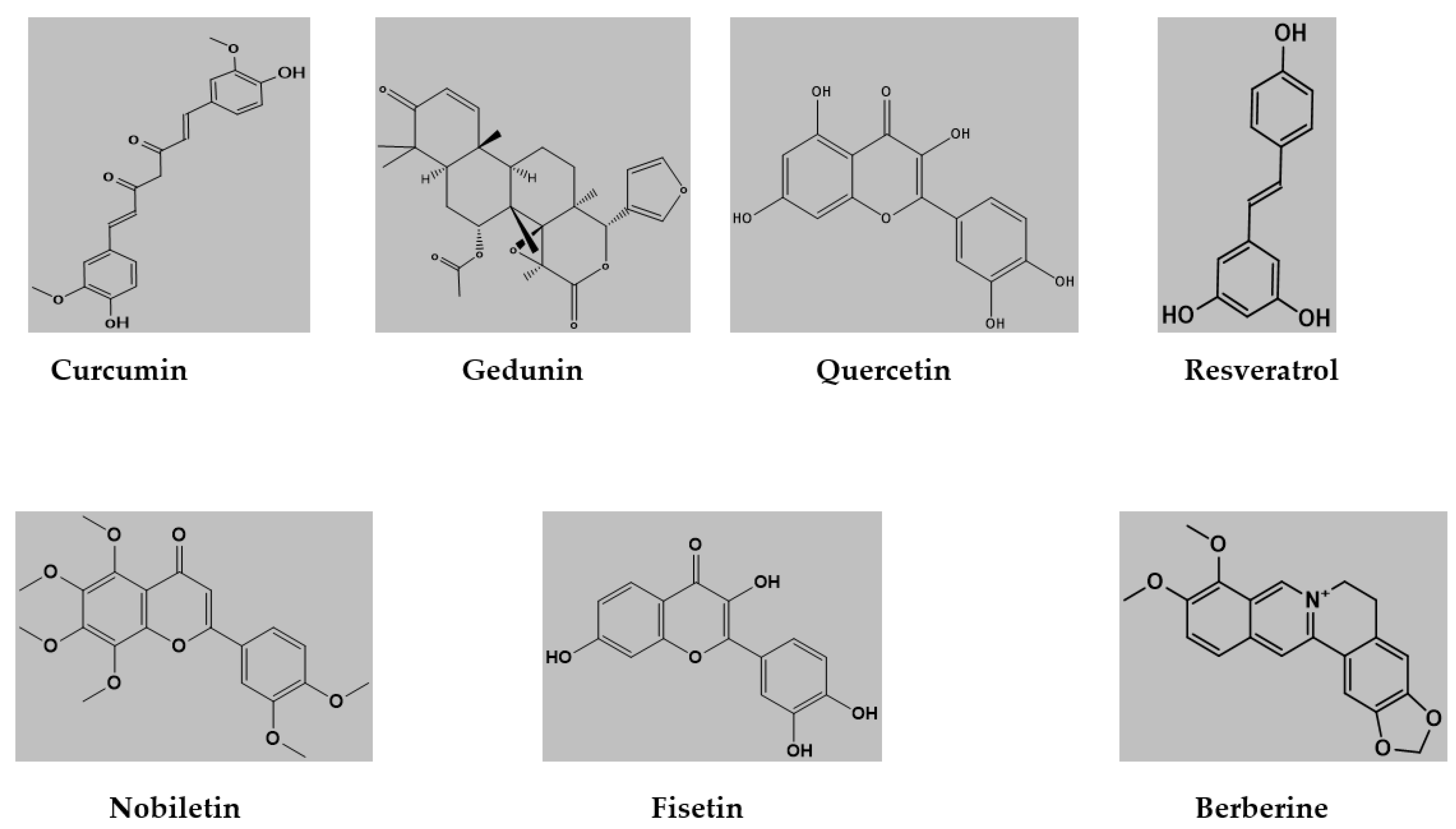
The docking calculations were performed on curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl) hepta-1,6-diene-3,5-dione), gedunin ([(1S,2R,4S,7S,8S,11R,12R,17R,19R)-7-(furan-3-yl)-1,8,12,16,16-pentamethyl-5,15-dioxo-3,6-dioxapentacyclo [9.8.0.02,4.02,8.012,17] nonadec-13-en-19-yl] acetate), quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one), resveratrol (5- [(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol), nobiletin (2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxychromen-4-one), Fisetin (2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one), berberine (16,17-dimethoxy-5,7-dioxa-13azoniapentacyclo [11.8.0.02,10.04,8.015,20]henicosa-1(13),2,4(8),9,14,16,18,20-octaene) (Figure 1).
Figure 1.
Structures of ligand molecules used for molecular docking.
4.1. Protein Preparation
The crystal structure of human Acetylcholinesterase (AChE) with the Protein Data Bank (PDB) ID 4PQE was accessed from the website (Available online: www.rcsb.org/, accessed on 2 February 2023) [122]. The structure was prepared by removing all water molecules, and the Kollman charges and polar hydrogens were added to the protein using AutoDockTools (ADT) version 1.5.7 [123,124,125,126,127]. For the molecular docking process, a grid box with a point spacing of 0.375 Å and dimensions of 40 × 40 × 40 was created. The grid box was centered at coordinates x = 4.764, y = 65.53, z = 56.856 to encompass both the peripheral and active site regions of human AChE. The prepared structure was saved in a dockable pdbqt format to facilitate the subsequent molecular docking analysis.
4.2. Ligand Preparation
The structures of seven ligands, along with the standard inhibitor donepezil, were obtained in SDF format from the PubChem database (Available online: www.pubchem.ncbi.nlm.nih.gov/, accessed on the 2 February 2023) [128]. These ligand structures were then converted into the pdb format using PyMoL Molecular Graphics System (Version 2.5.3). Additionally, Autodock tools (ADT; version 1.5.7) were utilized to convert both the ligand molecules and the protein into the dockable pdbqt format [129,130,131].
4.3. Molecular Docking
Molecular docking is a crucial approach employed to explore the active site of proteins and unravel the intricate interactions between ligands and biological molecules [132]. To provide a comprehensive overview of the ligand–receptor interactions, Table 1 categorizes the compounds based on their affinity ratings, offering valuable insights into their binding properties. To visualize these interactions and generate informative graphics, the software Discovery Studio Visualizer v3.0 (BIOVIA, 2021) was utilized [133].

Table 1.
Summary of molecular docking studies of phytochemical compounds against AChE.
The process of molecular docking allows us to delve into the structural details of ligand binding and assess the strength of their interactions with the target receptor. By examining the binding affinity, we can discern the potential efficacy and selectivity of the ligands in comparison to conventional inhibitors. This information aids in understanding the molecular mechanisms underlying the therapeutic effects of the compounds and provides a basis for further investigations.
4.4. ADME Studies
To assess the drug-likeness of the seven phytochemical compounds, absorption, distribution, and metabolism (ADME) studies were conducted [134]. The ADME properties were determined using the Swiss online ADME web Tool [135,136]. Furthermore, the relationship between the calculated lipophilicity (WLOGP) and the polar surface area (TPSA) of the compounds was analyzed using GraphPad Prism software v8.0 (GraphPad Software, San Diego, CA, USA). This analysis allowed for the evaluation of the blood–brain barrier (BBB) properties of the compounds [137].
5. Results and Discussion
5.1. Molecular Docking Results
The molecular docking results revealed that gedunin exhibited a higher binding affinity (−8.7 kcal/mol) for AChE compared to the standard AChE inhibitor (Figure 2), donepezil (−8.6 kcal/mol), as shown in Table 1. Following closely behind gedunin, the ligands fisetin, berberine, curcumin, quercetin, resveratrol, and nobiletin exhibited binding affinities of −8.2, −7.7, −7.5, −7.5, −7.0, and −6.9 kcal/mol, respectively, for AChE. Notably, nobiletin displayed the lowest binding affinity (−6.9 kcal/mol) among the studied ligands but interacted with crucial amino acid residues of AChE, as depicted in Figure 3. These findings align with previous studies indicating the inhibitory effect of nobiletin on AChE [138,139,140].
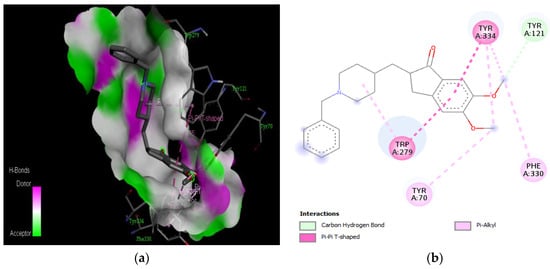
Figure 2.
Active site of the acetylcholinesterase (AchE) with donepezil: (a) H-bond interaction between donepezil and (AchE); (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
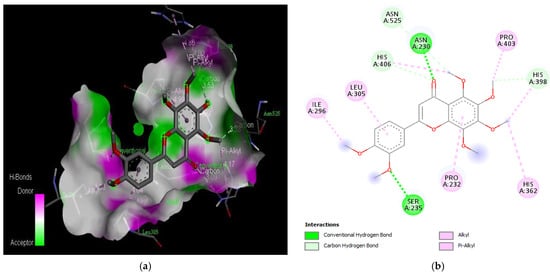
Figure 3.
Active site of the acetylcholinesterase (AchE) with nobiletin: (a) H-bond interactions; (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
Comparing the ligands to the standard inhibitor donepezil, gedunin, fisetin, berberine, and curcumin exhibited favorable affinities toward AChE (Figure 4, Figure 5, Figure 6 and Figure 7). Particularly, gedunin displayed the weakest binding energy among the ligands and even lower than the standard inhibitor, with a binding affinity of −8.7 kcal/mol. Furthermore, noteworthy interactions were observed between these four ligands and important amino acid residues at the active site of AChE [141]. For a better understanding of the interactions between these ligands and the active site of AChE, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9 present the 2D structures of AChE-ligand complexes, illustrating the hydrogen bond interactions.
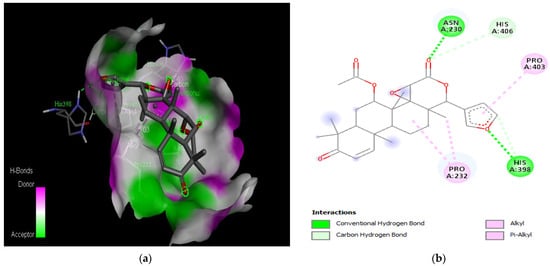
Figure 4.
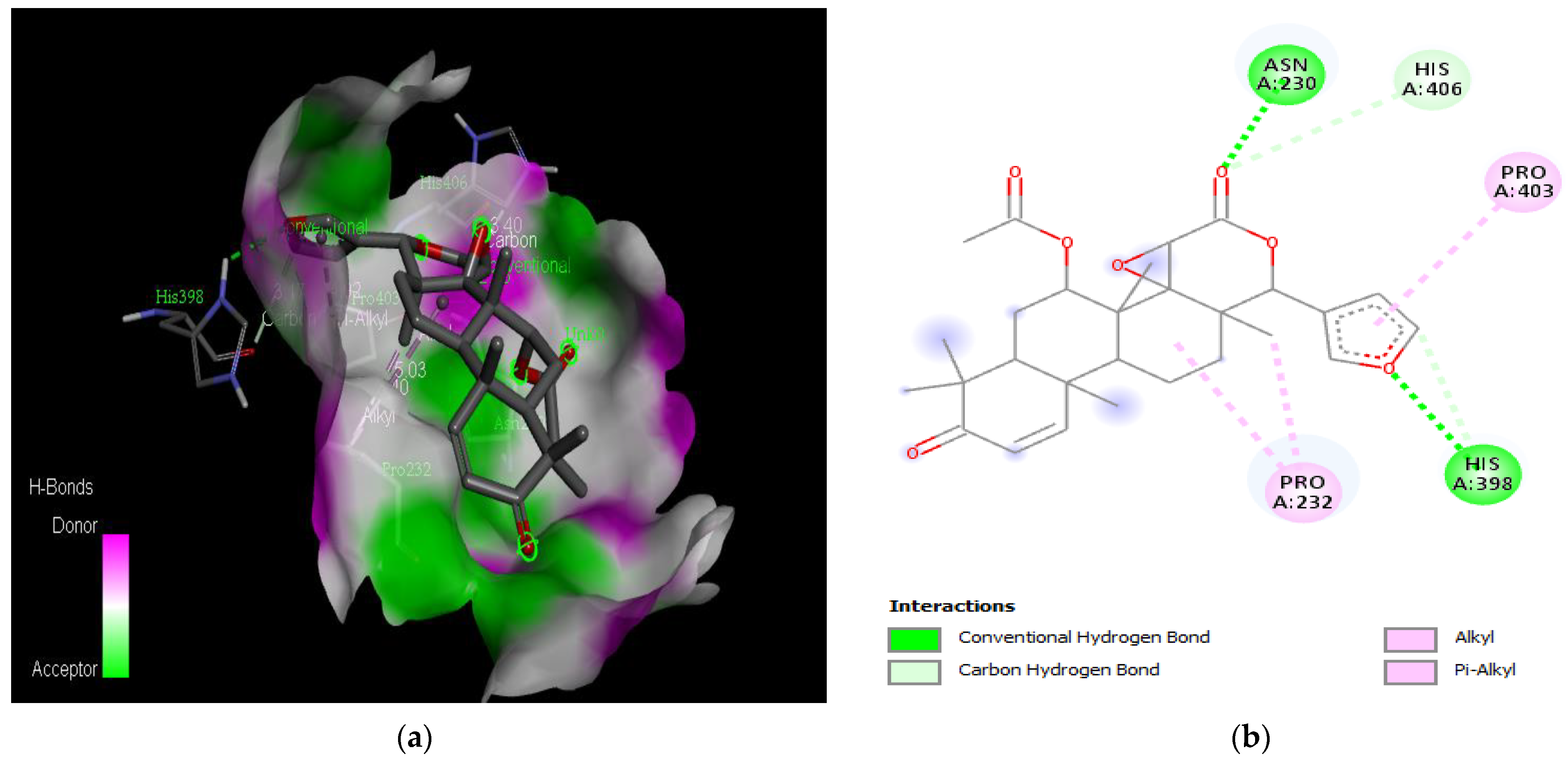
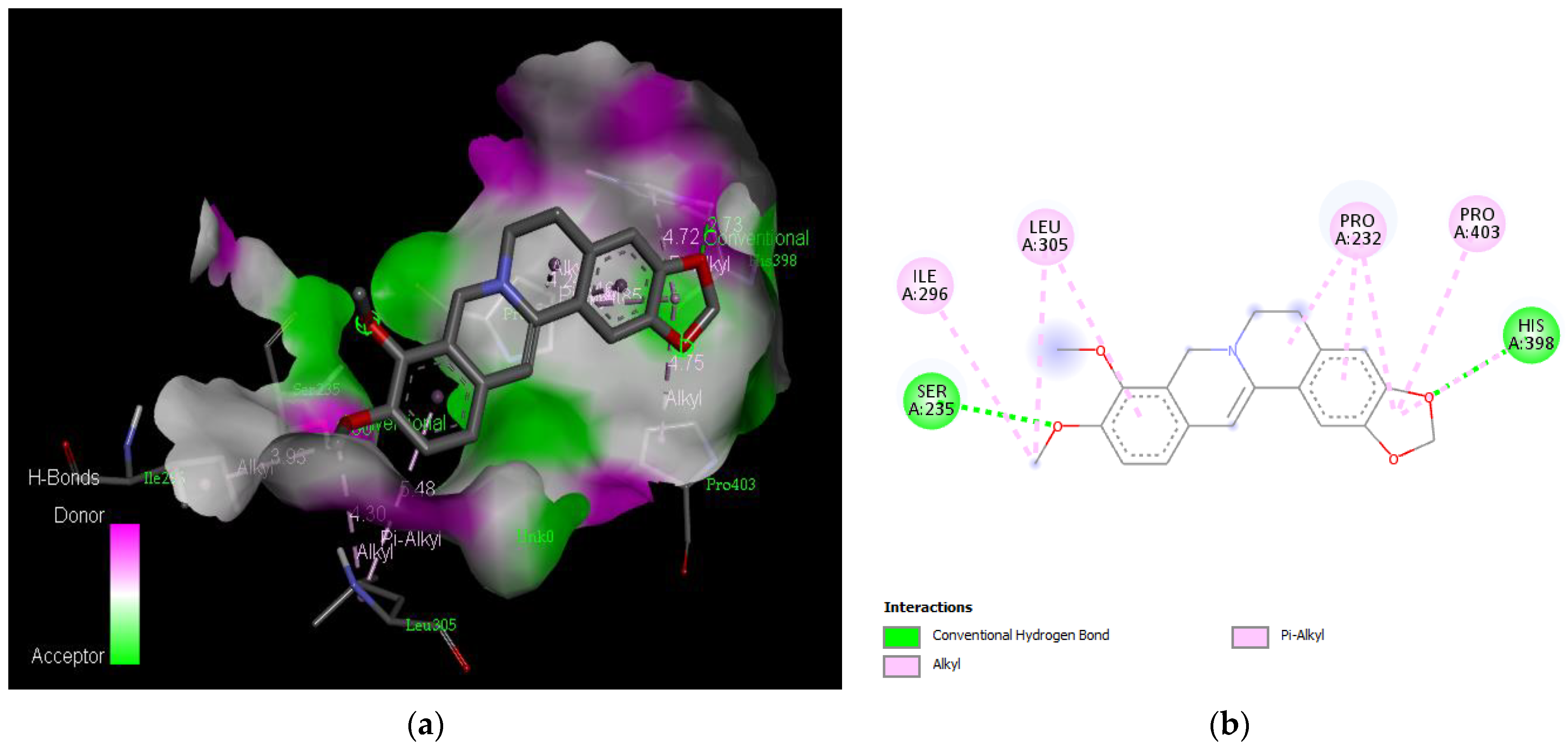
Active site of the acetylcholinesterase (AchE) with gedunin: (a) H-bond interactions; (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
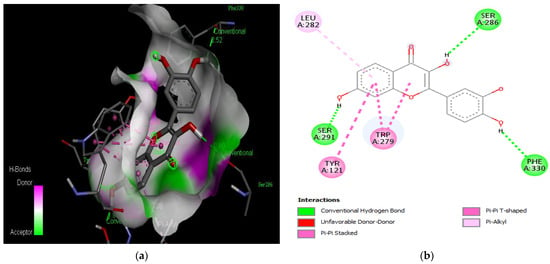
Figure 5.
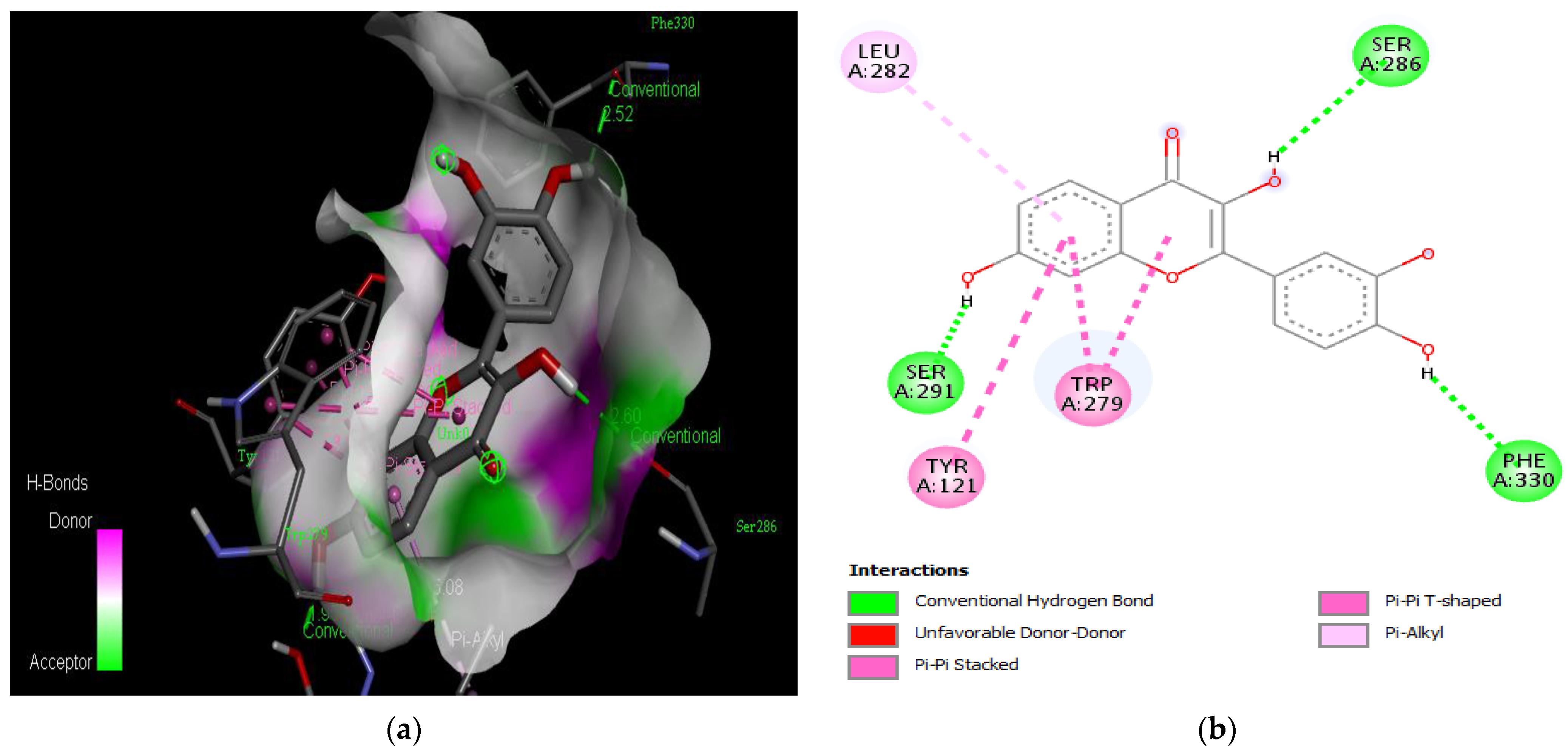
Active site of the acetylcholinesterase (AchE) with fisetin: (a) H-bond interactions; (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
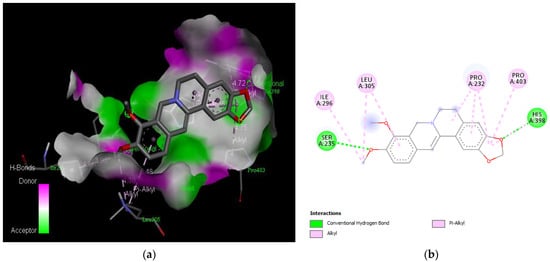
Figure 6.
Active site of the acetylcholinesterase (AchE) with berberine: (a) H-bond interactions; (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
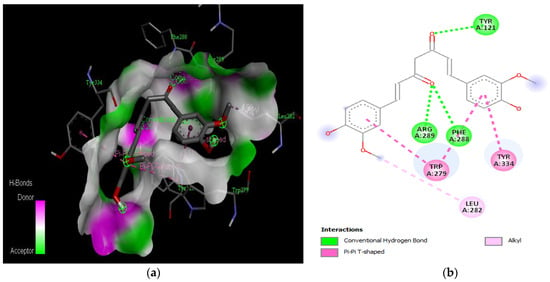
Figure 7.
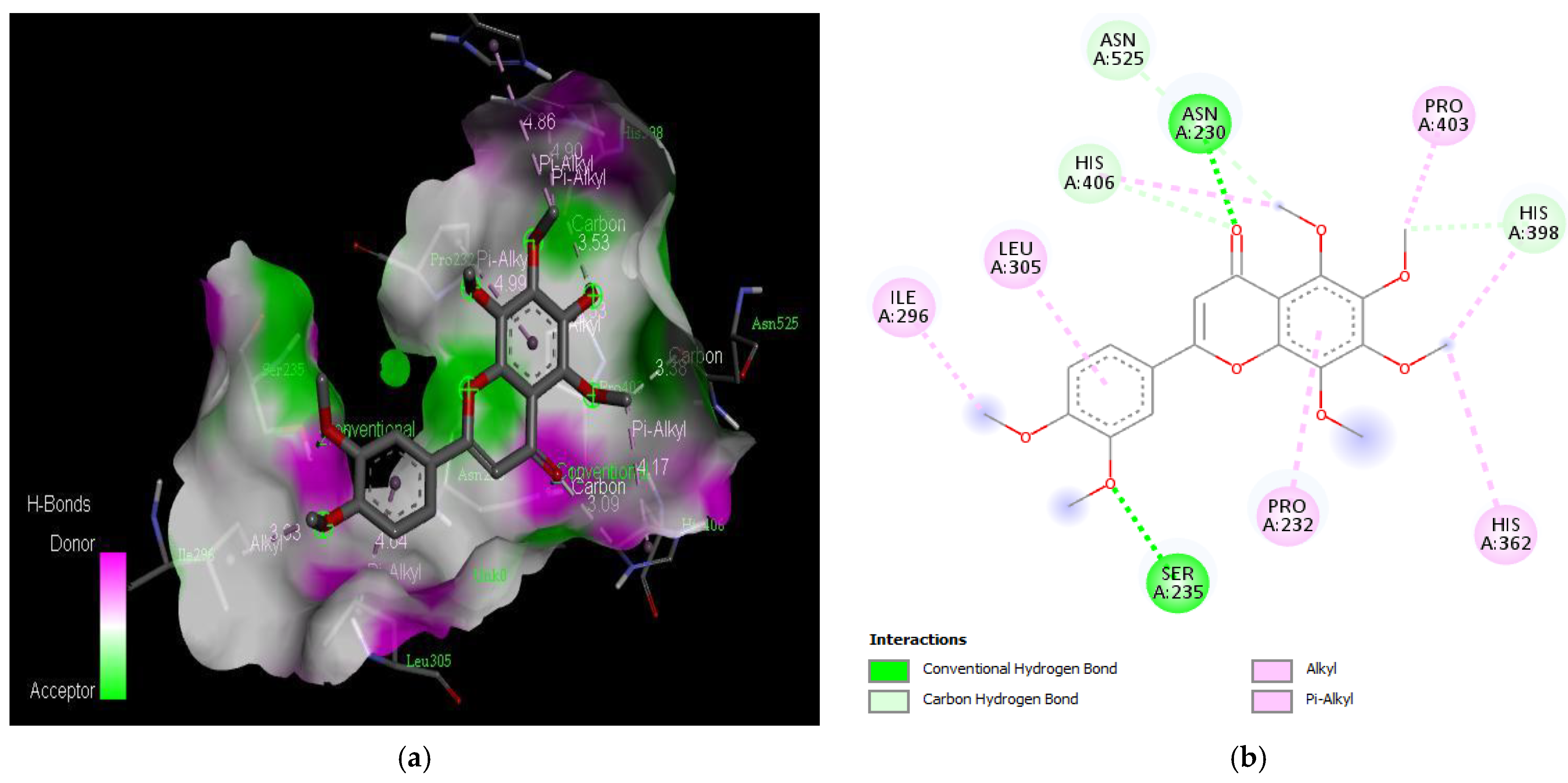
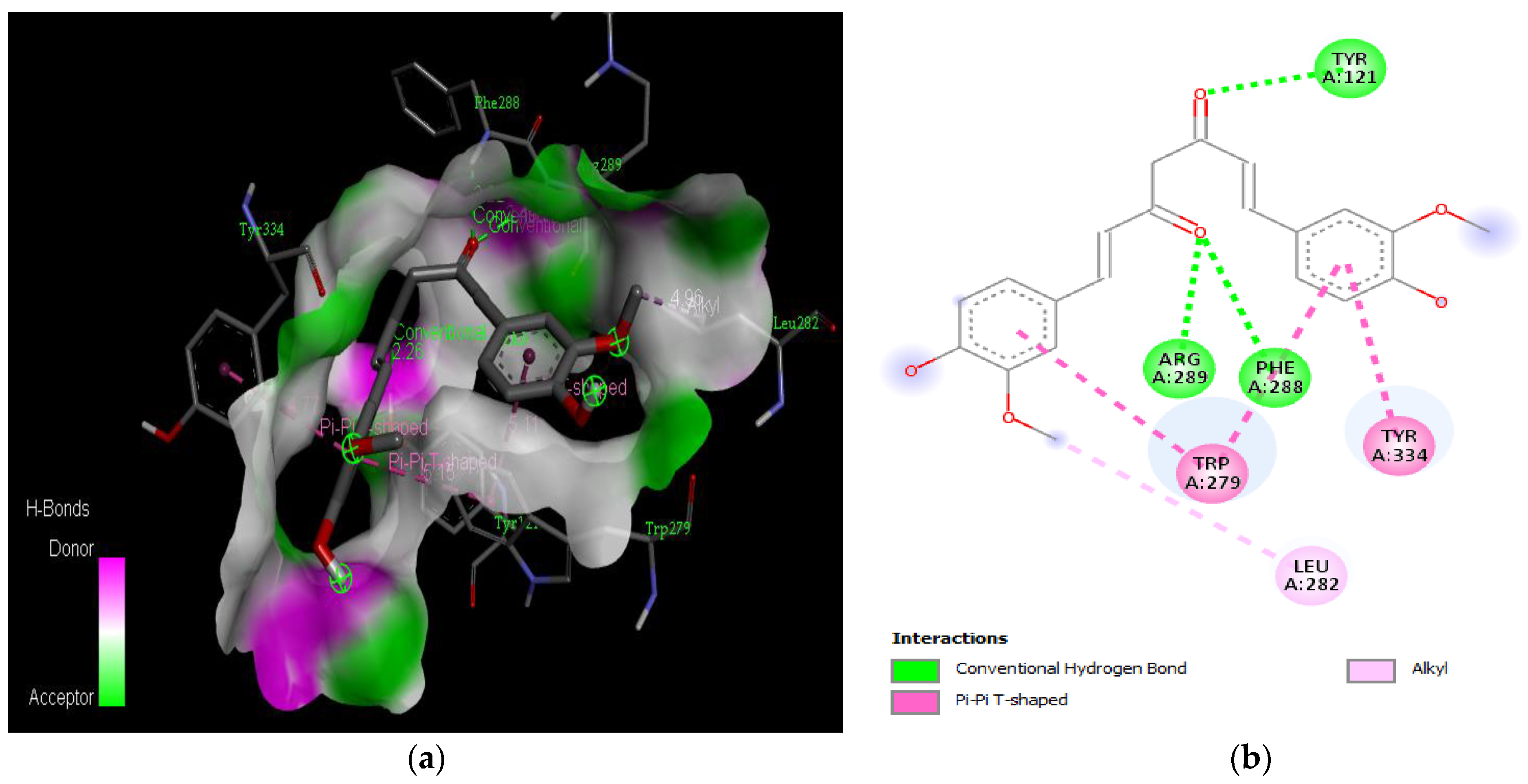
Active site of the acetylcholinesterase (AchE) with curcumin: (a) H-bond interactions; (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
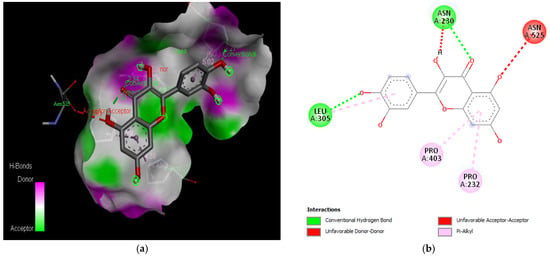
Figure 8.
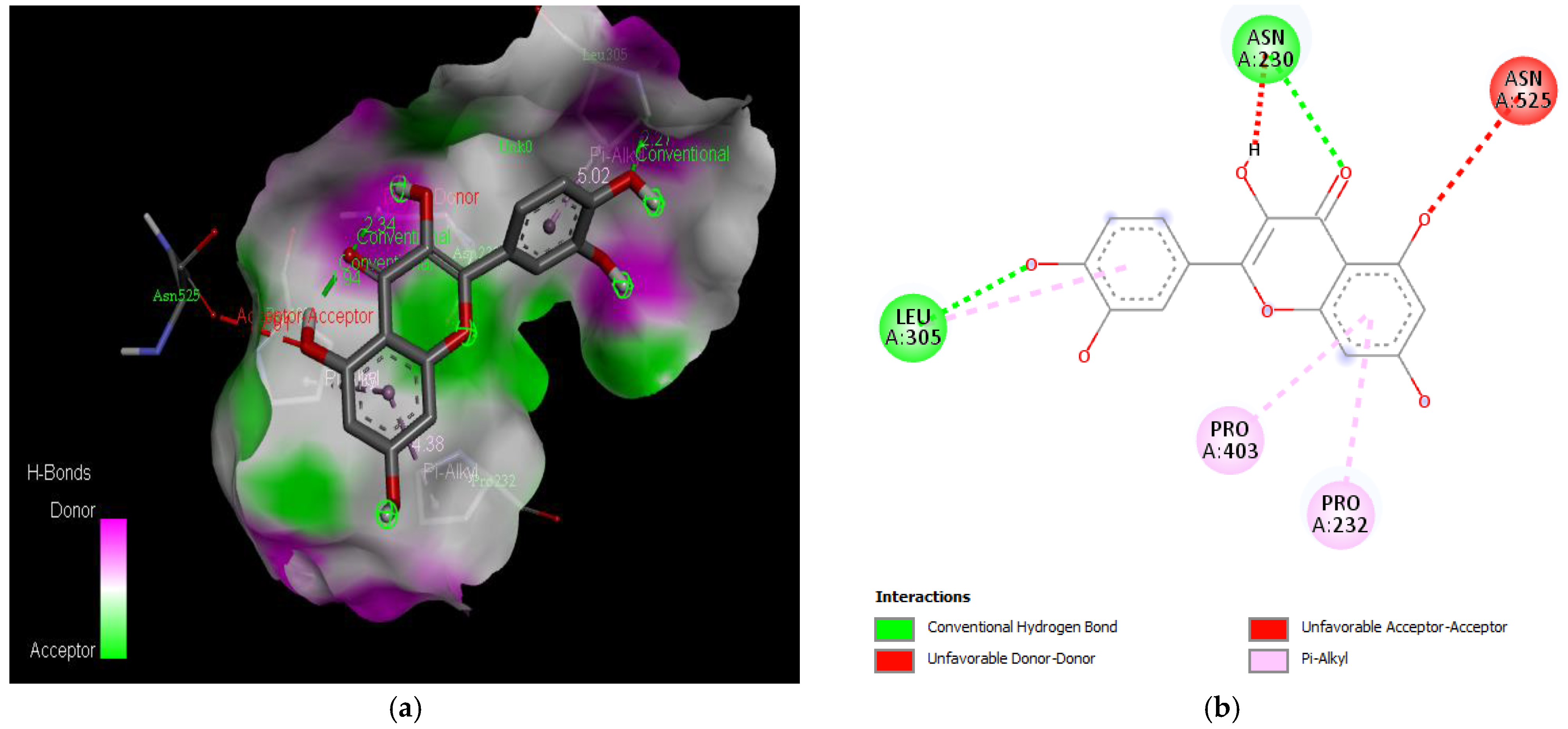
Active site of the acetylcholinesterase (AchE) with quercetin: (a) H-bond interactions; (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
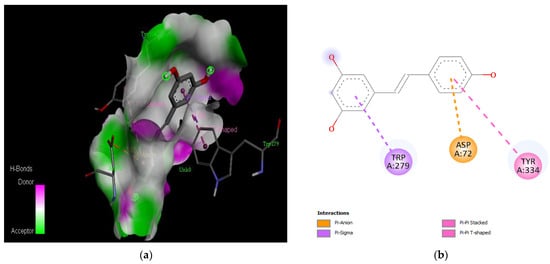
Figure 9.
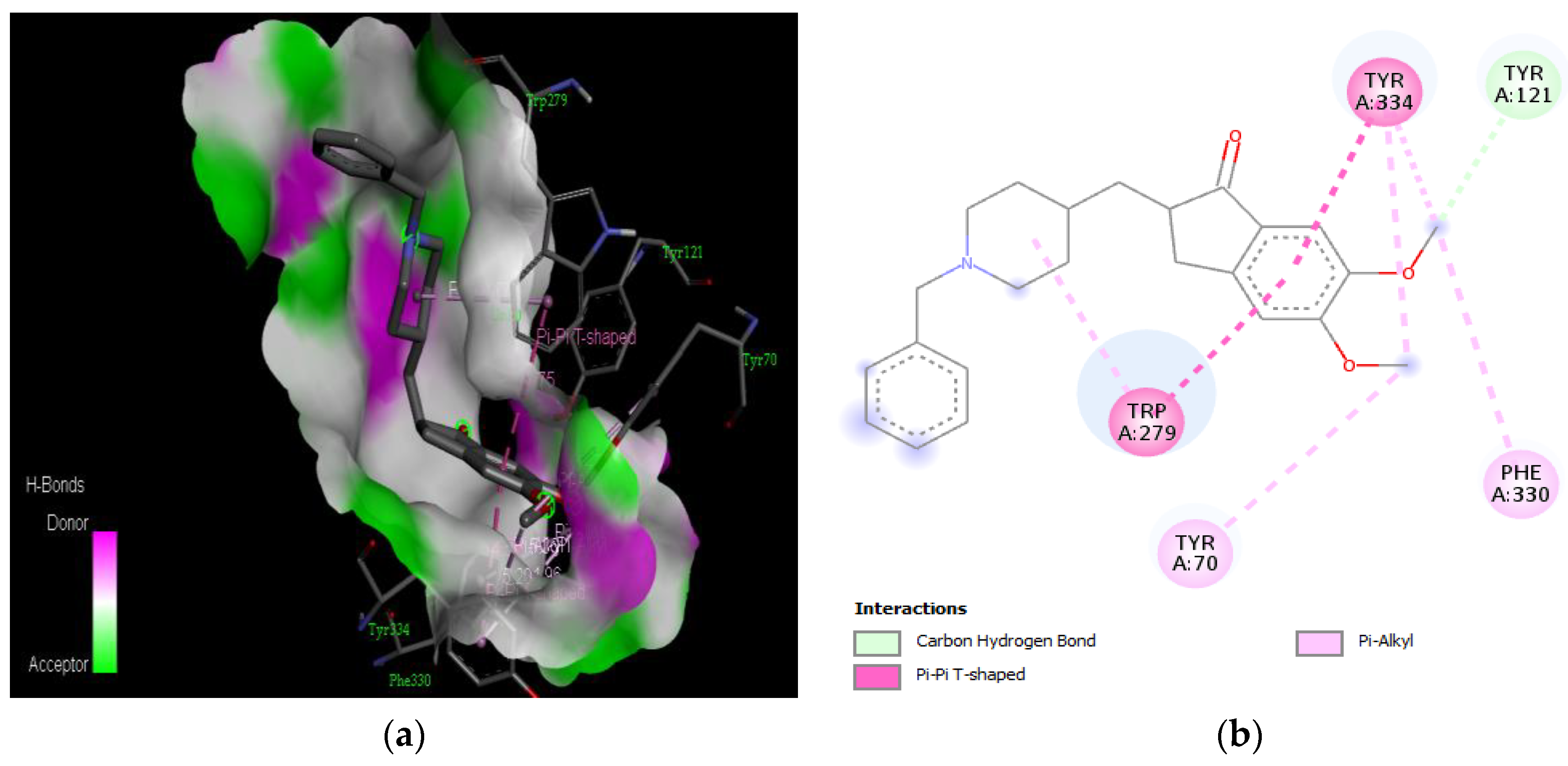
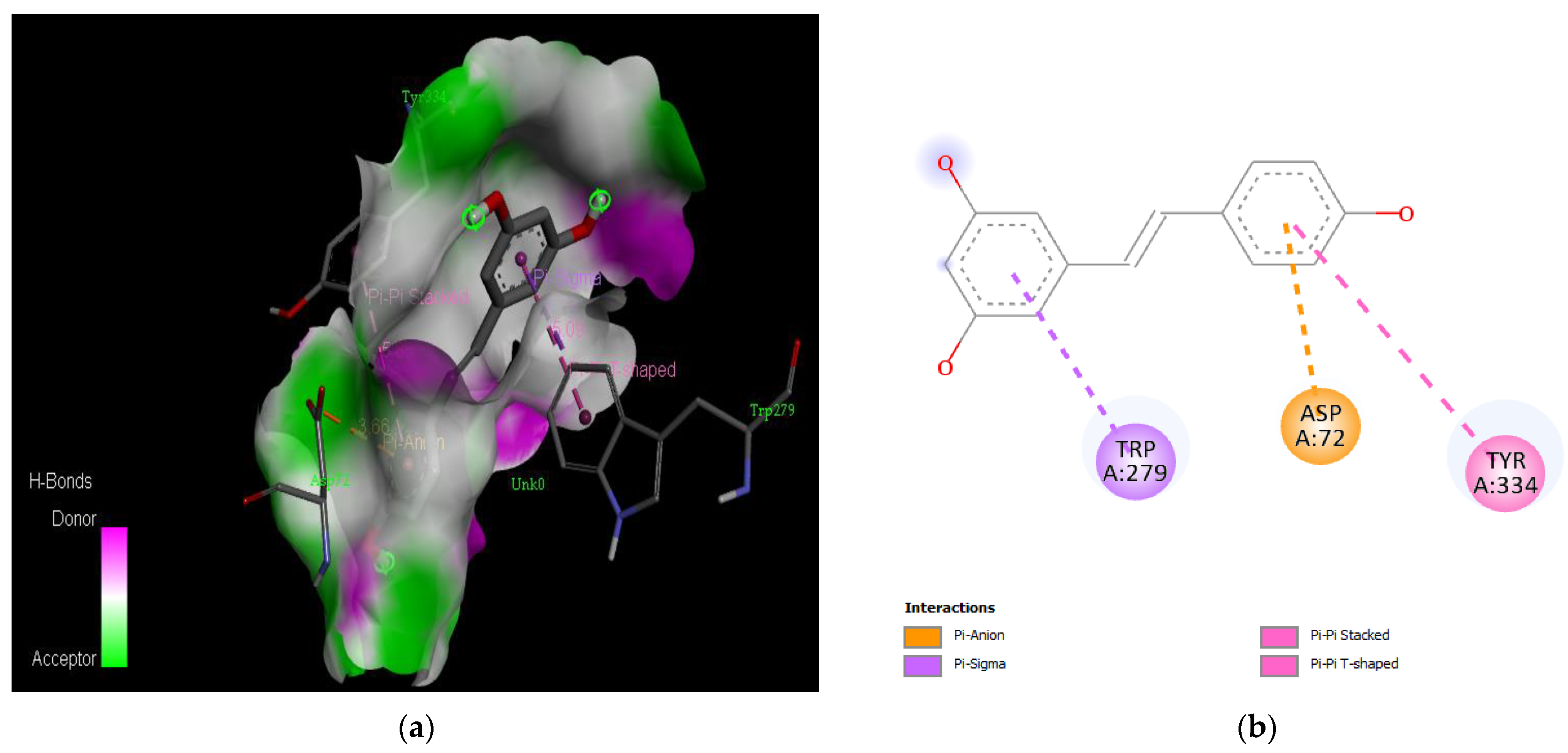
Active site of the acetylcholinesterase (AchE) with resveratrol: (a) H-bond interactions; (b) two-dimensional view the interactions between the ligand and the amino acids of the receptor.
Acetylcholinesterase (AChE) possesses a narrow gorge measuring about 20 Å, which consists of two main regions: the peripheral anionic site (PAS) located near the entrance and the catalytic active site (CAS) situated at the bottom [131]. The AChE structure contains several conserved aromatic amino acids, including Tyr 70, Asp 72, Trp 84, Gly 118, Gly 119, Tyr 121, Tyr 130, Ser 200, Ala 201, Trp 279, Phe 288, Phe 290, Glu 327, Phe 330, Phe 331, Tyr 334, and His 440 [142]. Inhibitors that target AChE hold promise for Alzheimer’s disease (AD) treatment, as they can bind to one or both of these binding sites [143].
The PAS of AChE is composed of amino acids Tyr 121, Trp 279, Tyr 70, Asp 72, and Tyr 334. It is responsible for cation-π interactions with the quaternary ammonium group of the substrate. The anionic site of AChE, comprising Trp 84, Tyr 130, Phe 330, and Phe 331, plays a role in inhibitor binding and interacts with the substrate. The proper orientation of acetylcholine within the gorge facilitates interactions with the anionic site [142]. In the case of gedunin, it forms multiple hydrogen bonds with key amino acid residues of AChE, specifically Asn 230 and His 398. Additionally, it engages in two pi-alkyl interactions with Pro 232, a pi-alkyl interaction with Pro 403, and a carbon–hydrogen bond interaction with His 406 (Figure 2b). Curcumin demonstrates two pi-pi T-shaped interactions with Trp 279, a pi-pi T-shaped interaction with Tyr 334, and a hydrogen bond interaction with Tyr 121, all of which involve important amino acid residues of the PAS (Figure 5b). Resveratrol exhibits a pi-sigma interaction with Trp 279 and a pi-pi T-shaped interaction with Tyr 334 (Figure 7b). Fisetin demonstrates a pi-pi stacked interaction with Tyr 121, two pi-pi T-shaped interactions with Trp 227, and a hydrogen bond interaction with Phe 330, which is a significant amino acid residue of the anionic site (AS) (Figure 3b).
5.2. Results of ADME Analysis
The findings of the ADME investigation on the seven compounds with high binding energy for acetylcholinesterase are summarized in Table 2. Notably, the results indicate that all seven compounds exhibit characteristics that make them suitable candidates for oral medication. Importantly, none of the compounds violated Lipinski’s rule, which implies that they possess favorable drug-like properties. Specifically, these compounds have a molecular weight of less than 500 Daltons, fewer than ten hydrogen bond acceptors, fewer than five hydrogen bond donors, and an octanol/water partition coefficient (MLogP) of less than five. Additionally, each of the seven compounds has a topological surface area (TPSA) smaller than 140 and fewer than ten rotatable bonds, complying with Veber’s criteria. These favorable ADME properties enhance the potential of these compounds as viable oral medications.

Table 2.
Physicochemical properties of phytochemical compounds with remarkable binding affinities against acetylcholinesterase.
Lipinski’s rule of five is a well-established standard in the field of drug discovery, outlining a set of physicochemical properties that characterize orally active compounds. According to Lipinski, compounds suitable for oral drug development generally have no more than one breach of the following criteria: (1) Fewer than five hydrogen bond donors (oxygen or nitrogen atoms with one or more hydrogen atoms), (2) Fewer than 10 hydrogen bond acceptors (oxygen or nitrogen atoms), (3) Molecular mass less than 500 Daltons, and (4) Octanol-water partition coefficient log P (MLogP) not exceeding five [123,144]. The seven compounds being studied in this research adhere to Lipinski’s criteria, with no more than one violation each, and they exhibit notably strong negative binding affinities. Additionally, the compounds were evaluated based on Veber’s rule, which takes into account the number of rotatable bonds and the polar surface area [145]. Significantly, none of the molecules violated Veber’s criteria, further bolstering their potential as promising candidates for oral drug development.
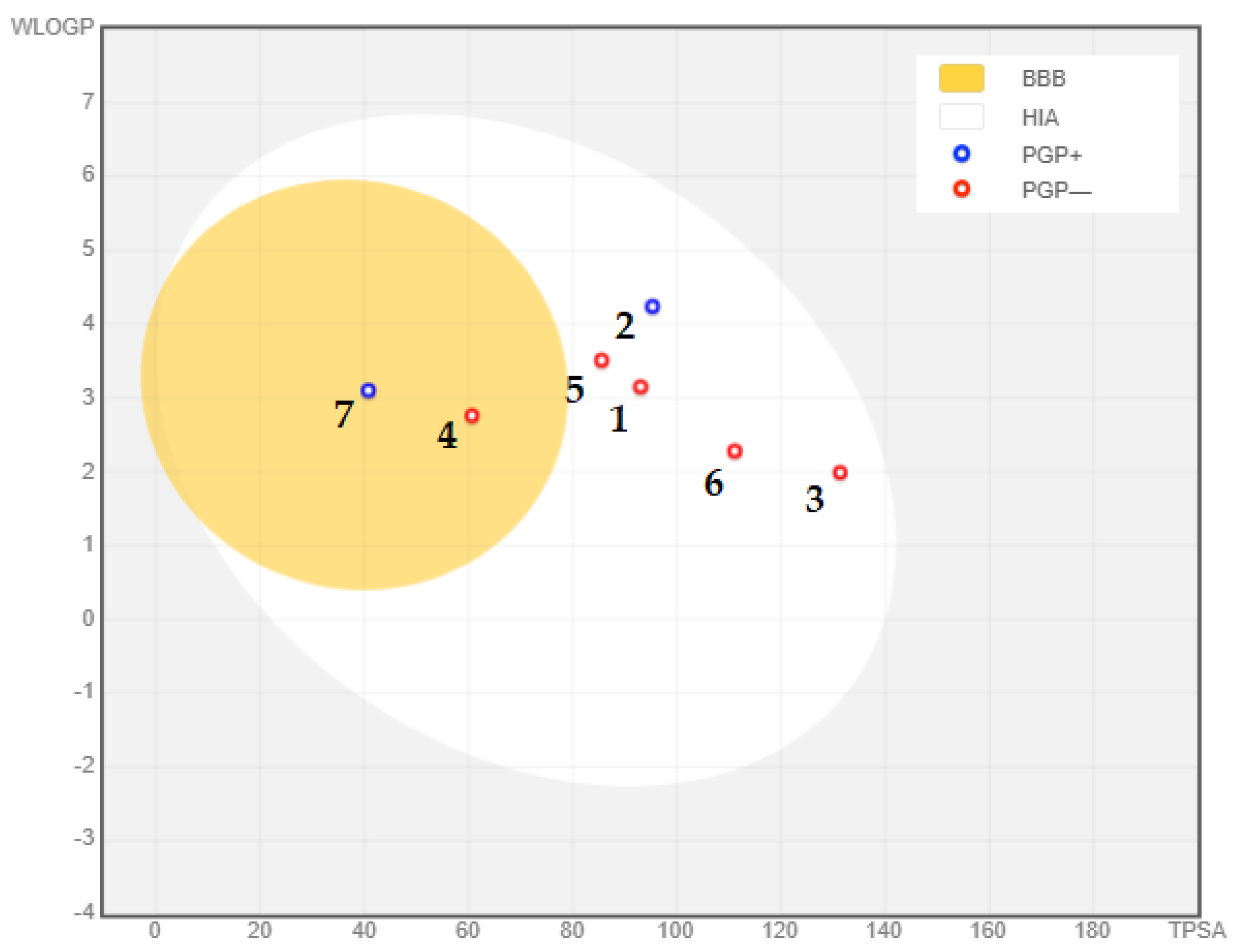
Considering the compounds’ potential to enter the central nervous system (CNS), it is essential to assess their ability to cross the blood–brain barrier (BBB). Typically, compounds that are moderately lipophilic (WLogP 0.4–6.0) and have moderate polarity (TPSA < 79 Å2) can penetrate the BBB [135]. In Table 2, only resveratrol (TPSA = 60.69 Å2, WLogP = 2.76) and berberine (TPSA = 40.80 Å2, WLogP = 3.10) meet these criteria, indicating their potential to cross the BBB. Additionally, most of the analyzed compounds were determined to be non-substrates of P-glycoprotein (PGP-) within the yellow zone, except for gedunin (two) and berberine (seven). This is illustrated in Figure 10, where two compounds fall within the yellow region (indicating PGP- non-substrates), while the remaining five compounds (BBB-) are located outside the yellow region. The BBB serves as a vital barrier between the systemic circulation and the CNS, protecting the brain through both biochemical and physical barriers, including enzymatic activity and active efflux [135,146]. Overcoming the challenges posed by the BBB is critical in the development of effective CNS drugs, and the ability of the selected compounds to cross the BBB holds importance in Alzheimer’s disease treatment [147]. Overall, the adherence of the compounds to Lipinski’s rule of five, their compliance with Veber’s rule, and their potential to cross the BBB indicate their viability as potential oral drug candidates for the treatment of Alzheimer’s disease.
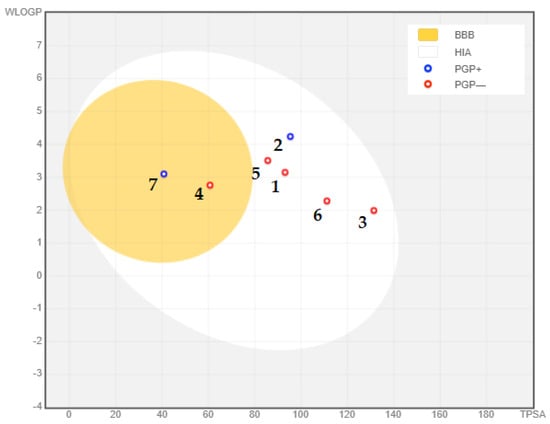
Figure 10.
Blood–brain barrier properties of phytochemical compounds with significantly remarkable binding affinity to acetylcholinesterase: (1) curcumin; (2) gedunin; (3) quercetin; (4) fisetin; (5) resveratrol; (6) nobiletin; (7) berberine. PGP+: P-glycoprotein substrate; PGP-: non-substrate of P-glycoprotein.
6. Conclusions
In recent decades, there has been a significant advancement in our understanding of psychotropic plants and their neuroprotective phytochemicals. These natural compounds have shown great potential in preclinical studies for the treatment of Alzheimer’s disease. However, it is crucial for patients to approach phytomedicines with caution, as not all natural substances are entirely harmless. While promising results have been obtained in animal models, therapeutic effectiveness and safety in humans cannot be assumed. Cholinesterase inhibition has emerged as an effective therapeutic strategy for neurodegenerative disorders, including Alzheimer’s disease. The findings from our study suggest that all seven compounds investigated have the potential to be considered as oral medication candidates. Notably, fisetin and berberine demonstrate reasonable binding affinity for acetylcholinesterase, but it is gedunin that exhibits the highest binding affinity compared to the standard inhibitor used. This highlights the potential of gedunin as a potent inhibitor of acetylcholinesterase. Moreover, resveratrol and berberine demonstrate the ability to cross the blood–brain barrier. This is an important characteristic as it enables the compounds to reach the central nervous system effectively. Therefore, all seven molecules identified in this study possess the capability of being orally administered, making them potentially more effective in the treatment of neurodegenerative diseases compared to the existing drug, donepezil. Overall, our findings contribute to the growing body of evidence supporting the potential of phytochemicals in the treatment of Alzheimer’s disease. However, further research and clinical studies are needed to validate their therapeutic effectiveness and safety in human patients.
Author Contributions
Conceptualization, A.F. and M.B.; methodology, A.F., A.O.T.A. and M.B.; software, A.F. and A.E.; validation, A.F., A.O.T.A., A.A. and M.B.; formal analysis, A.F., F.C. and A.E.; investigation, A.F., M.B. and M.K.P.; resources, A.F. and N.B.; data curation, A.F., F.C. and A.E.; writing—original draft preparation, A.F., M.B. and A.E.; writing—review and editing, A.F., H.Z., F.A.N., A.E. and M.K.P.; visualization, A.F., A.E., H.Z., F.A.N., M.B., A.A. and F.C.; supervision, A.O.T.A.; project administration, A.O.T.A.; funding acquisition, F.A.N., A.A. and M.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSP2023R379), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Acknowledgments
The authors extend their appreciation to Researchers Supporting Project number (RSP2023R379), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodini, M.; De Simone, M.S.; Caltagirone, C.; Carlesimo, G.A. Accelerated Long-Term Forgetting in Neurodegenerative Disorders: A Systematic Review of the Literature. Neurosci. Biobehav. Rev. 2022, 104815. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Bennett, B.D.; Babu-khan, S.; Kahn, S.; Elizabeth, A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase Cleavage of Alzheimer ’ s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Kocyigit, U.M.; Budak, Y.; Gürdere, M.B.; Ertürk, F.; Yencilek, B.; Taslimi, P.; Gülçin, İ.; Ceylan, M. Synthesis of Chalcone-Imide Derivatives and Investigation of Their Anticancer and Antimicrobial Activities, Carbonic Anhydrase and Acetylcholinesterase Enzymes Inhibition Profiles. Arch. Physiol. Biochem. 2018, 124, 61–68. [Google Scholar] [CrossRef]
- Mathew, M.; Subramanian, S. In Vitro Screening for Anti-Cholinesterase and Antioxidant Activity of Methanolic Extracts of Ayurvedic Medicinal Plants Used for Cognitive Disorders. PLoS ONE 2014, 9, e86804. [Google Scholar] [CrossRef] [PubMed]
- Ozmen Ozgun, D.; Gul, H.I.; Yamali, C.; Sakagami, H.; Gulcin, I.; Sukuroglu, M.; Supuran, C.T. Synthesis and Bioactivities of Pyrazoline Benzensulfonamides as Carbonic Anhydrase and Acetylcholinesterase Inhibitors with Low Cytotoxicity. Bioorg. Chem. 2019, 84, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Prasansuklab, A.; Tewin, T. Amyloidosis in Alzheimer’s Disease: The Toxicity of Amyloid Beta. Evid.-Based Complement. Altern. Med. 2013, 2013, 413808. [Google Scholar] [CrossRef]
- Gürbüz, P.; Martinez, A.; Pérez, C.; Martínez-González, L.; Göger, F.; Ayran, İ. Potential Anti-Alzheimer Effects of Selected Lamiaceae Plants through Polypharmacology on Glycogen Synthase Kinase-3β, β-Secretase, and Casein Kinase 1δ. Ind. Crops Prod. 2019, 138, 111431. [Google Scholar] [CrossRef]
- Griffiths, J.; Grant, S.G.N. Synapse Pathology in Alzheimer’s Disease. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 139, pp. 13–23. [Google Scholar]
- Chen, L.; Hu, L.; Zhao, J.; Hong, H.; Feng, F.; Qu, W.; Liu, W. Chotosan Improves Aβ1–42-Induced Cognitive Impairment and Neuroinflammatory and Apoptotic Responses through the Inhibition of TLR-4/NF-ΚB Signaling in Mice. J. Ethnopharmacol. 2016, 191, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Pozueta, J.; Lefort, R.; Shelanski, M.L. Synaptic Changes in Alzheimer’s Disease and Its Models. Neuroscience 2013, 251, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical Trials and Late-Stage Drug Development for Alzheimer’s Disease: An Appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H.; Zhu, B.; Cao, M.; Feng, T.; Sun, Z.; Du, G.; Zhao, Z. Advances and Applications of Fluids Biomarkers in Diagnosis and Therapeutic Targets of Alzheimer’s Disease. CNS Neurosci. Ther. 2023, 29, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P. Brain Imaging and Alzheimer’s Disease. Eur. Neuropsychopharmacol. 1993, 3, 168–169. [Google Scholar] [CrossRef]
- Ghosh, S.; Ali, R.; Verma, S. Aβ-Oligomers: A Potential Therapeutic Target for Alzheimer’s Disease. Int. J. Biol. Macromol. 2023, 239, 124231. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Houghton, P.J. Ethnobotanical Treatment Strategies Against Alzheimers Disease. Curr. Alzheimer Res. 2012, 9, 67–85. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.C.; Baudelaire, É.; Scher, J.; Dicko, A. Antioxidant and Antiacetylcholinesterase Activities of Different Granulometric Classes of Salix Alba (L.) Bark Powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Loizzo, M.; Tundis, R.; Menichini, F.; Menichini, F. Natural Products and Their Derivatives as Cholinesterase Inhibitors in the Treatment of Neurodegenerative Disorders: An Update. Curr. Med. Chem. 2008, 15, 1209–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Sato, S.; Dai, W.; Yamanaka, N. The Protective Effect of Hepatocyte Growth-Promoting Factor (PHGF) against Hydrogen Peroxide-Induced Acute Lung Injury in Rats. Med. Electron Microsc. 2001, 34, 92–102. [Google Scholar] [CrossRef]
- Candy, J.M.; Perry, R.H.; Perry, E.K.; Irving, D.; Blessed, G.; Fairbairn, A.F.; Tomlinson, B.E. Pathological Changes in the Nucleus of Meynert in Alzheimer’s and Parkinson’s Diseases. J. Neurol. Sci. 1983, 59, 277–289. [Google Scholar] [CrossRef]
- Taslimi, P.; Köksal, E.; Gören, A.C.; Bursal, E.; Aras, A.; Kılıç, Ö.; Alwasel, S.; Gülçin, İ. Anti-Alzheimer, Antidiabetic and Antioxidant Potential of Satureja Cuneifolia and Analysis of Its Phenolic Contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Rezende, F.A.G.G.; Sande, D.; Coelho, A.C.; Oliveira, G.P.; Boaventura, M.A.D.; Takahashi, J.A. Edible Flowers as Innovative Ingredients for Future Food Development: Anti-Alzheimer, Antimicrobial and Antioxidant Potential. Chem. Eng. Trans. 2019, 75, 337–342. [Google Scholar] [CrossRef]
- Falzon, C.C.; Balabanova, A. Phytotherapy: An Introduction to Herbal Medicine. Prim. Care Clin. Off. Pract. 2017, 44, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Persa, P.B.; Karimi, E. Alzheimer ’ S : Phytotherapy and the Most Important Herbs in the Treatment of Alzheimer’s. Plant Biotechnol. Persa 2020, 2, 61–62. [Google Scholar]
- Akram, M.; Riaz, M.; Munir, N.; Akhter, N.; Zafar, S.; Jabeen, F.; Ali Shariati, M.; Akhtar, N.; Riaz, Z.; Altaf, S.H.; et al. Chemical Constituents, Experimental and Clinical Pharmacology of Rosa Damascena: A Literature Review. J. Pharm. Pharmacol. 2020, 72, 161–174. [Google Scholar] [CrossRef]
- El Alami, A.; Chait, A. Enquête Ethnopharmacologique et Ethnobotanique Sur Les Plantes Médicinales Dans Le Haut Atlas Central Du Maroc. Alger. J. Nat. Prod. 2017, 5, 427–445. [Google Scholar] [CrossRef]
- Shikov, V.; Kammerer, D.R.; Mihalev, K.; Mollov, P.; Carle, R. Heat Stability of Strawberry Anthocyanins in Model Solutions Containing Natural Copigments Extracted from Rose (Rosa Damascena Mill.) Petals. J. Agric. Food Chem. 2008, 56, 8521–8526. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Cuevas, E.; Winterhalter, P.; Garcia-Parrilla, M.C.; Troncoso, A.M. Isolation, Identification, and Antioxidant Activity of Anthocyanin Compounds in Camarosa Strawberry. Food Chem. 2010, 123, 574–582. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, J.; Yang, F.; Li, S.; Ma, W.; Chang, X.; Yang, L. Neuroprotective Effects of Quercetin on Ischemic Stroke: A Literature Review. Front. Pharmacol. 2022, 13, 854249. [Google Scholar] [CrossRef]
- Yu, L.; Chen, C.; Wang, L.F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.R. Neuroprotective Effect of Kaempferol Glycosides against Brain Injury and Neuroinflammation by Inhibiting the Activation of NF-ΚB and STAT3 in Transient Focal Stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef]
- Karimipour, M.; Mardani, M.; Ghanadian, M.; Esmaeili, A.; Mohammadnejad, D.; Alaei, H.; Esfandiary, E. Neuroprotective Effects of Rosa Damascena Extract on Learning and Memory in a Rat Model of Amyloid-β-Induced Alzheimer‘s Disease. Adv. Biomed. Res. 2015, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Esfandiary, E.; Karimipour, M.; Mardani, M.; Alaei, H.; Ghannadian, M.; Kazemi, M.; Mohammadnejad, D.; Hosseini, N.; Esmaeili, A. Novel Effects of Rosa Damascena Extract on Memory and Neurogenesis in a Rat Model of Alzheimer’s Disease. J. Neurosci. Res. 2014, 92, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, Y.; Kato, M.; Karasawa, K. Methylnigakinone Content Determination and Geographical Origin Discrimination for P. Quassioides via Fluorescence Fingerprint and Principal Component Analyses. J. Pharm. Biomed. Anal. 2022, 219, 114932. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Gao, H.; Zhao, F.; Lin, H.W.; Pan, Y.M.; Zhou, G.X.; Yao, X.S. Anti-Inflammatory Alkaloids from the Stems of Picrasma Quassioides BENNET. Chem. Pharm. Bull. 2011, 59, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Shimogama, T.; Saiin, C.; Kim, H.S.; Wataya, Y.; Ihara, M. π-Delocalized β-Carbolinium Cations as Potential Antimalarials. Bioorg. Med. Chem. Lett. 2004, 14, 1689–1692. [Google Scholar] [CrossRef]
- Houël, E.; Bertani, S.; Bourdy, G.; Deharo, E.; Jullian, V.; Valentin, A.; Chevalley, S.; Stien, D. Quassinoid Constituents of Quassia Amara L. Leaf Herbal Tea. Impact on Its Antimalarial Activity and Cytotoxicity. J. Ethnopharmacol. 2009, 126, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Tang, Q.; Xu, J.; Wang, S.; Li, S.; Zou, X.; Cao, Z. Dehydrocrenatidine Inhibits Voltage-Gated Sodium Channels and Ameliorates Mechanic Allodia in a Rat Model of Neuropathic Pain. Toxins 2019, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Mohd Jamil, M.D.H.; Taher, M.; Susanti, D.; Rahman, M.A.; Zakaria, Z.A. Phytochemistry, Traditional Use and Pharmacological Activity of Picrasma quassioides: A Critical Reviews. Nutrients 2020, 12, 2584. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.; Hu, Y.; Du, T.; Zhu, H.; Chen, L.; Qu, W.; Zhang, J.; Xie, N.; Liu, W.; Feng, F.; et al. Effects of Picrasma Quassioides and Its Active Constituents on Alzheimer’s Disease in Vitro and in Vivo. Bioorg. Chem. 2019, 92, 103258. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Seong, Y.H. Inhibitory Effect of the Leaves and Stems of Actinidia Arguta on Aβ (25-35)-Induced Neuronal Cell Death and Memory Impairment. J. Biomed. Transl. Res. 2018, 19, 26–31. [Google Scholar] [CrossRef]
- Choi, J.J.; Park, B.; Kim, D.H.; Pyo, M.Y.; Choi, S.; Son, M.; Jin, M. Blockade of Atopic Dermatitis-like Skin Lesions by DA-9102, a Natural Medicine Isolated from Actinidia Arguta, in the Mgdeficiency Induced Dermatitis Model of Hairless Rats. Exp. Biol. Med. 2008, 233, 1026–1034. [Google Scholar] [CrossRef]
- Zuo, L.L.; Wang, Z.Y.; Fan, Z.L.; Tian, S.Q.; Liu, J.R. Evaluation of Antioxidant and Antiproliferative Properties of Three Actinidia (Actinidia Kolomikta, Actinidia Arguta, Actinidia Chinensis) Extracts in Vitro. Int. J. Mol. Sci. 2012, 13, 5506–5518. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, M.; Comeskey, D.J.; Wohlers, M.; McGhie, T.K. Characterization and Quantification of Anthocyanins in Red Kiwifruit (Actinidia Spp.). J. Agric. Food Chem. 2009, 57, 6856–6861. [Google Scholar] [CrossRef] [PubMed]
- Takano, F.; Tanaka, T.; Tsukamoto, E.; Yahagi, N.; Fushiya, S. Isolation of (+)-Catechin and (-)-Epicatechin from Actinidia Arguta as Bone Marrow Cell Proliferation Promoting Compounds. Planta Med. 2003, 69, 321–326. [Google Scholar] [CrossRef]
- Tan, C.; Wang, Z.; Feng, X.; Pan, B.; Irfan, M.; Liu, C. Transcriptomic and Metabolomics of Flavonoid Compounds in Actinidia Arguta Var. Arguta. J. King Saud Univ.-Sci. 2021, 33, 101605. [Google Scholar] [CrossRef]
- Ha, J.S.; Kim, J.M.; Park, S.K.; Kang, J.Y.; Lee, D.S.; Lee, U.; Kim, D.O.; Choi, S.G.; Heo, H.J. Anti-Amyloidogenic Properties of an Ethyl Acetate Fraction from: Actinidia Arguta in Aβ1-42-Induced ICR Mice. Food Funct. 2018, 9, 3264–3277. [Google Scholar] [CrossRef] [PubMed]
- Hanish Singh, J.C.; Alagarsamy, V.; Diwan, P.V.; Sathesh Kumar, S.; Nisha, J.C.; Narsimha Reddy, Y. Neuroprotective Effect of Alpinia Galanga (L.) Fractions on Aβ (25-35) Induced Amnesia in Mice. J. Ethnopharmacol. 2011, 138, 85–91. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hashimoto, Y. Antimicrobial and Bactericidal Activities of Esters of 2-Endo-Hydroxy-1,8-Cineole as New Aroma Chemicals. J. Agric. Food Chem. 2002, 50, 3522–3526. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Khan, M.A.; Malik, M.T. Hypoglycaemic Activity of Alpinia Galanga Rhizome and Its Extracts in Rabbits. Fitoterapia 2002, 73, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Salleh, M.; Yassin, M.; Chin, C.B.; Chen, L.L.; Sim, N.L.; Roxb, C.; Linn, K. Fungal Activity of the Essential Oils of Nine Zingiberaceae Species. Pharm. Biol. 2003, 41, 392–397. [Google Scholar] [CrossRef]
- Khattak, S.; Saeed-Ur-Rehman; Shah, H.U.; Khan, T.; Ahmad, M. In Vitro Enzyme Inhibition Activities of Crude Ethanolic Extracts Derived from Medicinal Plants of Pakistan. Nat. Prod. Res. 2005, 19, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Tanaka, N.; Uehara, A.; Iwashina, T. Flavonoids Profile, Taxonomic Data, History of Cosmetic Uses, Anti-Oxidant and Anti-Aging Potential of Alpinia Galanga (L.) Willd. Cosmetics 2020, 7, 89. [Google Scholar] [CrossRef]
- Tasleem, F.; Azhar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and Anti-Inflammatory Activities of Piper Nigrum L. Asian Pac. J. Trop. Med. 2014, 7, S461–S468. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and Therapeutic Potential of Black Pepper [Piper Nigrum (L.)] Essential Oil and Piperine: A Review. Clin. Phytosci. 2021, 7, 52. [Google Scholar] [CrossRef]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.S.; Koo, B.S.; et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Kuete, V.; Mihasan, M. Methanolic Extract of Piper Nigrum Fruits Improves Memory Impairment by Decreasing Brain Oxidative Stress in Amyloid Beta(1-42) Rat Model of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2014, 34, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, K.K.; Taha, E.M.; Rahim, S.M. Phenolic Profile, Antioxidant, and Antibacterial Effects of Ethanol and Aqueous Extracts of Rheum Ribes L. Roots. Der Pharm. Lett. 2015, 7, 26–30. [Google Scholar]
- Noori, S.; Kiasat, A.R.; Kolahi, M.; Mirzajani, R.; Seyyed Nejad, S.M. Determination of Secondary Metabolites Including Curcumin in Rheum Ribes L. and Surveying of Its Antioxidant and Anticancer Activity. J. Saudi Chem. Soc. 2022, 26, 101479. [Google Scholar] [CrossRef]
- El Nebrisi, E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Degirmenci, U.; Akkapulu, M.; Comelekoglu, U.; Balli, E.; Metin Ozcan, T.; Berköz, M.; Yalin, A.E.; Yalin, S. The Effect of Rheum Ribes L. On Oxidative Stress in Diabetic Rats. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 20200058. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, M.; Hojjati, M.R.; Fathpour, H.; Rabieic, Z.; Alibabaei, Z.; Basima, A. Effect of Rheum Ribes Hydro-Alcoholic Extract on Memory Impairments in Rat Model of Alzheimer’s Disease. Iran. J. Pharm. Res. 2015, 14, 1197–1206. [Google Scholar] [PubMed]
- Ibrahim, M.; Kaushik, N.; Sowemimo, A.; Chhipa, H.; Koekemoer, T.; Van De Venter, M.; Odukoya, O.A. Antifungal and Antiproliferative Activities of Endophytic Fungi Isolated from the Leaves of Markhamia Tomentosa. Pharm. Biol. 2017, 55, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.B.; Sowemimo, A.A.; Sofidiya, M.O.; Badmos, K.B.; Fageyinbo, M.S.; Abdulkareem, F.B.; Odukoya, O.A. Sub-Acute and Chronic Toxicity Profiles of Markhamia Tomentosa Ethanolic Leaf Extract in Rats. J. Ethnopharmacol. 2016, 193, 68–75. [Google Scholar] [CrossRef]
- Stanley, T. Research Paper. 10 Perform.-Based Proj. Sci. Classr. 2021, 4, 107–115. [Google Scholar] [CrossRef]
- Tantangmo, F.; Lenta, B.N.; Boyom, F.F.; Ngouela, S.; Kaiser, M.; Tsamo, E.; Weniger, B.; Rosenthal, P.J.; Vonthron-Sénécheau, C. Antiprotozoal Activities of Some Constituents of Markhamia Tomentosa (Bignoniaceae). Ann. Trop. Med. Parasitol. 2010, 104, 391–398. [Google Scholar] [CrossRef]
- Sofidiya, M.O.; Agunbiade, F.O.; Koorbanally, N.A.; Sowemimo, A.; Soesan, D.; Familusi, T. Antiulcer Activity of the Ethanolic Extract and Ethyl Acetate Fraction of the Leaves of Markhamia Tomentosa in Rats. J. Ethnopharmacol. 2014, 157, 1–6. [Google Scholar] [CrossRef]
- Sowemimo, A.; Samuel, F.; Fageyinbo, M.S. Anti-Inflammatory Activity of Markhamia Tomentosa (Benth.) K. Schum. Ex Engl. Ethanolic Leaf Extract. J. Ethnopharmacol. 2013, 149, 191–194. [Google Scholar] [CrossRef]
- Ionita, R.; Postu, P.A.; Beppe, G.J.; Mihasan, M.; Petre, B.A.; Hancianu, M.; Cioanca, O.; Hritcu, L. Cognitive-Enhancing and Antioxidant Activities of the Aqueous Extract from Markhamia Tomentosa (Benth.) K. Schum. Stem Bark in a Rat Model of Scopolamine. Behav. Brain Funct. 2017, 13, 5. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B.H. Catechin Polyphenols: Neurodegeneration and Neuroprotection in Neurodegenerative Diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Z.; Yang, R.; Guo, D.; Zheng, J. Anthraquinones from Hairy Root Cultures of Cassia Obtusifolia. Phytochemistry 1998, 49, 1623–1625. [Google Scholar] [CrossRef]
- Wu, D.T.; Liu, W.; Han, Q.H.; Wang, P.; Xiang, X.R.; Ding, Y.; Zhao, L.; Zhang, Q.; Li, S.Q.; Qin, W. Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia Obtusifolia). Molecules 2019, 24, 2817. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wang, Y.; Wang, Q.; Yang, W.J.; Gu, Y.; Wang, R.; Song, X.M.; Wang, X.J. Quality Evaluation of Semen Cassiae (Cassia Obtusifolia L.) by Using Ultra-High Performance Liquid Chromatography Coupled with Mass Spectrometry. J. Sep. Sci. 2012, 35, 2054–2062. [Google Scholar] [CrossRef]
- Leung, S.W.; Lai, J.H.; Wu, J.C.; Tsai, Y. Neuroprotective Effects of Emodin against Ischemia / Reperfusion Injury through Activating ERK-1/2 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 2899. [Google Scholar] [CrossRef]
- Dong, H.K.; Byung, H.Y.; Kim, Y.W.; Lee, S.; Bum, Y.S.; Ji, W.J.; Hyoung, J.K.; Yong, S.L.; Jae, S.C.; Sun, Y.K.; et al. The Seed Extract of Cassia Obtusifolia Ameliorates Learning and Memory Impairments Induced by Scopolamine or Transient Cerebral Hypoperfusion in Mice. J. Pharmacol. Sci. 2007, 105, 82–93. [Google Scholar] [CrossRef]
- Boozari, M.; Butler, A.E.; Sahebkar, A. Impact of Curcumin on Toll-like Receptors. J. Cell. Physiol. 2019, 234, 12471–12482. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the Active Substance of Turmeric: Its Effects on Health and Ways to Improve Its Bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Kouhpeikar, H.; Butler, A.E.; Bamian, F.; Barreto, G.E.; Majeed, M.; Sahebkar, A. Curcumin as a Therapeutic Agent in Leukemia. J. Cell. Physiol. 2019, 234, 12404–12414. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Monteiro-Alfredo, T.; Silva, S.; Matafome, P. Curcumin Derivatives for Type 2 Diabetes Management and Prevention of Complications. Arch. Pharm. Res. 2020, 43, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Hosseiniyan Khatibi, S.M.; Khalilov, R.; Negahdari, R.; Zununi Vahed, S.; Maleki Dizaj, S. Anti-Microbial Activity of Curcumin Nanoformulations: New Trends and Future Perspectives. Phyther. Res. 2020, 34, 1926–1946. [Google Scholar] [CrossRef]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid Lipid Curcumin Particles Provide Greater Anti-Amyloid, Anti-Inflammatory and Neuroprotective Effects than Curcumin in the 5xFAD Mouse Model of Alzheimer’s Disease. BMC Neurosci. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Bisht, S.; Maitra, A.; Maitra, A.; Lahiri, D.K. Neuroprotective and Neurorescue Effects of a Novel Polymeric Nanoparticle Formulation of Curcumin (NanoCurcTM) in the Neuronal Cell Culture and Animal Model: Implications for Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 23, 61–77. [Google Scholar] [CrossRef]
- Samy, D.M.; Ismail, C.A.; Nassra, R.A.; Zeitoun, T.M.; Nomair, A.M. Downstream Modulation of Extrinsic Apoptotic Pathway in Streptozotocin-Induced Alzheimer’s Dementia in Rats: Erythropoietin versus Curcumin. Eur. J. Pharmacol. 2016, 770, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Zheng, B.W.; Guo, Y.; Zhao, J.; Zhao, J.Y.; Ma, X.W.; Jiang, Z.F. Antioxidative and Neuroprotective Effects of Curcumin in an Alzheimer’s Disease Rat Model Co-Treated with Intracerebroventricular Streptozotocin and Subcutaneous D-Galactose. J. Alzheimer’s Dis. 2016, 52, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-G.; Luo, X.-D. Correction to Meliaceous Limonoids: Chemistry and Biological Activities. Chem. Rev. 2012, 112, 2591. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Mitsui, K.; Fukaya, H.; Takeya, K. Fluorinated and Rearranged Gedunin Derivatives. Phytochem. Lett. 2012, 5, 486–489. [Google Scholar] [CrossRef]
- Nagini, S. Neem Limonoids as Anticancer Agents: Modulation of Cancer Hallmarks and Oncogenic Signaling, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 36, ISBN 9780128022153. [Google Scholar]
- Tom, S.; Rane, A.; Katewa, A.S.; Chamoli, M.; Matsumoto, R.R.; Andersen, J.K.; Chinta, S.J. Gedunin Inhibits Oligomeric Aβ1–42-Induced Microglia Activation Via Modulation of Nrf2-NF-ΚB Signaling. Mol. Neurobiol. 2019, 56, 7851–7862. [Google Scholar] [CrossRef] [PubMed]
- Sampson, L.; Rimm, E.; Hollman, P.C.H.; De Vries, J.H.M.; Katan, M.B. Flavonol and Flavone Intakes in US Health Professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Wach, A.; Pyrzyńska, K.; Biesaga, M. Quercetin Content in Some Food and Herbal Samples. Food Chem. 2007, 100, 699–704. [Google Scholar] [CrossRef]
- Sandeep, M.S.; Nandini, C.D. Influence of Quercetin, Naringenin and Berberine on Glucose Transporters and Insulin Signalling Molecules in Brain of Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2017, 94, 605–611. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Balen, G.P.; Van Den Berg, D.J.; Bast, A.; Van Der Vijgh, W.J.F. Influence of Iron Chelation on the Antioxidant Activity of Flavonoids. Biochem. Pharmacol. 1998, 56, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I.B.; Dcrozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and Free Radical Scavenging Mechanisms of Inhibitory Action of Rutin and Quercetin in Lipid Peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin Inhibits Expression of Inflammatory Cytokines through Attenuation of NF-ΚB and P38 MAPK in HMC-1 Human Mast Cell Line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Booyse, F.M.; Pan, W.; Grenett, H.E.; Parks, D.A.; Darley-Usmar, V.M.; Bradley, K.M.; Tabengwa, E.M. Mechanism by Which Alcohol and Wine Polyphenols Affect Coronary Heart Disease Risk. Ann. Epidemiol. 2007, 17, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective Effect of Quercetin in Primary Neurons against Aβ(1-42): Relevance to Alzheimer’s Disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ashrafpour, M.; Parsaei, S.; Sepehri, H. Quercetin Improved Spatial Memory Dysfunctions in Rat Model of Intracerebroventricular Streptozotocin-Induced Sporadic Alzheimer’sdisease. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 411–415. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.S.; Gacar, N.; Utkan, T.; Gacar, G.; Scarpace, P.J.; Tumer, N. Protective Effects of Resveratrol on Aging-Induced Cognitive Impairment in Rats. Neurobiol. Learn. Mem. 2016, 131, 131–136. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant Effects of Resveratrol in the Cardiovascular System. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J.H. Potential of Resveratrol in Anticancer and Anti-Inflammatory Therapy. Nutr. Rev. 2008, 66, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Yazir, Y.; Utkan, T.; Gacar, N.; Aricioglu, F. Resveratrol Exerts Anti-Inflammatory and Neuroprotective Effects to Prevent Memory Deficits in Rats Exposed to Chronic Unpredictable Mild Stress. Physiol. Behav. 2015, 138, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gupta, Y.K. Chronic Treatment with Trans Resveratrol Prevents Intracerebroventricular Streptozotocin Induced Cognitive Impairment and Oxidative Stress in Rats. Life Sci. 2002, 71, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, D.; Zhang, P.; Wang, X.; Du, G.; Brennan, C.; Li, S.; Ho, C.T.; Zhao, H. Antioxidant Protection of Nobiletin, 5-Demethylnobiletin, Tangeretin, and 5-Demethyltangeretin from Citrus Peel in Saccharomyces Cerevisiae. J. Agric. Food Chem. 2018, 66, 3155–3160. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive Effects of Nobiletin on Hyperleptinemia and Colitis-Related Colon Carcinogenesis in Male ICR Mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin Restoring β-Amyloid-Impaired CREB Phosphorylation Rescues Memory Deterioration in Alzheimer’s Disease Model Rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a Citrus Flavonoid, Improves Cognitive Impairment and Reduces Soluble Aβ Levels in a Triple Transgenic Mouse Model of Alzheimer’s Disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary Intakes of Flavonols, Flavones and Isoflavones by Japanese Women and the Inverse Correlation between Quercetin Intake and Plasma LDL Cholesterol Concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids Protect Neuronal Cells from Oxidative Stress by Three Distinct Mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Akaishi, T.; Morimoto, T.; Shibao, M.; Watanabe, S.; Sakai-Kato, K.; Utsunomiya-Tate, N.; Abe, K. Structural Requirements for the Flavonoid Fisetin in Inhibiting Fibril Formation of Amyloid β Protein. Neurosci. Lett. 2008, 444, 280–285. [Google Scholar] [CrossRef]
- Sagara, Y.; Vanhnasy, J.; Maher, P. Induction of PC12 Cell Differentiation by Flavonoids Is Dependent upon Extracellular Signal-Regulated Kinase Activation. J. Neurochem. 2004, 90, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Singh, R.; Sharma, D. Antiepileptic Effect of Fisetin in Iron-Induced Experimental Model of Traumatic Epilepsy in Rats in the Light of Electrophysiological, Biochemical, and Behavioral Observations. Nutr. Neurosci. 2017, 20, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Shim, I.; Lee, H.; Hahm, D.H. Phellodendron Amurense and Its Major Alkaloid Compound, Berberine Ameliorates Scopolamine-Induced Neuronal Impairment and Memory Dysfunction in Rats. Korean J. Physiol. Pharmacol. 2012, 16, 79–89. [Google Scholar] [CrossRef]
- Xuan, W.-T.; Wang, H.; Zhou, P.; Ye, T.; Gao, H.-W.; Ye, S.; Wang, J.-H.; Chen, M.-L.; Song, H.; Wang, Y.; et al. Berberine Ameliorates Rats Model of Combined Alzheimer’s Disease and Type 2 Diabetes Mellitus via the Suppression of Endoplasmic Reticulum Stress. 3 Biotech 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine Acutely Activates the Glucose Transport Activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Shi, X.; Li, X.; Hong, J.; Chen, J.; Gu, W.; Lu, X.; Xu, G.; Ning, G. Effect of Traditional Chinese Medicine Berberine on Type 2 Diabetes Based on Comprehensive Metabonomics. Talanta 2010, 81, 766–772. [Google Scholar] [CrossRef]
- Peng, W.H.; Hsieh, M.T.; Wu, C.R. Effect of Long-Term Administration of Berberine on Scopolamine-Induced Amnesia in Rats. Jpn. J. Pharmacol. 1997, 74, 261–266. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.A.; El-moneam, N.A. Neuroprotective Effect of Berberine against Environmental Heavy Metals-Induced Neurotoxicity and Alzheimer’s-like Disease in Rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef]
- Güzel, E.; Koçyiğit, Ü.M.; Taslimi, P.; Erkan, S.; Taskin, O.S. Biologically Active Phthalocyanine Metal Complexes: Preparation, Evaluation of α-Glycosidase and Anticholinesterase Enzyme Inhibition Activities, and Molecular Docking Studies. J. Biochem. Mol. Toxicol. 2021, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elbouzidi, A.; Taibi, M.; Ouassou, H.; Ouahhoud, S.; Ou-Yahia, D.; Loukili, E.H.; Aherkou, M.; Mansouri, F.; Bencheikh, N.; Laaraj, S. Exploring the Multi-Faceted Potential of Carob (Ceratonia siliqua var. Rahma) Leaves from Morocco: A Comprehensive Analysis of Polyphenols Profile, Antimicrobial Activity, Cytotoxicity against Breast Cancer Cell Lines, and Genotoxicity. Pharmaceuticals 2023, 16, 840. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Ou-Yahia, D.; Dalli, M.; Bellaouchi, R.; Tikent, A.; Roubi, M.; Gseyra, N.; Asehraou, A.; Hano, C.; et al. Assessment of the Antioxidant and Antimicrobial Potential of Ptychotis Verticillata Duby Essential Oil from Eastern Morocco: An In Vitro and In Silico Analysis. Antibiotics 2023, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Mendie, L.E.; Hemalatha, S. Molecular Docking of Phytochemicals Targeting GFRs as Therapeutic Sites for Cancer: An In Silico Study. Appl. Biochem. Biotechnol. 2022, 194, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; El Hachlafi, N.; Al-Mijalli, S.H.; Jeddi, M.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G.; et al. Phytochemical Profile, Assessment of Antimicrobial and Antioxidant Properties of Essential Oils of Artemisia Herba-Alba Asso., and Artemisia Dracunculus L.: Experimental and Computational Approaches. J. Mol. Struct. 2023, 1294, 136479. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; El Hachlafi, N.; El Kamili, T.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G. Integrated Analysis of Antimicrobial, Antioxidant, and Phytochemical Properties of Cinnamomum Verum: A Comprehensive In Vitro and In Silico Study. Biochem. Syst. Ecol. 2023, 110, 104700. [Google Scholar] [CrossRef]
- Ishola, A.A.; Oyinloye, B.E.; Ajiboye, B.O.; Kappo, A.P. Molecular Docking Studies of Flavonoids from Andrographis Paniculata as Potential Acetylcholinesterase, Butyrylcholinesterase and Monoamine Oxidase Inhibitors towards the Treatment of Neurodegenerative Diseases. Biointerface Res. Appl. Chem. 2021, 11, 9871–9879. [Google Scholar] [CrossRef]
- Kandsi, F.; Elbouzidi, A.; Lafdil, F.Z.; Meskali, N.; Azghar, A.; Addi, M.; Hano, C.; Maleb, A.; Gseyra, N. Antibacterial and Antioxidant Activity of Dysphania Ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches. Antibiotics 2022, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Elbouzidi, A.; Ouassou, H.; Aherkou, M.; Kharchoufa, L.; Meskali, N.; Baraich, A.; Mechchate, H.; Bouhrim, M.; Idir, A.; Hano, C.; et al. LC–MS/MS Phytochemical Profiling, Antioxidant Activity, and Cytotoxicity of the Ethanolic Extract of Atriplex Halimus L. against Breast Cancer Cell Lines: Computational Studies and Experimental Validation. Pharmaceuticals 2022, 15, 1156. [Google Scholar] [CrossRef] [PubMed]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Grujić-Letić, N. Species of the Genus Salix L.: Biochemical Screening and Molecular Docking Approach to Potential Acetylcholinesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef]
- Fan, J.; Fu, A.; Zhang, L. Progress in Molecular Docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Shakil, S.; Haneef, M. A Simple Click by Click Protocol to Perform Docking: Autodock 4.2 Made Easy for Non-Bioinformaticians. EXCLI J. 2013, 12, 830–857. [Google Scholar]
- Paul Gleeson, M.; Hersey, A.; Hannongbua, S. In-Silico ADME Models: A General Assessment of Their Utility in Drug Discovery Applications. Curr. Top. Med. Chem. 2011, 11, 358–381. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Ishola, A.A.; Adewole, K.E. In Silico Screening of Anticholinesterase Alkaloids for Cyclooxygenase-2 (COX-2) and Matrix Metalloproteinase 8 (MMP-8) Inhibitory Potentials as Multi-Target Inhibitors of Alzheimer’s Disease. Med. Chem. Res. 2019, 28, 1704–1717. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.H.; Wang, T.M.; Kang, T.G.; Sun, H.Y.; Pei, W.H.; Song, H.P.; Zhang, H. A Strategy to Discover Selective α-Glucosidase/Acetylcholinesterase Inhibitors from Five Function-Similar Citrus Herbs through LC-Q-TOF-MS, Bioassay and Virtual Screening. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1174, 122722. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a Citrus Flavonoid That Improves Memory Impairment, Rescues Bulbectomy-Induced Cholinergic Neurodegeneration in Mice. J. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; De Luca, D.; Colica, C.; Menichini, F. Evaluation of Citrus Aurantifolia Peel and Leaves Extracts for Their Chemical Composition, Antioxidant and Anti-Cholinesterase Activities. J. Sci. Food Agric. 2012, 92, 2960–2967. [Google Scholar] [CrossRef]
- Mishra, A. Molecular Docking Analysis of Modified Gedunin from Neem with Snake Venom Enzymes. Bioinformation 2021, 17, 776–783. [Google Scholar] [CrossRef]
- Bajda, M.; Wiȩckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Nguyen, T.C.V.; Nguyen, N.S.; Nguyen, D.M.; Nguyen, T.T.H.; Le, M.T.; Thai, K.M. Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Appl. Sci. 2016, 6, 198. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.T.; Clark, D.E. In Silico Predictions of Blood-Brain Barrier Penetration: Considerations to “Keep in Mind”. J. Pharmacol. Exp. Ther. 2005, 315, 477–483. [Google Scholar] [CrossRef]
- Pardridge, W.M. Alzheimer’s Disease Drug Development and the Problem of the Blood-Brain Barrier. Alzheimer’s Dement. 2009, 5, 427–432. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).